Anti-GnRH Neutralizing Antibodies Produce Testosterone Ablation and Tumor Shrinkage in Prostate Cancer Models
Article Information
Jesús A. Junco1*, Franklin Fuentes1, Lesvia Calzada1, Eddy Bover1, Eulogio Pimentel2, Yovisleidis López1, Roberto Basulto1, Hilda Garay2, Osvaldo Reyes2, Angel Cid-Arregui4, Maria D. Castro1, Rafael Martínez2, Niurka Arteaga1, Ana Campal1, Ayni Rodríguez3, Andrés Serradelo1, Eduardo Hernández1, Mirialis Arias, Raúl González1, Matilde López2, Gisell Bebert5, Gerardo Guillén2
Center for Genetic Engineering and Biotechnology of Camaguey. Ave Finlay y Circunvalación Norte, CP 70100, Camaguey, Cuba
Center for Genetic Engineering and Biotechnology. Ave 31 e/ 158 y 190, Playa, P.O. Box 6162. Habana 10600, Cuba
Camaguey Medical University. Carretera Central Oeste entre Madame Curie y Calle 9. Camagüey. CP 70100. Camaguey, Cuba
German Cancer Research Center Im Neuenheimer Feld 280, 69120 Heidelberg, Germany
Universidad de Camaguey Ignacio Agramonte. Carr. Circunv. Norte Km. 5. Camaguey C.P.74650. Cuba
*Corresponding Author: Dr. Jesús Arturo Junco Barranco, Prostate Cancer Vaccine Department, Centre for Genetic Engineering and Biotechnology of Camaguey, Cuba
Received: 18 June 2020; Accepted: 25 July 2020; Published: 29 August 2020
Citation: Jesús A. Junco, Franklin Fuentes, Lesvia Calzada, Eddy Bover, Eulogio Pimentel, Yovisleidis López, Roberto Basulto, Hilda Garay, Osvaldo Reyes, Angel Cid-Arregui, Maria D. Castro, Rafael Martínez, Niurka Arteaga, Ana Campal, Ayni Rodríguez, Andrés Serradelo, Eduardo Hernández, Mirialis Arias, Raúl González, Matilde López, Gisell Bebert, Gerardo Guillén. Anti-GnRH Neutralizing Antibodies Produce Testosterone Ablation and Tumor Shrinkage in Prostate Cancer Models. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 333-348.
View / Download Pdf Share at FacebookAbstract
Background: Vaccines based on modified GnRH peptide variants can be an alternative treatment for advanced prostate cancer. However, the efficacy of the GnRH-based vaccine variants has been limited by their insufficient immunogenicity.
Methods: The current vaccine based on the modified peptide GnRHm1-TT peptide has been formulated in the different oil adjuvants; Montanide ISA 51 and Montanide ISA 51VG.
Results: Experiments carried out in healthy animal models demonstrated to produce significant anti-GnRH antibody levels (p<0.01) that led to testosterone ablation (p<0.001), and in correspondence produced prostate atrophy (p<0.001). Here, it has been also studied the effect of different GnRHm1-TT peptide doses on its efficacy in different healthy animals. The immunization of Copenhagen rats implanted with the hormone-sensitive autologous Dunning R3327-H prostate tumor caused inhibition of prostate tumor growth in and resulted in over two-fold increase in survival compared to placebo (p<0.01). In vitro studies in the COS-7-ratGnRH model using sera from healthy animals immunized with the GnRHm1-TT/Montanide ISA 51VG vaccine candidate, proved that the anti-GnRH antibodies generated were able to neutralize natural GnRH hormone and inhibit signaling through its receptor.
Conclusions: We conclude that the GnRHm1-TT peptide formulated in Montanide ISA 51 VG is a suitable vaccine candidate that induce an effective anti GnRH immune response in different animal species and in correspondence produce a significant drop of testosterone levels, atrophy of prostate and tumor shrinkage in the Dunning R3327-H tumor model.
Keywords
Prostate cancer immunotherapy; GnRH based vaccines; Adjuvant combination; Hormone ablation; Hormone sensitive prostate cancer
Prostate cancer immunotherapy articles; GnRH based vaccines articles, Adjuvant combination articles, Hormone ablation articles, Hormone sensitive prostate cancer articles
Prostate cancer immunotherapy articles Prostate cancer immunotherapy Research articles Prostate cancer immunotherapy review articles Prostate cancer immunotherapy PubMed articles Prostate cancer immunotherapy PubMed Central articles Prostate cancer immunotherapy 2023 articles Prostate cancer immunotherapy 2024 articles Prostate cancer immunotherapy Scopus articles Prostate cancer immunotherapy impact factor journals Prostate cancer immunotherapy Scopus journals Prostate cancer immunotherapy PubMed journals Prostate cancer immunotherapy medical journals Prostate cancer immunotherapy free journals Prostate cancer immunotherapy best journals Prostate cancer immunotherapy top journals Prostate cancer immunotherapy free medical journals Prostate cancer immunotherapy famous journals Prostate cancer immunotherapy Google Scholar indexed journals GnRH based vaccines articles GnRH based vaccines Research articles GnRH based vaccines review articles GnRH based vaccines PubMed articles GnRH based vaccines PubMed Central articles GnRH based vaccines 2023 articles GnRH based vaccines 2024 articles GnRH based vaccines Scopus articles GnRH based vaccines impact factor journals GnRH based vaccines Scopus journals GnRH based vaccines PubMed journals GnRH based vaccines medical journals GnRH based vaccines free journals GnRH based vaccines best journals GnRH based vaccines top journals GnRH based vaccines free medical journals GnRH based vaccines famous journals GnRH based vaccines Google Scholar indexed journals Adjuvant combination articles Adjuvant combination Research articles Adjuvant combination review articles Adjuvant combination PubMed articles Adjuvant combination PubMed Central articles Adjuvant combination 2023 articles Adjuvant combination 2024 articles Adjuvant combination Scopus articles Adjuvant combination impact factor journals Adjuvant combination Scopus journals Adjuvant combination PubMed journals Adjuvant combination medical journals Adjuvant combination free journals Adjuvant combination best journals Adjuvant combination top journals Adjuvant combination free medical journals Adjuvant combination famous journals Adjuvant combination Google Scholar indexed journals Hormone ablation articles Hormone ablation Research articles Hormone ablation review articles Hormone ablation PubMed articles Hormone ablation PubMed Central articles Hormone ablation 2023 articles Hormone ablation 2024 articles Hormone ablation Scopus articles Hormone ablation impact factor journals Hormone ablation Scopus journals Hormone ablation PubMed journals Hormone ablation medical journals Hormone ablation free journals Hormone ablation best journals Hormone ablation top journals Hormone ablation free medical journals Hormone ablation famous journals Hormone