Aflatoxin Detection and Quantification in Stored Cowpea Seeds in Ibadan, Nigeria
Article Information
Oredoyin A Ogungbemile, Peter M Etaware*, Adegboyega C Odebode
Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Nigeria
*Corresponding author: Peter M Etaware, Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Nigeria
Received: 26 December 2019; Accepted: 31 December 2019; Published: 03 January 2020
Citation:
Oredoyin A Ogungbemile, Peter M Etaware, Adegboyega C Odebode. Aflatoxin Detection and Quantification in Stored Cowpea Seeds in Ibadan, Nigeria. Journal of Biotechnology and Biomedicine 3 (2020): 010-017.
View / Download Pdf Share at FacebookAbstract
Aflatoxins were reportedly detected in the kidney of 58% of deceased children caused by kwashiorkor at Obafemi Awolowo University Teaching Hospital, Ile-Ife. The incidence of kwashiorkor in West Africa has since been associated with aflatoxin contamination of human diets. Although, more researches need to be conducted to proper ascertain the level of menace caused by Aflatoxin ingestion in humans, the frequency of occurrence on stored cowpea seeds in Nigeria was the aim of this research. The procedure described by Association of Official Analytical Chemists AOAC [1] was used in the detection and quantification of Aflatoxins present in stored cowpea seeds in Ibadan, Nigeria. Aflatoxins B1, B2, G1 and G2 were detected in all cowpea samples analyzed. Samples collected from Oja-Oba market were found to contain the highest amount Aflatoxins i.e. B1 (1.5 × 10-2 µg/g), B2 (0.80 × 10-2 µg/g), G1 (0.60 × 10-2 µg/g), and G2 (1.0 × 10-2 µg/g) compared to those found in other market locations, the control setup and WHO [2] standards (P ≤ 0.05). The result of this study calls for public health concern due to the high level of Aflatoxins found in cowpea seeds from local markets in Ibadan, Oyo State, Nigeria. Utmost care should be taken to minimize or totally eliminate postharvest pathogens that produce mycotoxins in stored grains and pulses.
Keywords
Aflatoxins, Kwashiorkor, Stored cowpea seeds, Local markets, Postharvest pathogens
Aflatoxins articles Aflatoxins Research articles Aflatoxins review articles Aflatoxins PubMed articles Aflatoxins PubMed Central articles Aflatoxins 2023 articles Aflatoxins 2024 articles Aflatoxins Scopus articles Aflatoxins impact factor journals Aflatoxins Scopus journals Aflatoxins PubMed journals Aflatoxins medical journals Aflatoxins free journals Aflatoxins best journals Aflatoxins top journals Aflatoxins free medical journals Aflatoxins famous journals Aflatoxins Google Scholar indexed journals Kwashiorkor articles Kwashiorkor Research articles Kwashiorkor review articles Kwashiorkor PubMed articles Kwashiorkor PubMed Central articles Kwashiorkor 2023 articles Kwashiorkor 2024 articles Kwashiorkor Scopus articles Kwashiorkor impact factor journals Kwashiorkor Scopus journals Kwashiorkor PubMed journals Kwashiorkor medical journals Kwashiorkor free journals Kwashiorkor best journals Kwashiorkor top journals Kwashiorkor free medical journals Kwashiorkor famous journals Kwashiorkor Google Scholar indexed journals Stored cowpea seeds articles Stored cowpea seeds Research articles Stored cowpea seeds review articles Stored cowpea seeds PubMed articles Stored cowpea seeds PubMed Central articles Stored cowpea seeds 2023 articles Stored cowpea seeds 2024 articles Stored cowpea seeds Scopus articles Stored cowpea seeds impact factor journals Stored cowpea seeds Scopus journals Stored cowpea seeds PubMed journals Stored cowpea seeds medical journals Stored cowpea seeds free journals Stored cowpea seeds best journals Stored cowpea seeds top journals Stored cowpea seeds free medical journals Stored cowpea seeds famous journals Stored cowpea seeds Google Scholar indexed journals Local markets articles Local markets Research articles Local markets review articles Local markets PubMed articles Local markets PubMed Central articles Local markets 2023 articles Local markets 2024 articles Local markets Scopus articles Local markets impact factor journals Local markets Scopus journals Local markets PubMed journals Local markets medical journals Local markets free journals Local markets best journals Local markets top journals Local markets free medical journals Local markets famous journals Local markets Google Scholar indexed journals Postharvest pathogens articles Postharvest pathogens Research articles Postharvest pathogens review articles Postharvest pathogens PubMed articles Postharvest pathogens PubMed Central articles Postharvest pathogens 2023 articles Postharvest pathogens 