Antimicrobial activity of green bio-synthesized silver nanoparticles using extracts of Pittosporum senacia
Article Information
Gunness Deepshi, Lisa Ah-Shee-Tee, Haadiyah Hosany, Vishwakalyan Bhoyroo*
Department of Agricultural and Food Science, Faculty of Agriculture, University of Mauritius, Réduit, Mauritius
*Corresponding Author: Vishwakalyan Bhoyroo, Department of Agricultural and Food Science, Faculty of Agriculture, University of Mauritius, Réduit, Mauritius
Received: 06 June 2020; Accepted: 13 July 2020; Published: 12 August 2020
Citation:
Gunness Deepshi, Lisa Ah-Shee-Tee, Haadiyah Hosany, Vishwakalyan Bhoyroo. Antimicrobial activity of green bio-synthesized silver nanoparticles using extracts of Pittosporum senacia. Journal of Biotechnology and Biomedicine 3 (2020): 104-110.
View / Download Pdf Share at FacebookAbstract
Compared to traditional production method (chemical method), biological synthesis of nanoparticles tend to be cheaper since no chemical reducing agents are required, secondary metabolites easily reduced metal ions to nanoparticles in simple steps. The present study compared the antimicrobial activities of both chemically and biologically produced nanoparticles. Aqueous extract of Pittosporum senacia, an endemic medicinal plant, was used to produce silver nanoparticle. Sodium citrate was used as reducing agent in the chemical production of silver nanoparticle. Produced silver nanoparticles were then characterised using UV-Vis spectroscopy. Absorption peak were produced at 400 nm by both type of nanoparticles indicating their spherical nature. Peak at 600 nm was also observed for the biosynthesized nanoparticle denoting other shapes of nanoparticles or possible aggregation. Phytochemical test revealed the presence of saponins, carbohydrates and phenols that were responsible for the reduction and stabilisation of biosynthesized silver nanoparticle. The antimicrobial activities of both type of synthesized nanoparticle were tested against both Gram positive and Gram negative bacteria and a fungus. Excellent antimicrobial activities were recorded against Escherichia coli using both chemically and biologically synthesized silver nanoparticles, reporting inhibition zone of 20.0 ± 0.0 mm and 21.67 ± 1.15 mm respectively. No synergistic interaction was found between biologically synthesized nanoparticles and antibiotic, chloramphenicol. This study demonstrated that there was no significant difference in the antimicrobial properties of both type of nanoparticle and Pittosporum senacia leaves can be effectively used to synthesize silver nanoparticles.
Keywords
Silver nanoparticle, Antimicrobial, Chemical synthesis, Biosynthesis, Pittosporum senacia
Silver nanoparticle articles Silver nanoparticle Research articles Silver nanoparticle review articles Silver nanoparticle PubMed articles Silver nanoparticle PubMed Central articles Silver nanoparticle 2023 articles Silver nanoparticle 2024 articles Silver nanoparticle Scopus articles Silver nanoparticle impact factor journals Silver nanoparticle Scopus journals Silver nanoparticle PubMed journals Silver nanoparticle medical journals Silver nanoparticle free journals Silver nanoparticle best journals Silver nanoparticle top journals Silver nanoparticle free medical journals Silver nanoparticle famous journals Silver nanoparticle Google Scholar indexed journals Chemical synthesis articles Chemical synthesis Research articles Chemical synthesis review articles Chemical synthesis PubMed articles Chemical synthesis PubMed Central articles Chemical synthesis 2023 articles Chemical synthesis 2024 articles Chemical synthesis Scopus articles Chemical synthesis impact factor journals Chemical synthesis Scopus journals Chemical synthesis PubMed journals Chemical synthesis medical journals Chemical synthesis free journals Chemical synthesis best journals Chemical synthesis top journals Chemical synthesis free medical journals Chemical synthesis famous journals Chemical synthesis Google Scholar indexed journals Antimicrobial articles Antimicrobial Research articles Antimicrobial review articles Antimicrobial PubMed articles Antimicrobial PubMed Central articles Antimicrobial 2023 articles Antimicrobial 2024 articles Antimicrobial Scopus articles Antimicrobial impact factor journals Antimicrobial Scopus journals Antimicrobial PubMed journals Antimicrobial medical journals Antimicrobial free journals Antimicrobial best journals Antimicrobial top journals Antimicrobial free medical journals Antimicrobial famous journals Antimicrobial Google Scholar indexed journals Biosynthesis articles Biosynthesis Research articles Biosynthesis review articles Biosynthesis PubMed articles Biosynthesis PubMed Central articles Biosynthesis 2023 articles Biosynthesis 2024 articles Biosynthesis Scopus articles Biosynthesis impact factor journals Biosynthesis Scopus journals Biosynthesis PubMed journals Biosynthesis medical journals Biosynthesis free journals Biosynthesis best journals Biosynthesis top journals Biosynthesis free medical journals Biosynthesis famous journals Biosynthesis Google Scholar indexed journals Pittosporum senacia articles Pittosporum senacia Research articles Pittosporum senacia review articles Pittosporum senacia PubMed articles Pittosporum senacia PubMed Central articles Pittosporum senacia 2023 articles Pittosporum senacia 2024 articles Pittosporum senacia Scopus articles Pittosporum senacia impact factor journals Pittosporum senacia Scopus journals Pittosporum senacia PubMed journals Pittosporum senacia medical journals Pittosporum senacia free journals Pittosporum senacia best journals Pittosporum senacia top journals Pittosporum senacia free medical journals Pittosporum senacia famous journals Pittosporum senacia Google Scholar indexed journals "Phytochemical screening articles Phytochemical screening Research articles Phytochemical screening review articles Phytochemical screening PubMed articles Phytochemical screening PubMed Central articles Phytochemical screening 2023 articles