ablation Google Scholar indexed journals Hormone sensitive prostate cancer articles Hormone sensitive prostate cancer Research articles Hormone sensitive prostate cancer review articles Hormone sensitive prostate cancer PubMed articles Hormone sensitive prostate cancer PubMed Central articles Hormone sensitive prostate cancer 2023 articles Hormone sensitive prostate cancer 2024 articles Hormone sensitive prostate cancer Scopus articles Hormone sensitive prostate cancer impact factor journals Hormone sensitive prostate cancer Scopus journals Hormone sensitive prostate cancer PubMed journals Hormone sensitive prostate cancer medical journals Hormone sensitive prostate cancer free journals Hormone sensitive prostate cancer best journals Hormone sensitive prostate cancer top journals Hormone sensitive prostate cancer free medical journals Hormone sensitive prostate cancer famous journals Hormone sensitive prostate cancer Google Scholar indexed journals malignant disease articles malignant disease Research articles malignant disease review articles malignant disease PubMed articles malignant disease PubMed Central articles malignant disease 2023 articles malignant disease 2024 articles malignant disease Scopus articles malignant disease impact factor journals malignant disease Scopus journals malignant disease PubMed journals malignant disease medical journals malignant disease free journals malignant disease best journals malignant disease top journals malignant disease free medical journals malignant disease famous journals malignant disease Google Scholar indexed journals Immuno-neutralization articles Immuno-neutralization Research articles Immuno-neutralization review articles Immuno-neutralization PubMed articles Immuno-neutralization PubMed Central articles Immuno-neutralization 2023 articles Immuno-neutralization 2024 articles Immuno-neutralization Scopus articles Immuno-neutralization impact factor journals Immuno-neutralization Scopus journals Immuno-neutralization PubMed journals Immuno-neutralization medical journals Immuno-neutralization free journals Immuno-neutralization best journals Immuno-neutralization top journals Immuno-neutralization free medical journals Immuno-neutralization famous journals Immuno-neutralization Google Scholar indexed journals adenocarcinomas articles adenocarcinomas Research articles adenocarcinomas review articles adenocarcinomas PubMed articles adenocarcinomas PubMed Central articles adenocarcinomas 2023 articles adenocarcinomas 2024 articles adenocarcinomas Scopus articles adenocarcinomas impact factor journals adenocarcinomas Scopus journals adenocarcinomas PubMed journals adenocarcinomas medical journals adenocarcinomas free journals adenocarcinomas best journals adenocarcinomas top journals adenocarcinomas free medical journals adenocarcinomas famous journals adenocarcinomas Google Scholar indexed journals immunogenicity articles immunogenicity Research articles immunogenicity review articles immunogenicity PubMed articles immunogenicity PubMed Central articles immunogenicity 2023 articles immunogenicity 2024 articles immunogenicity Scopus articles immunogenicity impact factor journals immunogenicity Scopus journals immunogenicity PubMed journals immunogenicity medical journals immunogenicity free journals immunogenicity best journals immunogenicity top journals immunogenicity free medical journals immunogenicity famous journals immunogenicity Google Scholar indexed journals testosterone articles testosterone Research articles testosterone review articles testosterone PubMed articles testosterone PubMed Central articles testosterone 2023 articles testosterone 2024 articles testosterone Scopus articles testosterone impact factor journals testosterone Scopus journals testosterone PubMed journals testosterone medical journals testosterone free journals testosterone best journals testosterone top journals testosterone free medical journals testosterone famous journals testosterone Google Scholar indexed journals
Article Details
Abbreviations:
GnRH- Gonadotrophin Releasing Hormone; VSSP- Very Small Size Proteoliposomes; IP- Inositol Phosphate. CFA- Complete Freund Adjuvant; IFA- Incomplete Freund adjuvant
1. Introduction
Prostate cancer is the most commonly diagnosed malignant disease in men in the western hemisphere, with more than 650 000 new cases per year [1, 2]. Most prostate adenocarcinomas is androgen dependent [3, 4], which means that androgenic suppression is an effective strategy for treatment. Immuno-neutralization of GnRH [5-10] and GnRH constructs resembling multiple or recombinant peptide antigens [11-13] are alternatives to hormone therapy to treat prostate cancer. The development of therapeutic vaccines against prostate cancer faces pressing problems, such as low antigenic immunogenicity, self-tolerance, and heterogeneity of results among individuals [14, 15]. Bringas et al. 2000, [16], produced a GnRH peptide variant in which L-glycine at the sixth position was substituted for L-proline, in order to increase rigidity of the peptide and facilitate its recognition by the immune system. Besides, to increase its immunogenicity, they synthesized the GnRH peptide linked to a helper T epitope from the tetanus toxin (TT). This fusion peptide, named GnRHm1-TT, proved very effective for castration of prepuberal pigs when it was emulsified in Freund´s adjuvant (FA). However, considering the toxicity of FA and its unique authorization in certain animal experiments, it was necessary to search for new adjuvants with sufficient immunogenic capacity in healthy individuals, as well as in cancer patients. Thus, a number of adjuvants have been developed and tested during the past years [17-21].
Moreover, the dose of the peptide vaccine is a sensitive issue awaiting solution, considering that although the vaccine dose is not calculated by body surface area, factors like species, age, and genetic heterogeneity have a critical influence on the immune [22-25]. Here, we tested Montanide ISA 51 and Montanide ISA 51 VG for their adjuvant efficacy as compared to FA. Both produced a favorable anti-GnRH response with the GnRHm1-TT peptide. In addition, the influence of the peptide dose on the immune response and the biological effect on animals of different species were determined in successive steps, along with the immune neutralizing capacity of the antibodies generated. Finally, the anti tumor potential of the GnRHm1-TT peptide was determined with the Dunning R3327-H prostate tumor model implanted in Copenhagen rats.