2024 articles Postharvest pathogens Scopus articles Postharvest pathogens impact factor journals Postharvest pathogens Scopus journals Postharvest pathogens PubMed journals Postharvest pathogens medical journals Postharvest pathogens free journals Postharvest pathogens best journals Postharvest pathogens top journals Postharvest pathogens free medical journals Postharvest pathogens famous journals Postharvest pathogens Google Scholar indexed journals Mycotoxin articles Mycotoxin Research articles Mycotoxin review articles Mycotoxin PubMed articles Mycotoxin PubMed Central articles Mycotoxin 2023 articles Mycotoxin 2024 articles Mycotoxin Scopus articles Mycotoxin impact factor journals Mycotoxin Scopus journals Mycotoxin PubMed journals Mycotoxin medical journals Mycotoxin free journals Mycotoxin best journals Mycotoxin top journals Mycotoxin free medical journals Mycotoxin famous journals Mycotoxin Google Scholar indexed journals Moore Plantation articles Moore Plantation Research articles Moore Plantation review articles Moore Plantation PubMed articles Moore Plantation PubMed Central articles Moore Plantation 2023 articles Moore Plantation 2024 articles Moore Plantation Scopus articles Moore Plantation impact factor journals Moore Plantation Scopus journals Moore Plantation PubMed journals Moore Plantation medical journals Moore Plantation free journals Moore Plantation best journals Moore Plantation top journals Moore Plantation free medical journals Moore Plantation famous journals Moore Plantation Google Scholar indexed journals Analytical Chemists articles Analytical Chemists Research articles Analytical Chemists review articles Analytical Chemists PubMed articles Analytical Chemists PubMed Central articles Analytical Chemists 2023 articles Analytical Chemists 2024 articles Analytical Chemists Scopus articles Analytical Chemists impact factor journals Analytical Chemists Scopus journals Analytical Chemists PubMed journals Analytical Chemists medical journals Analytical Chemists free journals Analytical Chemists best journals Analytical Chemists top journals Analytical Chemists free medical journals Analytical Chemists famous journals Analytical Chemists Google Scholar indexed journals Aspergillus flavus articles Aspergillus flavus Research articles Aspergillus flavus review articles Aspergillus flavus PubMed articles Aspergillus flavus PubMed Central articles Aspergillus flavus 2023 articles Aspergillus flavus 2024 articles Aspergillus flavus Scopus articles Aspergillus flavus impact factor journals Aspergillus flavus Scopus journals Aspergillus flavus PubMed journals Aspergillus flavus medical journals Aspergillus flavus free journals Aspergillus flavus best journals Aspergillus flavus top journals Aspergillus flavus free medical journals Aspergillus flavus famous journals Aspergillus flavus Google Scholar indexed journals Agriculture articles Agriculture Research articles Agriculture review articles Agriculture PubMed articles Agriculture PubMed Central articles Agriculture 2023 articles Agriculture 2024 articles Agriculture Scopus articles Agriculture impact factor journals Agriculture Scopus journals Agriculture PubMed journals Agriculture medical journals Agriculture free journals Agriculture best journals Agriculture top journals Agriculture free medical journals Agriculture famous journals Agriculture Google Scholar indexed journals
Article Details
1. Introduction
Cowpea is an essential crop with multipurpose usage among the locals of Nigeria, which made the country famous (1st) in both production and consumption (Adegbite and Amusa, [3]). The seeds and leaves play pertinent roles in Ethno-medicine, they are used in the treatment of various ailments such as swellings and infections, tooth aches, insect stings (Brink and Belay, [4]) and common cold (Siddhuraju and Becker, [5]). The roots are also potent cure for chest pain, epilepsy, dysmenorrheal and constipation (Brink and Belay, [4]). However, most seeds (cowpea seeds inclusive) are highly susceptible to microbial contamination under poor storage conditions (Frisvad et al., 2006; Etaware and Etaware, [6-8]). Fungi and insects play significant roles in the reduction of seed quality during storage (Richard et al., 2009; Etaware, 2019 [8, 9]). The Food and Agriculture Organization (FAO) estimated over 25% of the world’s cereals contaminated by mycotoxins (FAO [10]). Mycotoxins are secondary metabolites secreted by fungi, which can cause diseases or death in humans and other animals when ingested in large quantity (Bennett and Klich, 2003; Bandyopadhyay et al., [11, 12]).