2. Materials and Methods
2.1 Generation and synthesis of GnRHm1-TT synthetic peptide
The GnRHm1-TT fusion peptide was generated during the synthesis by substitution of the L-glycine amino acid in the sixth position in the natural GnRH sequence (QHWSYGLRPG), by an L-proline (QHWSYPLRPG). The construct was completed with the addition of the QYIKANSKFIGITEL tetanus toxoid epitope, using the solid phase method as described by Hougten et al. [26].
2.2 Immunogenicity study of the GnRHm1-TT peptide in rats using different oily adjuvants
An immunogenicity study was conducted in 8-12 week-old male Copenhagen rats to evaluate the influence of different adjuvants. The animals were kept under controlled environmental conditions, at 20±2°C, 65% relative humidity, and 14 h light/10 h dark. Access to water and sterile feed was ad libitum. Materials used in the experiment were Complete and incomplete Freund´s adjuvants (CFA, IFA), from SIGMA, San Luis, USA; Montanide ISA 51 adjuvant; and Montanide ISA 51 VG adjuvant (Seppic, Paris, France). For emulsion preparation, the GnRHm1-TT peptide was resuspended in distilled water at a final concentration of 750 µg in 250 µL, and a similar volume of the adjuvant was added on a 50/50 proportion. The emulsion was made at 3500 r.p.m. for 30 minutes. The adjuvant-vaccine emulsion was administered subcutaneously on four dorsal spots of each animal.
2.3 Blood drawing and serum collection
Blood (approximately 200 µL) was drawn through puncture of the retro orbital venous sinus. Then it was centrifuged at 3200 r.p.m. for 15 minutes. The serum samples were stored at -20°C, until use.
2.4 Dosage scale-up study of the GnRHm1-TT peptide in healthy adult Copenhagen rats
Healthy adult rats like the ones described in item 2.2 were used in the study. Each group comprised 10 animals. Three doses of the GnRHm1-TT peptide (125, 300 and 750 µg) were tested in a fortnightly immunization scheme. The immunogen was prepared as described for the immunogenicity in rats.
2.5 Determination of anti-GnRH antibody titers by enzyme-linked immunosorbent assay (ELISA)
An indirect ELISA developed at the CIGB Vaccine Laboratory was used for seroconversion and titration assays of anti-GnRH antibodies. The samples were considered as positive seroconversion when they reached 0.197 OD, at 492 nM. Titers above 1: 50 were considered positive.
2.6 Determination of testosterone levels
The serum testosterone levels were determined using the TESTO CT2 commercial kit (CisBio, International, France). The samples to be measured were applied according to the insert instructions.
2.7 Evaluation of target organs
For histological evaluation of target organs, the testicles and prostates from each animal were surgically removed; then they were weighed in analytical balance (Sartorius). Organ weight was normalized according to the animal body weight.
2.8 Immunogenicity study in rabbits
This study included 15 New Zealand rabbits of approximately 4 kg. Five animals were immunized with 750 μg of the GnRHm1-TT peptide, and another five with 1 mg of the peptide. A control group of five animals were injected with the placebo substance. This placebo contained all the component of the vaccine except the GnRH peptide. The preparation and administration of immunogens was made according to previous descriptions for rats. The blood was drawn fortnightly from the dorsal vein of the ear using a disposable syringe (Terumo, Switzerland). The seroconversion and titration assays were performed according to description for rats. The testosterone levels were determined as previously described.
2.9 Immunogenicity study in monkeys
Two Macacus irus and two Macacus rhesus monkeys were immunized with 2.5 mg of the GnRHm1-TT peptide, emulsified in Montanide ISA 51. In each case, one Macacus received the placebo substance. The emulsion was administered intramuscularly (IM) every two weeks and. Testosterone determined as described previously. All protocols for the work with laboratory animals followed the guidelines of the Cuban Council for Animal Care and were approved by the Animal Care Committee at the Center for Genetic Engineering and Biotechnology in Havana, Cuba.
2.10 Antiserum inhibition studies on the accumulation of inositol phosphate (IP)
The inhibiting capacity of IP production by the antibodies generated against the natural GnRH hormone was checked by measuring the accumulation of IP in COS-7 cells. Briefly, the study consisted in incubating different concentrations of anti-GnRH sera produced in rabbit, using 10-9 M of natural GnRH, which were added to COS-7 cells previously labeled with tritium. COS-7 cells transfected to
express the GnRH receptor were incubated overnight in 0.5 mL of DMEM medium (Life Technologies, Inc.), antibiotics and myo-[2-3H] inositol 1μCi/well. The effective amount of antibodies was measured by incubation with growing concentrations of pure anti-GnRH antibody fractions purified with protein A Sepharose.
2.1 1 Studies in rodents using the Dunning R3327-H prostate tumor model implanted in Copenhagen rats
The study was made in male Copenhagen rats in which fragments of the Dunning R3327 tumor had been implanted previously, according to Finstad et al. 2004 (11). The therapeutic intervention (immunizations and castration) was initiated when the tumors were about 10 mm. The experiment included three groups (i) immunized with the GnRHm1-TT/ Montanide vaccine (n=9); (ii) animals inoculated with placebo (n=7); and (iii) surgically castrated animals (n=10). This experiment was carried out according to the Cuban and German guidelines established for laboratory animals.
Statistical analysis
The Kolmogorov-Smirnov test was made in order to check if the data were normally distributed; then the Bartlet or maximum F test was made to determine variance homogeneity. One way or bifactorial analyses of variance (ANOVA) were made to check equality between group averages. Later on, the Dunn multiple-range test was made for multiple comparisons. The T-Student test was performed to check equality between the means of the unmatched groups. The Kruskal Wallis or Mann-Whitney U tests were made when the data did not comply with variance homogeneity. Prism Graph Pad. 4.0/1995, for Windows, (StatSoft, Inc) was used for that purpose.
3. Results
3.1 Immunization with the GnRHm1-TT peptide using different oily adjuvants in adult rats
The first goal of this study was to determine the efficacy of the immunization of healthy adult animals with the GnRHm1-TT peptide emulsified in oily adjuvants (Montanide ISA 51 and Montanide ISA 51VG), compared to universal FA, as previously assayed in the Vaccines Laboratory [21-27].
The analysis of the anti GnRH antibody titers showed that from day 30 of immunization, the levels of anti GnRH antibodies began to increase in all the experimental groups and on day 45, they presented titers of 1: 1500. These titers continued to increase until reaching double (1: 3000) (p<0.01) on day 60 and remained similar until the end of the trial. There were not significant differences among the adjuvants immunogenicity using the GnRHm1-TT peptide. On the other hand, the evaluation of the changes in testosterone levels showed that from day 45 of immunization, a noticeable drop in testosterone levels was observed in the three experimental groups. These values became significant from day 60, when they dropped to values between 2 and 2.5nmol / L. The highest depletion of testosterone levels were observed on day 75, when all the experimental groups showed testosterone levels in castration values (p<0.01) (Figure 1).
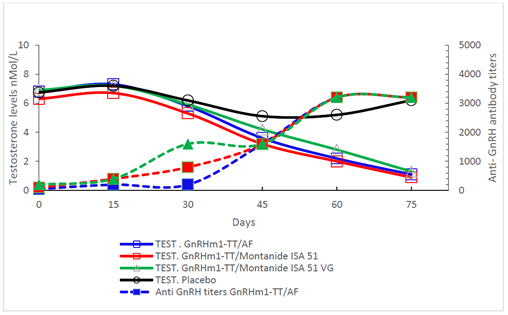
Figure 1: Anti-GnRH antibodies and Testosterone levels correspondence in adult Copenhagen rats immunized with the GnRHm1-TT peptide emulsified in different adjuvants. For anti GnRH antibody titration, rat sera was analyzed through an indirect ELISA using dilutions from 1:50 to 1:10 000 and their valor are represented in the right axis. Testosterone levels were measured in nmol/L using a radioimmunoassay and are represented in the left axis of the graph. Each point of the curve represent the mean of the triplicates of a mixture of sera of 10 animals. Anti GnRH titers are represented through dashed lines and full symbols and Testosterone levels as continue lines and open symbols as described in the Figure legend. The anti GnRH antibodies and Testosterone levels that belong to the same group are denoted with the same color.
Once the immunization scheme was completed in rats immunized with the formulations of GnRHm1-TT in the described adjuvants, the animals were slaughtered to evaluate the effect produced by the immunization on the target organs. Resection and weighing of the prostate of the animals of the group immunized with the GnRHm1-TT peptide in AF, showed a 40% reduction in the average weight of the prostate compared to the non-immunized controls, while when the GnRHm1-TT peptide was administered formulated in Montanide ISA 51 and Montanide ISA51 VG, a more drastic reduction was observed, which resulted in a 50% decrease in prostate weight compared to placebo (p <0.01). However, no statistically significant difference was observed between the treated groups when performing the Kruskal Wallis test followed by Dunn's multiple comparison (Figure 2).
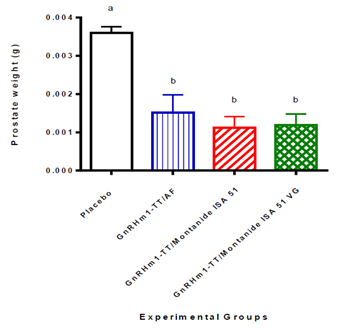
Figure 2: Variation of the weight of the prostate in Copenhagen rats immunized with the GnRHm1-TT peptide emulsified in different oily adjuvants. The bars represents the mean of 10 animals ± SD. Different letters signify a statistically significant difference between the experimental groups for a difference of (p <0.01) obtained from the Kruskal Wallis test followed by a Dunn multiple comparison test. The experimental groups are represented on the abscissa axis and the normalized organ weight is shown on the ordinate axis.
3.2 Evaluation of the influence of the GnRHm1-TT peptide dose on the immune response of healthy adult animals
The purpose of the immunization experiments described below was to evaluate the dosage scale-up effects of GnRHm1-TT peptide on different species of healthy adult animals. For that purpose, Montanide ISA 51 VG was used in the rest of experiments having in mind this is produced using fatty acid from plant source.
3.2.1 Immunization of healthy adult Copenhagen rats: The assay was first carried out in healthy adult rats, with doses of 125, 300, and 750µg of the GnRH m1-TT peptide. Each dose was administered fortnightly. Figure 3, shows that the immunization with 750 µg dose of the GnRHm1-TT peptide adjuvated in Montanide ISA 51 VG, produced anti-GnRH antibody titers reaching an average of 1: 3000 from day 30 until the end of experiment on day 75(P<0.01). However, when the 125 and 300µg doses of the peptide were used, seroconversion was not observed in most animals. In concordance, measurements of serum testosterone levels showed that only the 750 mg dose of the peptide caused a significant reduction of testosterone below castration levels (p<0.001) at the end of the study. Although the 300μg dose caused a significant reduction of more than half of the initial values of testosterone for the two cases (p<0.05), it did not induce castration values (Figure 3).
Evaluation of the effect on the prostate of immunization with the three described peptide dose levels (125, 300 and 750 µg) showed a poor influence of the 125 µg dose on the reduction of prostate weight. At the same time, it demonstrated the significant, albeit discrete, effect of the 300 µg dose of the peptide on prostate size and weight relative to placebos (p <0.05). However, the administration of 750 µg of the GnRHm1-TT peptide produced a decrease of approximately 50% in the weight of the prostate at the sacrifice of the animals with respect to the placebos (p <0.01) (Figure 4). Determination of the difference between treatments was performed using an analysis of variance and Dunn's multiple range test.
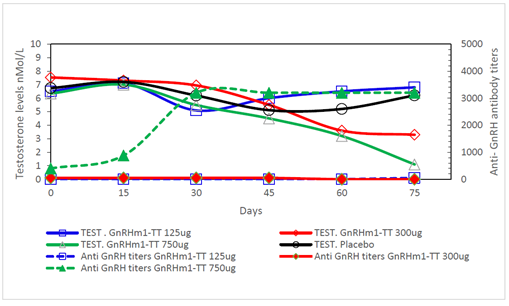
Figure 3: Anti-GnRH antibodies and Testosterone levels correspondence in adult Copenhagen rats immunized with different doses of the GnRHm1-TT peptide emulsified in Montanide ISA 51 VG adjuvant. For anti GnRH antibody titration, rat sera was analyzed through an indirect ELISA using dilutions from 1:50 to 1:5000 and its value is represented in the right axis. Testosterone levels were measured in nmol/L using a radioimmunoassay and are represented in the left axis of the graph. Each point of the curve denote the mean of the triplicate of a mixture of sera of 10 animals. Anti GnRH titers are represented through dashed lines and full symbols and Testosterone levels as continue lines and open symbols as described in the Figure legend. The anti GnRH antibodies and Testosterone levels that belong to the same group are denoted with the same color.