Mycotoxin contamination of various foodstuff and animal feeds are a major problem in the tropics and subtropics, where climatic conditions and storage practices are favorable for fungal growth (Quiroga et al., 2009; Shukla et al., 2009; Salari et al., [13-15]). Aflatoxins (Figure 1) are one of the most lethal and poisonous mycotoxins present in stored grains (Manafi and Khosravinia, [16]). They are produced by toxigenic strains of Aspergillus and other related moulds (Mazaheri, 2009; Da Costa et al., [17, 18]). Studies carried out by the International Agency for Research on Cancer (IARC) led to the classification of aflatoxins as human carcinogen (IARC [19]). Aflatoxins can interfere with vaccine-induced immunity (Farag, [20]), cause immunosuppression, impaired growth, damaged liver, and death in livestock or man (John and Steve, [21]). The liver is the main target; therefore, the level of damage depends on the reaction with DNA, RNA, enzymes and proteins (John and Steve, [21]), as illustrated in (Figure 2). Agricultural products such as groundnut, maize and cowpea are major targets for Aspergillus species (Whitlow and Hagler, [22]). Aflatoxins are Thermostable (heat resistant) and are not completely eliminated by normal cooking procedure (Kishore et al., [23]).
The incidence of kwashiorkor in West Africa has been associated with aflatoxin contamination of human diets (Tiffany, [24] . Severe cases of aflatoxicosis were recorded in Kenya in 2002 (CAST [25]), 317 people were reported ill, while 125 people died (CDC, 2004; Lewis et al., [26, 27]). In 1974, 100 out 0f 400 people died from eating contaminated maize- based cereals in India Lawley, [28]. According to Reddey et al. [29], these deaths may have resulted from immune toxicity in which lymphocytes immune functions were suppressed or disturbed by these toxins. Aflatoxins were reportedly detected in the kidney of 58% of deceased children by kwashiorkor at Obafemi Awolowo University Teaching Hospital, Ile-Ife (Oyelami et al., [30]). In a similar report by Onyemelukwe et al. [31], aflatoxins were found in both the sera and the blood of children with kwashiorkor. These data indicate that higher aflatoxins were more frequently detected in sera and urine of patients with kwashiorkor, as compared to the healthy control within similar age groups (Onyemelukwe et al., [31]. Although, more researches need to be conducted to proper ascertain the level of menace caused by Aflatoxin ingestion in humans, placing a priority on the management of aflatoxigenic fungi and their frequency of occurrence on stored cowpea seeds in Nigeria is imminent in order to safeguard human lives.
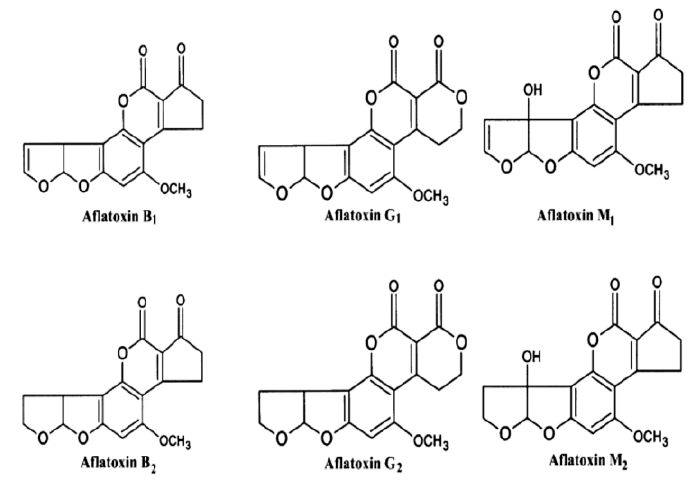
Figure 1: Chemical structures of Aflatoxins B1, B2, G1, G2, M1 and M2 (Zain, [32]).