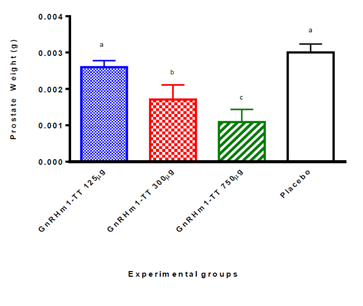
Figure 4: Variation of the weight of the prostate in Copenhagen rats immunized with different doses of the GnRHm1-TT peptide adjuvanted in Montanide ISA 51 VG. After the conclusion of the experiment, the rats were sacrificed and the prostate of all rats dissected and weighed in analytical balance. The resulting values were normalized against the weight of each animal. The bars represents the mean of 10 animals ± SD. Different letters signify a statistically significant difference between the experimental groups for a difference of (p <0.01) obtained from the Kruskal Wallis test followed by a Dunn multiple comparison test.
3.2.2 Immunization of New Zealand rabbits and Macacus irus monkeys: The main purpose of rabbit and monkey trials was to explore the influence of a GnRHm1-TT peptide dose increase on anti-GnRH humoral responses in two heavier and larger animal species with different HLA haplotypes. As a result of immunizing rabbits with the 750ug and 1mg doses of the GnRHm1-TT peptide adjuvant in Montanide ISA 51 VG, it was found that on day 45 both doses produced anti-GnRH antibody titers of 1: 1600 (p<0.05). However, thereafter, while animals immunized with the 750ug dose of GnRHm1-TT reached titers of 1: 6000 (p<0.01), the 1mg dose produced a significant increase in these titers to 1: 12000 between days 60 and 75 of started the experiment (p<0.001). Both peptide doses caused a significant reduction of testosterone up to castration levels on day 75, when the final evaluation of the experiment was made (Figure 5).
The immunization experiment with monkeys showed that all vaccinated animals generated anti-GnRH antibody responses with titers that gradually increased from 1: 3000 on day 45 of the experiment to 1: 6000 in the final evaluation. Analyses of testosterone levels in serum showed that on the 45th day following the beginning of the immunization scheme, there was a 50% reduction a reduction in testosterone levels from approximately 20 nmol/L to 11.2 ± 0.6 nmol/L, (p<0.05). At the end of the experiment a significant reduction of the mean testosterone values was observed to reach castration levels 1.625 ± 0.22 nmol/L (p<0.001) (Figure 6).
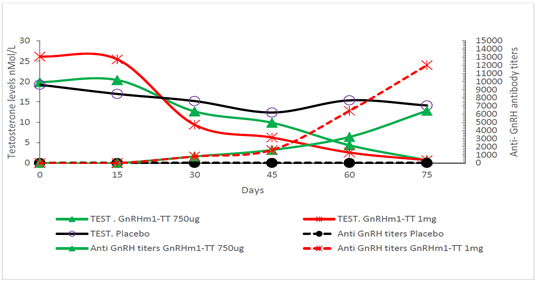
Figure 5: Anti-GnRH antibody titration and Testosterone determination in rabbits immunized with different doses of the GnRHm1-TT peptide formulated in Montanide ISA 51VG. The animals were immunized SC on days 0, 15, 30 and 45. For anti GnRH antibody titration, rat sera was analyzed through an indirect ELISA using dilutions from 1:50 to 1:15 000 and their valor are represented in the right axis. Testosterone levels were measured in nmol/L using a radioimmunoassay and are represented in the left axis of the graph. Each point of the curve represent the mean of the duplicate of a mixture of sera of 5 animals. Anti GnRH titers are represented through dashed lines and full symbols and Testosterone levels as continue lines and open symbols as described in the Figure legend. The anti GnRH antibodies and Testosterone levels that belong to the same group are denoted with the same color. The comparisons between the groups in each time point were made through a non-parametric test of Kruskal Wallis followed by a Dunn test. Significant differences are represented in the graph.
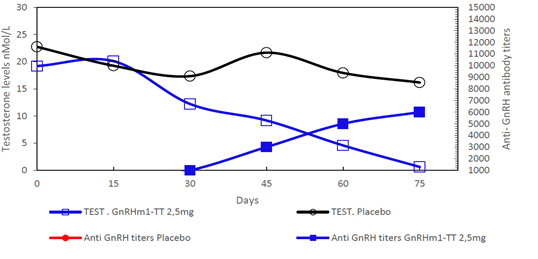
Figure 6: Anti-GnRH humoral response and Testosterone levels detected in Macacus irus monkeys immunized with increasing doses of the vaccine candidate GnRHm1-TT/ Montanide ISA 51 VG. Animals were immunized four times with 2.5 mg of GnRHm1-TT peptide by IM route. For anti GnRH antibody titration, sera from the monkeys was analyzed through an indirect ELISA using dilutions from 1:50 to 1:15 000 and their valor are represented in the right axis. Testosterone levels were measured in nmol/L using a radioimmunoassay and are represented in the left axis of the graph. Each point of the curve represent the mean of the duplicate of a mixture of sera of 4 animals. Anti GnRH titers are represented through dashed lines and full symbols and Testosterone levels as continue lines and open symbols as described in the Figure legend. The anti GnRH antibodies and Testosterone levels that belong to the same group are denoted with the same color. For anti GnRH antibody comparison the non-parametric Mann-Whitney U test was used while Testosterone values were compared using the Student t Newman-Keuls test.
3.3 Determination of anti GnRH neutralizing activity of sera from rabbits immunized with the GnRHm1-TT/Montanide ISA 51VG vaccine
The rabbit sera with high anti-GnRH antibody titers from the animals immunized with the 1mg dose were used to determine if the purified anti-GnRH antibodies could neutralize natural GnRH hormone signaling in COS-7 mammal cells transfected with the rat GnRH receptor. Concentrations of anti-GnRH antibodies between 12.5 and 6.25μg/mL caused a significant reduction of intracellular inositol phosphate (IP), which decreased between 5 and 6-fold in relation to the signal produced by the natural GnRH used as the positive control (P<0.001). Antibody concentrations between 3.1 and 0.76 μg/mL also caused a significant reduction in IP production, compared to the control, though in lower proportions (p<0.01). Concentrations below 0.76 μg/mL did not cause a significant inhibition of signaling through the rat GnRH receptor, expressed in the COS-7 system. However, contrary to what would be expected, anti-GnRH antibody concentrations above 12.5 μg/mL did not produce greater IP reduction (data not shown). The pre-immune sera served as experimental control and showed no effect on GnRH signaling. The Kruskal-Wallis and the Duncan's multiple comparison tests were used for statistical analysis (Figure 7).
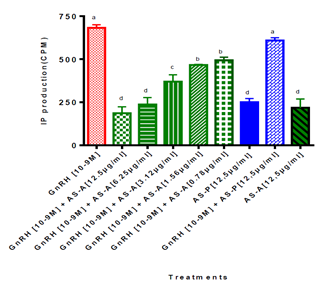
Figure 7: Inhibition of the effect of GnRH on IP production in COS-7 cells. COS-7 cells transfected with the rat GnRH receptor were seeded on 24-well culture plates in the presence of GnRH and decreasing concentrations from 12.5 to 0.78 μg / mL of polyclonal antibodies anti GnRH or pre-immune sera obtained from rabbits immunized with 1 mg doses of the GnRHm1-TT peptide in Montanide ISA 51 VG adjuvant. GnRH (10-9M) with no antibody was used as control. Different letters represent significant differences obtained through the Kruskal-Wallis test and Dunn's multiple comparison. AS-A: refers to purified anti-GnRH policlonal antibodies obtained in rabbits and AS-P refers to Immunoglobulins purified from non immunized rabbits. All of them were were semipurified using Protein A Sepharose.
3.4 Therapeutic efficacy of GnRHm1-TT/ Montanide ISA 51 VG vaccine in the Dunning R3327-H prostate tumor model
After exploring the feasibility of an effective immune response with the GnRHm1-TT/Montanide ISA 51 VG vaccine candidate in different healthy mammal models in this study, the next step was to determine its efficacy in a model that resembled human prostate cancer. The test to check the therapeutic capacity of the vaccine candidate was performed in adult male Copenhagen rats with subcutaneously implanted Dunning R3327-H hormone-sensitive prostate tumor. As a result of immunization with GnRHm1-TT/Montanide ISA 51 vaccine, 8/9 rats developed significant levels of anti-GnRH antibodies 90 days after beginning the immunization scheme (p<0.05). The evaluation of anti-GnRH antibody titers on the 90th day showed average values of 1: 450, whereas the determination of testosterone levels evidenced a remarkable decline of its values up to castration levels in the same number of rats (8/9) (p<0.01) (Table 1). The analysis of accumulated survival probability showed that whereas on the 90th day of the experiment, all animals in the placebo group had to be sacrificed due to increased tumor size, while most animals in the immunized and castrated groups showed slow tumor growth, with more than a two-fold increased survival in comparison to the placebo group (p<0.01) (Table 1).
|
Number of the imm.animal |
Time to Anti GnRH seroconversion (days) |
anti GnRH titers (day 90) after immunization |
Testosterone (nmol/L) (day 90) |
Survival (in days) |
|
32- |
60 |
1/ 200e |
1,70 ± 0,04a |
150b |
|
36- |
45 |
1/ 400c |
0,07 ± 0,02a |
150b |
|
43 |
45 |
1/400c |
0,01 ± 0,00a |
265a |
|
46- |
45 |
1/800b |
0,07 ± 0,02a |
195b |
|
49- |
45 |
1/ 800b |
0,00 ± 0,00a |
265a |
|
51- |
45 |
1/ 1600a |
0,00 ± 0,00a |
95c |
|
55- |
45 |
1/200d |
0,84 ± 0,28a |
60c |
|
56- |
60 |
1/100d |
0,18 ± 0,01a |
265a |
|
60- |
- |
0/00 |
1,9 0 ± 0,3b |
85c |
Different letters denote significant diffrences according using the following test: Mann Whitney U test for anti GnRH antibodies, a simple ANOVA for testosterone and the Log Rank test for survival determination.
Table 1: Effects of immunization with the vaccine GnRHm1-TT regarding to anti GnRH antibody production, testosterone levels and survival of rats implanted with allogeneic Dunning R3327-H tumor.
4. Discussion
Several vaccines based on modified GnRH peptide variants have been tested since the early 1990s [5, 7]. However, an important limitation has been their low immunogenicity due, at least in part, to the small size of the peptide [28] and its structural preservation in all mammals [29]. Adjuvants are essential components of vaccines that may help overcome this problem. The most common adjuvants for human vaccines are still based on aluminium [30, 31]. The Montanide ISA 51 and Montanide ISA 51 VG adjuvants are similar to IFA and have been approved for clinical trials with human vaccines [32- 34]. In this paper, we report a comparative study of the biological activity of the GnRHm1-TT synthetic peptide formulated in Montanide ISA 51 and Montanide ISA 51 VG adjuvants compared to FA. The latter is the reference adjuvant to induce Th1 humoral and cell-mediated responses, but it is highly toxic [35-39].
The fact that Montanide ISA 51 and Montanide ISA 51 VG equaled FA in terms of induction of anti-GnRH antibody titers in experimental animals was unexpected, considering that CFA, besides oils and emulsifying agents, has quite a high number of components with a known immunoenhancing capacity, such as fragments of mycobacteria, DNA, CpG, LPS and others [31, 40-42]. The elevated production of antibodies against natural GnRH induced by the GnRHm1-TT peptide both in FA and the two Montanide types in the animals also evidenced the immunogenic capacity of the peptide and its potential to evade the physiological tolerance mechanisms when these adjuvants were used.
Our results with the GnRHm1-TT peptide vaccine showed that along with the antibody immune responses there was a drop in the testosterone levels and a reduction in prostate size detected at sacrifice in comparison to the animals of the placebos group (p<0.05). The comparable behavior observed with the Montanide ISA 51 and Montanide ISA 51VG adjuvants proved their equivalence, and therefore, their usefulness to induce enhanced immunity in animal models and probably in men. The above described immunogenicity studies showed the feasibility of generating an effective immune response in rats with the 750 ug dose of the GnRHm1-TT peptide in Montanide ISA 51 VG. However, it was important to study the necessary minimum dose to achieve the best biological effect. The 750µg dose of the GnRHm1-TT peptide was by far the most effective to achieve the highest biological response, based on antibody concentration, the effect on testosterone levels, and its atrophying capacity on prostate.