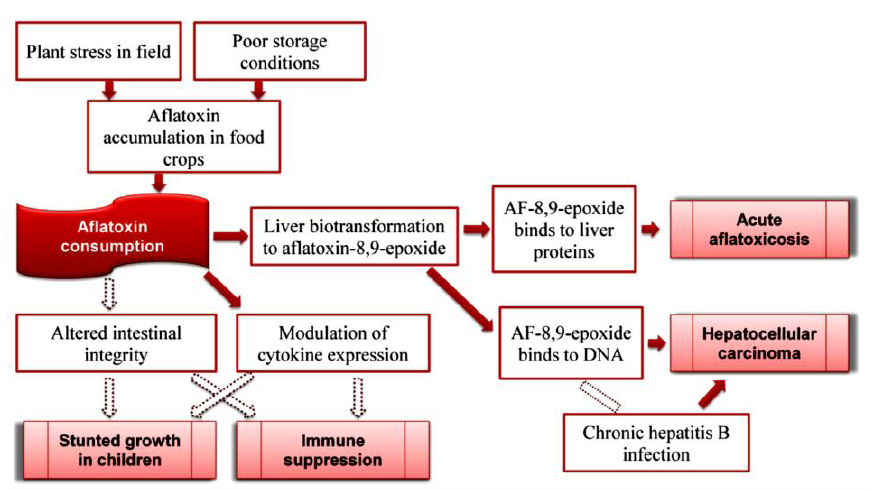
Figure 2: Aflatoxin and disease pathways in humans. Source: Wu [33].
2. Methodology
2.1 Sample collection
A total of 500g of cowpea seeds were obtained from three (3) randomly selected store houses in Sasa, Bodija and Oja-Oba markets in Ibadan, Oyo state, Nigeria (Test Locations) and pure breed healthy samples from the Institute of Agricultural Research and Training (IAR&T), Moore Plantation, Ibadan, Oyo State, Nigeria (Control Location). The samples were aseptically packaged in sterile sample collection bags, labeled appropriately and transferred to the Mycology/Pathology Laboratory of the Department of Botany, University of Ibadan, Ibadan, Oyo State, Nigeria and the toxin detection Centre of the Institute of Agriculture Research and Training (IAR&T), Moore Plantation, Ibadan, Oyo State. for further analysis.
2.2 Detection of Aflatoxin in cowpea seeds
The procedure described by Association of Official Analytical Chemists AOAC [1] was used for this analysis.
2.2.1 Procedure
The cowpea samples were sorted and pulverized. A total of 1g of aqueous sample was pipetted into 100ml conical flask; 2.5ml of distilled water and 25ml of chloroform was added.
The flask was covered with a stopper and rocked in a shaker for 30 minutes after which the solution obtained was filtered using Whatman filter paper no.1.
About 10ml of each filtrate was collected and evaporated to dryness to a volume of 5ml in water bath.
Chloroform (1ml) was spotted and 0.2ml of the reconstituted extract was placed on a pre-coated 20 x 20cm Thin Layer Chromatography (TLC) plate along with Aflatoxin standards of known concentration.
The spotted TLC plate was developed in an equilibrated tank containing chloroform: acetone (9:1 v/v).
The developed TLC plate was air dried at ambient temperature (28 ± 2ºc) and detection of aflatoxins was done under UV light at wavelength of 360nm.
A colour change from blue to yellow upon exposure to aqueous sulphuric acid (50:50 v/v) confirms the presence of Aflatoxin B1.
Aflatoxin B2 is a dihydro-derivative of Aflatoxin B1 which will produce a colour change from pale blue to deep yellowish upon exposure to aqueous sulphuric acid (50:50). This confirms the presence of Aflatoxin B2.
Aflatoxin G1 fluoresces yellowish green upon exposure to UV light while Aflatoxin G2 fluoresces pale yellowish green upon exposure to same UV light.
2.3 Quantification of Aflatoxin in cowpea seeds
The procedure described by Association of Official Analytical Chemists AOAC [1] was used for this analysis.
2.3.1 Procedure
TLC plates of O.5µm were used for the quantification. 0.8ml of initially extracted cowpea samples was applied to the plate as bands.
The preparative TLC plates were developed in an equilibrated tank for aflatoxin extraction.
When the solvent front has risen to about three- quarter of the total length of the plate, the plate was taken out of the tank and examine under UV light.
Once the area containing the toxin of interest is located after UV light examination, it is scrapped off, elute with chloroform and filtered using Whatman filter paper No.1.