Although the immunogenicity results achieved in rabbits and monkeys corroborated the existence of a correspondence between high anti-GnRH antibody titers and the reduction of testosterone levels, they failed to prove the existence of a linear correlation between them. In that sense, the results tend to show that when the anti-GnRH hormone antibodies reach critical levels to form about 100% of immunocomplexes with the circulating GnRH, the testosterone levels drop strongly. Accordingly, it has been previously reported that the immunocastration capacity of the antibodies depends on the speed of their appearance and existence in sufficient concentrations to achieve the biological effect [43], rather than on their isotype or affinity. The Macacus irus monkeys share more than 95% genetic homology with humans [44], so the immunization study with them provided evidence to which extent a significant increase of the peptide dose may affect the immune response. These results are very relevant to take decisions on the peptide dose for other species including humans, depending on their immunogenic capacity and toxicological properties.
The Dunning R3327-H tumor model was used to evaluate the therapeutic effect of the GnRHm1-TT peptide adjuvanted in Montanide ISA 51 VG, since it shares similar features to the prostate cancer described in humans [45]. After four immunizations, seroconversion against the natural GnRH hormone was observed in 88% of the rats immunized, which demonstrated the feasibility of consistent humoral immune response despite the presence of the already-established tumor. Interestingly, although higher antibody titers were accompanied by a strong reduction in testosterone levels and inhibition of tumor growth, in some cases high antibody titers and testosterone depletion showed no important inhibition of the tumor growth, which thus demonstrated certain loss of hormone sensitivity. On the contrary, in some immunized animals where low anti-GnRH antibody titers were observed, there was a significant ablation of testosterone, which was associated with a marked inhibition of tumor growth (Table 1). These results coincided with the ones achieved for vaccine candidate UBICTh® (11).
According to the results of the GnRHm1-TT vaccine adjuvanted in Montanide ISA 51 in different animal models, a good correlation may be inferred between anti-GnRH antibody levels, testosterone depletion, the effect on target organs found in healthy animals and the tumor inhibition observed in Copenhagen rats with the implanted Dunning R3327-H tumor model. To corroborate that the effects described corresponded directly to the hypothesis that the immunoneutralizing capacity of the anti-GnRH antibodies generated through active immunization with the GnRHm1-TT/Montanide ISA 51 vaccine was responsible for testosterone ablation, six previously purified serum samples from rabbits immunized with GnRHm1-TT/Montanide ISA 51 were used in a final trial. Then they were exposed to the COS-7-ratGnRH mammal cell model at different concentrations to explore its inhibitory capacity to produce IP. The gradual decrease of IP concentrations detected in this trial, as concentrations of the anti-GnRH polyclonal antibody increased up to 12.5 μg/mL evidenced the neutralizing capacity of natural GnRH developed by the antibodies produced through the GnRHm1-TT/Montanide ISA 51 vaccine. Besides, it explained the direct relationship found in previous studies among anti-GnRH antibody production, testosterone ablation, and atrophy of the prostate and testicles of animals immunized with the Heberprovac vaccine candidate.
4. Conclusions
Immunization using the synthetic GnRHm1-TT peptide in an emulsion of Montanide ISA 51 adjuvant with animal or plant components, produced a significant prostate and testicle size reduction in rats (p<0.005). A dose scale-up of the synthetic peptide of up to 1mg demonstrated higher immunogenicity, and produced greater atrophy of the target organs than the 750µg dose. However, a 2.5 mg dose scale-up in Macacus irus monkeys did not show higher antibody titers or advantage on testosterone depletion. The anti-GnRH antibodies generated by immunization with the GnRHm1-TT/.Montanide ISA 51 VG vaccine candidate in rabbits showed the neutralizing capacity of natural GnRH up to a 0.78µg/mL concentration, according to the results achieved in the in vitro experiments. The immunocastration effect of the GnRHm1-TT/Montanide ISA 51 formulation led to growth inhibition of the Dunning R3327-H hormone-sensitive prostate tumor model in rats, when the animals were treated therapeutically with the vaccine candidate. The effects of immunocastration corresponded to better survival advantage.
Acknowledgements
We would like to thanks The German Academic Exchange Service (DAAD) by the funds provided to permit the development of experiments in the Dunning R3327-H model at DKFZ, Heidelberg, Germany.
Declaration of Interest
The authors declare no conflict of interests.
References
- Schröder FH. Prostate cancer around the world. An overview. Urol Oncol. 28 (2010): 663-667.
- Braga SFM, de Souza MC, Cherchiglia ML. Time trends for prostate cancer mortality in Brazil and its geographic regions: An age-period-cohort analysis. Cancer Epidemiol 50 (2017): 53-59.
- Pienta KJ, Brandley D. Mechanisms underlaying the androgen-independent prostate cancer. Clin. Can. Res 12 (2006): 1665-1671.
- Crona DJ, Milowsky MI, Whang YE. Androgen receptor targeting drugs in castration-resistant prostate cancer and mechanisms of resistance. Clin Pharmacol Ther. 98 (2015): 582-589.
- Talwar GP. Vaccines for control of fertility and hormone-dependent cancers. Immunology and Cell Biology 75 (1997): 184-189.
- Talwar GP, Hemant, Vyas HK, et al. Gonadotropin-releasing hormone/human chorionic gonadotropin β based recombinant antibodies and vaccines. Journal of Reproductive immunology 83 (2009): 158-163.
- Simms M.S, Scholfield DP, Jacobs E, et al. Anti-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br. J. Cancer 83 (2000): 443-446.
- Xu J, Zhu Z, Wu J, et al. Immunization with a recombinant GnRH vaccine conjugated to heat shock protein 65 inhibits tumor growth in orthotopic prostate cancer mouse model. Cancer Lett 259 (2008): 240-250.
- Aguilar FF, Barranco JJ, Fuentes EB, et al. Very small size proteoliposomes (VSSP) and Montanide combination enhance the humoral immuno response in a GnRH based vaccine directed to prostate cancer. Vaccine 30 (2012): 6595-6599.