The extract was evaporated to dryness in a water bath and reconstitute with 3ml chloroform.
The 3ml reconstituted solution and aflatoxin standard of 20µg/ml concentration was used to read Absorbance or Optical Density on an ultraviolet Spectrophotometer (Cecil Instrument CE505) at a wavelength of 360nm. Aflatoxin concentration in µg/g was calculated using the formula stated below:
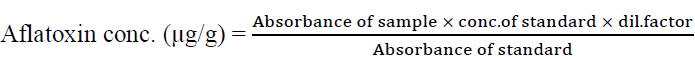
3. Data analysis
The data obtained from the research was organized and analyzed using Costat 6.451 statistical software. Tables and graphs was prepared using excel worksheets and the homogeneity of means was determined by Duncan Multiple Range Test (DMRT). Data was represented as means and standard deviation.
4. Results
4.1 Aflatoxin detection in stored cowpea seeds from Ibadan, Nigeria
The laboratory analysis conducted showed that Aflatoxins B1, B2, G1 and G2 were detected in all the cowpea samples collected from Sasa, Bodija and Oja-Oba markets in Ibadan, Oyo State, Nigeria (Table 1).
4.2 Aflatoxins quantification and characterization
Cowpea seeds collected from Oja-Oba market was found to contain the highest amount Aflatoxins i.e. B1 (1.5 x 10-2µg/g), B2 (0.80 x 10-2µg/g), G1 (0.60 x 10-2µg/g), and G2 (1.0 x 10-2µg/g) which were significantly different (P≤0.05) from those found in cowpea samples from other market locations, the control setup and [2] WHO recommended standards (Table 2). The Aflatoxin G1 and G2 contents of stored cowpea samples from Sasa market [G1 (0.20 x 10-2µg/g) and G2 (0.50 x 10-2µg/g), respectively] and Bodija market [G1 (0.40 x 10-2µg/g)], respectively) were not statistically significant (P≤0.05) from the levels found in the control setup (G1 (0.20 x 10-2µg/g), and G2 (0.30 x 10-2µg/g), respectively) and WHO standards (G1 (0.30 x 10-2µg/g), and G2 (0.30 x 10-2µg/g), respectively) at P≤0.05 (Table 2).
|
Location |
Aflatoxin |
|||
|
B1 |
B2 |
G1 |
G2 |
|
|
Sasa Market |
+ |
+ |
+ |
+ |
|
Bodija Market |
+ |
+ |
+ |
+ |
|
Oja-Oba Market |
+ |
+ |
+ |
+ |
+ = Aflatoxin detected; - = Aflatoxin not detected
Table 1: Aflatoxins detected in cowpea seeds from local markets in Ibadan, Nigeria.
|
Samples |
Purpose |
Location |
Aflatoxin (x 10-2µg/g) |
|||
|
B1 |
B2 |
G1 |
G2 |
|||
|
Stored Cowpea Seeds |
Public Consumption |
Sasa |
1.00 ± 0.00b |
0.50 ± 0.10ab |
0.20 ± 0.00b |
0.50 ± 0.00bc |
|
Bodija |
0.70 ± 0.10b |
0.50 ± 0.00ab |
0.40 ± 0.00b |
0.60 ± 0.00b |
||
|
Oja-Oba |
1.50 ± 0.10a |
0.80 ± 0.10a |
0.60 ± 0.00a |
1.00 ± 0.10a |
||
|
Control |
IAR&T |
0.40 ± 0.00c |
0.30 ± 0.00b |
0.20 ± 0.10b |
0.30 ± 0.10c |
|
|
Fresh |
Standard |
WHO |
0.30 ± 0.10c |
0.30 ± 0.10b |
0.30 ± 0.10b |
0.30 ± 0.10c |
Means with the same alphabets down the column are not significantly different at P≤0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only. Note: WHO – World Health Organization
Table 2: Aflatoxin quantification and characterization of cowpea seeds from Ibadan, Nigeria.
5. Discussion
Aflatoxins were detected in all the cowpea samples from Ibadan, Oyo State, Nigeria. This could be due to improper handling during harvest and poor storage conditions. This was earlier reported by Esuruoso [34] who stated that Cowpea seeds exported from Western Nigeria in the early 1970s were reported to harbor moulds such as Aspergillus flavus, A. niger, Fusarium vertilliodes, F. solani, Penicillium digitatum and Rhizopus sp. etc. which are capable of mycotoxin production. This however points to a potential health hazards to human beings and animals. Aflatoxins B1, B2, G1, and G2 were detected in all the cowpea seeds used for this study. Aflatoxin contamination was higher than the tolerable limit stipulated by the Standard Organization of Nigeria (SON) and World Health Organization WHO) in majority of the cowpea samples collected. The highest Aflatoxin levels were found in cowpea samples from Oja-Oba market, while the lowest was found in Sasa market, which was within the tolerable limit (in some cases). The levels of aflatoxin contamination in cowpea seeds in the present study may be attributed to several factors among which are favorable environmental conditions and poor storage facilities among other factors. The presence of these aflatoxins in cowpea seeds should be monitored intermittently to avoid serious health hazards or even death to humans and other consumers of cowpea seeds and other cowpea products. A similar trend of Aflatoxin infiltration of stored cowpea seeds had earlier been reported by Seenappa [35] who investigated Aspergillus species infection and aflatoxin production in some cowpea lines in Tanzania. However, Houssou et al., [36] detected only Aflatoxin B1 in samples of cowpea seeds collected after three (3) months of storage in the republic of Benin, West Africa.
6. Conclusion
The result of this study raised a major public health concern due to the high level of Aflatoxins found in stored cowpea seeds collected from local markets in Ibadan, Oyo State, Nigeria. Utmost care should be taken to minimize or totally eliminate postharvest pathogens that produce mycotoxins in stored grains and pulses. Proper handling and processing of cowpea and cowpea products should be encouraged. Farmers and marketers should be trained on appropriate techniques for harvesting, handling and storage. To avoid domestic hazard from consumption of contaminated cowpea seeds, the National Agency for Food, Drug Administration and Control (NAFDAC) and other food agencies in Nigeria should conduct regular check-ups or quarantine services for cowpea seeds in circulation within and around markets in Nigeria.
References
- Association of Official Analytical Chemist. Official methods of analysis (23rd edition Volume II). Natural Toxins, Method number 991.31(AOAC, International) (2005): 456.
- World Health Organization (WHO). Mycotoxins in African foods: Implications to food safety and health. AFRO Newsletter WHO Food Safety (FOS) Issue 2, July (2006).
- Adegbite AA, Amusa NA. The major economic field diseases of cowpea in the humid agro-ecologies of South-Western Nigeria. African Journal of Biotechnology 7 (2008): 4706-4712.
- Brink M, Belay G. Plant Resources of Tropical Africa, Cereals and Pulses. Backhuys Publishers, CTA Wageningen, Netherlands 1 (2006): 298.
- Sidduraju P, Becker K, et al. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Journal of Food Chemistry 101 (2007): 10-19.
- Frisvad JC, Thrane U, Samson RA, et al. Important mycotoxins and the fungi which produce them. Advances in Experimental Medicine Biology 571 (2006): 3-31.
- Etaware PM. Stereotyping Fungi Affecting Stored Melon Seeds within Local Markets in Lagos, Nigeria. Journal of Applied Microbiological Research 2 (2019): 14-20.
- Etaware EU, Etaware PM. Conservation of the functional properties of okra powder by local storage techniques. Journal of Nanotechnological Research 1 (2019): 136-143.
- Richard E, Heutte N, Bouchart V, et al. Evaluation of fungal contamination and mycotoxin production in maize silage. Animal Feed Science and Technology 148 (2009): 309-320.
- Food and Agricultural Organization (FAO). Accessed on 08 June 2017 at 15:36pm GMT (2011).
- Bennett JW, Klich M. Mycotoxins. Clinical Microbiological Review 16 (2003): 497-516.
- Bandyopadhyay R, Kumar M, Leslie J. Food Additives and Contaminants 24 (2007): 1109-1114.
- Quiroga EN, Sampietro DA, Sgariglia MA, et al. Antimycotic Activity of 5_- prenylisoflavanones of the Plant Geoffroea decorticans, against Aspergillus International Journal of Food Microbiology 132 (2009): 42-46.
- Shukla R, Kumar A, Singh P, et al. Efficacy of Lippa alba (Mill.) N. Brown Essential Oil and Its Monoterpene Aldehyde Constituents against Fungi Isolated from Some Edible Legume Seeds and Aflatoxin B1 Production. International Journal of Food Microbiology 135 (2009): 165-170.