- Barranco JA, Millar RP, Fuentes F, et al. Gradual reduction of testosterone using a gonadotropin-releasing hormone vaccination delays castration resistance in a prostate cancer model. Oncol Lett 12 (2016): 963-970.
- Finstad CL, Wang CY, Kowalsky J, et al. Synthetic luteinizing hormone releasing hormone (LHRH) vaccine for effective androgen deprivation and its application to prostate cancer immunotherapy. Vaccine 22 (2004): 1300-1313.
- Xu J, Zu Z, Duan P, et al. Protein Expression and Purification. Protein 50 (2006): 163-170.
- Gupta JC, Hada RS, Sahai P, et al. Development of a novel recombinant LHRH fusion protein for therapy of androgen and estrogen dependent cancers. Protein Expr Purif 134 (2017): 132-138.
- Ko EC, Wang X, Ferrone S. Immunotherapy of malignant diseases. Challenges and strategies. Int Arch Allergy Immunol 132 (2003): 294-309.
- Lage A, Perez R, Fernandez LE. Therapeutic cancer vaccines: at midway between immunology and pharmacology. Curr Cancer Drug Targets 5 (2005): 611-627.
- Bringas R, Basulto R, Reyes O. et al. Vaccine preparation for the reversible immunocastration of mammals. PCT patent N0. A61K37/38, C07K 7/00 (WO1998027111A1) (2000).
- Sierra GV, Campa HC, Varcacel NM, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14 (1991): 195-207.
- Pérez O, Bracho G, Lastre M, Mora N, et al. Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen-associated molecular pattern. Immunol Cell Biol 82 (2004): 603-610.
- McKee SJ, Bergot AS, Leggatt GR. Recent progress in vaccination against human papillomavirus-mediated cervical cancer. Rev Med Virol. 1 (2015): 54-71.
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 16 (2016): 1154-1168.
- Mathan TSM, Textor J, Sköld AE, et al. Harnessing RNA sequencing for global, unbiased evaluation of two new adjuvants for dendritic-cell immunotherapy. Oncotarget 8 (2017): 19879-19893.
- Ladd A, Tsong YY, Lok J, et al. Active immunization against LHRH: I. Effects of conjugation site and dose. Am J Reprod Immunol 22 (1990): 56-63.
- Sad S, Talwar GP, Raghupathy R. Influence of the genetic background and carrier protein on the antibody response to GnRH. J Reprod Immunol. 19 (1991): 197-207.
- Ferro VA, Khan MA, McAdam D, et al. Efficacy of an anti-fertility vaccine based on mammalian gonadotrophin releasing hormone (GnRH-I) a histological comparison in male animals. Vet Immunol Immunopathol 101 (2004): 73-86.
- Siel D, Vidal S, Sevilla R, Paredes R, Carvallo F, Lapierre L, Maino M, Pérez O, Sáenz L. Effectiveness of an immunocastration vaccine formulation to reduce the gonadal function in female and male mice by Th1/Th2 immune response. Theriogenology 2016 86 (6): 1589-98.
- Aponte PM, Gutierrez-Reinoso MA, Sanchez-Cepeda EG, et al. Active immunization against GnRH in pre-pubertal domestic mammals: testicular morphometry, histopathology and endocrine responses in rabbits, guinea pigs and ram lambs. Animal 24 (2017): 1-10.
- Hougten RA, De Graw ST, Bray MK, et al. Simultaneous multiple peptide Synthesis: The rapid preparation of large numbers of discrete peptides for biological, immunological and methodologicals studies. Biotecniques 4 (1986): 522-526.
- Basulto R, Milanes C, Rojas A, et al. Effects of immunization against GnRH in the structure and function of testicles in adult dogs. Biotecnología Aplicada 20 (2003): 20-24.
- Schally AV, Arimura A, Baba Y, et al. Isolation and properties of the FSH and LHreleasing hormone, Biochem. Biophys. Res. Commun 43 (1971): 393-399.
- Millar RP, Pawson AJ, Morgan K, et al. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Frontiers in Neuroendocrinology 29 (2008): 17-35.
- Aucouturier J, Dupuis L, Deville S, et al. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines 1 (2002): 111-118.
- Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. International Workshop on Vaccine Adjuvants and Glycoconjugates 12 (2006): 44-45.
- Kumar S, Jones TR, Oakley MS, et al. CpG oligodeoxynucleotide and Montanide ISA 51 adjuvant combination enhanced the protective efficacy of a subunit malaria vaccine. Infect Immun 72 (2004): 949-957.
- Kanako Iseki, Hiromi Matsunaga, Nobukazu Komatsu, et al. Evaluation of a new oil adjuvant for use in peptide-based cancer vaccination. Cancer Science 101 (2010): 2087-2293.
- Stephane Ascarateil, Aude Puget, Marie-Eve Koziol. Safety data of Montanide ISA 51 VG and Montanide ISA 720 VG, two adjuvants dedicated to human therapeutic vaccines. J Immunother Cancer 3 (2015): P428.
- Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science 271 (1996): 1728-1730.
- Audibert FM, Lise LD. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today 14 (1993): 281-284.
- Allison AC. The mode of action of immunological adjuvants. Dev Biol Stand 92 (1998): 3-11.
- Ahmad A, Ganaie MP, Shrivastava VK. The effect of active immunization with gonadotropin releasing hormone conjugate (GnRH-BSA) on gonadosomatic indices (GSI) and sperm parameters in mice. Iranian Journal of Reproductive Medicine 6 (2008): 119-123.
- Mesa C, Fernandez L.E. Challenges facing adjuvants for cancer immunotherapy. Immunol Cell Biol 2004 82 (6): 644–50.
- Mesa C, de Leon J, Fernandez LE. Very small size proteoliposomes derived from Neisseria meningitidis: An effective adjuvant for generation of CTL responses to peptide and protein antigens Vaccine 24 (2006): 2692-2699.
- Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine 25 (2007): 3752-3762.
- Miller LA, Takwar GP, Killiam GJ. Contraceptive effect of a recombinant GnRH vaccine in adult female pigs. Proced 22nd vertebrate pest. Conf. (2006): 106-109.
- Salamanca-Gómez F. El genoma del chimpancé. Gac Méd Méx 141 (2005): 543-44.
- Isaacs JT, Isaacs WB, Feitz WF, et al. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancer. Prostate 9 (1986): 261-281.


 Impact Factor: * 4.1
Impact Factor: * 4.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 