- Salari R, Najafi MBH, Boroushaki MT, et al. Assessment of the Microbiological Quality and Mycotoxin Contamination of Iranian Red Pepper Spice. Journal of Agricultural Science and Technology 14 (2012): 1511-1521.
- Manafi M, Khosravinia H. Effects of Aflatoxin on the Performance of Broiler Breeders and Its Alleviation through Herbal Mycotoxin Binder. Journal Agricultural Science and Technology 15 (2013): 55-63.
- Mazaheri M. Determination of aflatoxins in imported rice to Iran. Food Chemistry and Toxicology 47 (2009): 2064-2066.
- Da Costa CL, Geraldo MR, Arroteia CC, et al. In vitro activity of neem oil on Aspergillus flavus growth, sporulation viability of spores, morphology and aflatoxin B1 and B2. Advances in Biosciences and Biotechnology 1 (2010): 292-299.
- International Agency for Research on Cancer (IARC). Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monotoring and Evaluation of Carcinogens causing Risks to Humans 82 (2002): 1-556.
- Farag DM. Aflatoxins: Awareness and control. Dubai International Food Safety Conference (2008): 1-55.
- John P, Steve E. Mycotoxin contamination of corn – what it is, what it does to pigs and what can be done about it. Iowa state University Extension. IPIC 12 (2010): 1-7.
- Whitlow LW, Hagler WM. Mycotoxin contamination of feedstuffs – an additional stress factor for Diary cattle. www.cals.ncsu.edu/.../myco~1.pd... Retrieved 10 November, 2016 (2013): 32.
- Kishore KG, Pande S, Manjula K, et al. Occurrence of mycotoxins and toxigenic fungi in groundnut (Arachis hypogaea L.) seeds in Andhra Pradesh, India. Plant Pathology Journal 18 (2002): 204-209.
- Tiffany I. The implication of aflatoxin contamination for local food safety in Senegal (2013).
- Council for Agricultural Science and Technology (CAST). Mycotoxins: risks in plant, animal, and human systems. Task Force Report, Ames, IA (2003): 139.
- Centers for Disease Control and Prevention (CDC). Outbreak of aflatoxin poisoning eastern and central provinces, Kenya, January–July, 2004. MMWR Morb Mortal Wkly Rep 53 (2004): 790-792.
- Lewis L, Onsongo M, Njapau H, et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in Eastern and Central Kenya. Environmental Health Perspective 113 (2005): 1763-1767.
- Lawley R. Aflatoxins. Food Safety Watch. https//:www.foodsafetywatch.org/facts. Retrieved March 22, 2017 (2013).
- Reddy VK, Srinivas M, Reddy AR, et al. Plant extracts and the management of aflatoxin production by Aspergillus flavus. International Journal of Pharmaceutical and Biological Sciences 2 (2011): 492-498.
- Oyelami OA, Maxwell S, Adelusola KA, et al. Aflatoxins in autopsy kidney specimens from children in Nigeria. Journal of Toxicology and Environmental Health 55 (1998): 317-323.
- Onyemelukwe GC, Ogoina D, Ibiam GE, et al. Aflatoxins in body fluids and food of Nigerian children with Protein- Energy Malnutrition (PEM). African Journal of Food, Agriculture and Nutritional Development 12 (2012): 6553-6566.
- Zain ME. Impact of mycotoxins on human and animals, Journal of Saudi chemical society 5 (2011): 129-144.
- Wu F. The global burden of disease caused by foodborne aflatoxin. WHO Commissioned Report, Foodborne Disease Burden Epidemiology Reference Group (FERG) (2010).
- Esuruoso OF. Seed-borne fungi of cowpea (Vigna unguiculata) in Western Nigeria. Nigeria Journal of Plant Produce. 2 (1975): 87-90.
- Seenappa M, Keswani CL, Kundya TM. Aspergillus infection and aflatoxin production in some cowpea (Vigna unguiculata (L.) Walp.) lines in Tanzania. 83 (1983): 103-106.
- Houssou PA, Ahuhuendo BC, Jacobsem M. Natural infection of cowpea by toxigenic fungi and mycotoxins in Benin, West Africa. Journal of Stored Products and Research 45 (2009): 40-44.


 Impact Factor: * 5.3
Impact Factor: * 5.3 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 