Phytochemical screening 2024 articles Phytochemical screening Scopus articles Phytochemical screening impact factor journals Phytochemical screening Scopus journals Phytochemical screening PubMed journals Phytochemical screening medical journals Phytochemical screening free journals Phytochemical screening best journals Phytochemical screening top journals Phytochemical screening free medical journals Phytochemical screening famous journals Phytochemical screening Google Scholar indexed journals " spectroscopy articles spectroscopy Research articles spectroscopy review articles spectroscopy PubMed articles spectroscopy PubMed Central articles spectroscopy 2023 articles spectroscopy 2024 articles spectroscopy Scopus articles spectroscopy impact factor journals spectroscopy Scopus journals spectroscopy PubMed journals spectroscopy medical journals spectroscopy free journals spectroscopy best journals spectroscopy top journals spectroscopy free medical journals spectroscopy famous journals spectroscopy Google Scholar indexed journals Endemic medicinal articles Endemic medicinal Research articles Endemic medicinal review articles Endemic medicinal PubMed articles Endemic medicinal PubMed Central articles Endemic medicinal 2023 articles Endemic medicinal 2024 articles Endemic medicinal Scopus articles Endemic medicinal impact factor journals Endemic medicinal Scopus journals Endemic medicinal PubMed journals Endemic medicinal medical journals Endemic medicinal free journals Endemic medicinal best journals Endemic medicinal top journals Endemic medicinal free medical journals Endemic medicinal famous journals Endemic medicinal Google Scholar indexed journals Phytochemical articles Phytochemical Research articles Phytochemical review articles Phytochemical PubMed articles Phytochemical PubMed Central articles Phytochemical 2023 articles Phytochemical 2024 articles Phytochemical Scopus articles Phytochemical impact factor journals Phytochemical Scopus journals Phytochemical PubMed journals Phytochemical medical journals Phytochemical free journals Phytochemical best journals Phytochemical top journals Phytochemical free medical journals Phytochemical famous journals Phytochemical Google Scholar indexed journals Chemical production articles Chemical production Research articles Chemical production review articles Chemical production PubMed articles Chemical production PubMed Central articles Chemical production 2023 articles Chemical production 2024 articles Chemical production Scopus articles Chemical production impact factor journals Chemical production Scopus journals Chemical production PubMed journals Chemical production medical journals Chemical production free journals Chemical production best journals Chemical production top journals Chemical production free medical journals Chemical production famous journals Chemical production Google Scholar indexed journals
Article Details
1. Introduction
Silver nanoparticles have received considerable attention due to their distinct physical, chemical and biological properties, attributed to their large surface area and increased number of atoms at the surface [1]. The latter can be produced by both chemical and biological means, however, the chemical approach includes certain drawbacks. Dramatic modifications in the structure, size and stability of the nanoparticle can arise by small changes in the synthetic parameters [2]. Biosynthetic methods offer an alternative to complex chemical approach. This study employed Pittosporum senacia extract as reducing and stabilizing agent to produce silver nanoparticles. Silver nanoparticles are known for its myriad application such as antimicrobial, anti-inflammatory, catalytic, larvicidal and wound healing activities [3]. However, it is also true that the properties of silver nanoparticles are strongly influenced by its shape, size and size distribution [1]. This study investigated the significant difference in antimicrobial activities of both chemically and biologically synthesized nanoparticles.
2. Materials And Methods
2.1 Sample collection and identification
Fresh Pittosporum senacia leaves were collected and identified at the Mauritius herbarium, with an accession number of MAU0022466. The leaves were washed with tap water to remove any dust or debris. It was washed a second time with distilled water and dried for 21 days to remove moisture.
2.2 Sample extraction
5 g of leaf powder was mixed in 100 ml of distilled water and then heated for 10 minutes at approximately 80°C. The leaf decoction was allowed to cool at room temperature and then filtered using Whatman No. 1 filter paper and stored at 4°C until further use [4].
2.3 Biosynthesis of silver nanoparticles
1 mM of silver ions was prepared by dissolving 0.017 g of silver nitrate in 100 ml of distilled water. 10 ml of 1 mM of silver nitrate was taken in a 50-ml conical flask; then 1 ml, 2 ml, 3 ml, 4 ml and 5 ml of Pittosporum senacia extract was added separately to it. The final volume was 15 ml, so distilled water was added to top up. The solution was left for incubation in the dark, to prevent any light reaction [4].
2.4 Chemical synthesis of silver nanoparticles
A modified protocol [5] was used for the chemical production of silver nanoparticles. 10 ml of 1 mM of silver nitrate was boiled while stirring vigorously and 2 ml of sodium citrate was added to it. The solution was stirred until any color change was observed and left for cooling at room temperature.
2.5 Characterization of silver nanoparticles
The Ultraviolet-visible spectroscopy (Jenway 7305 Spectrophotometer) was used to confirm the formation of silver nanoparticles [6]. The absorbance was measured at a wavelength of 200 to 700 nm after both 5 min and 60 min incubation process. Color change was also observed visually after addition of plant extract to silver nitrate solution.
2.6 Purification of silver nanoparticles
Both the biologically and chemically synthesized silver nanoparticles were centrifuged in Centrifuge-MIKRO 22R, at 10, 000 rpm for 30 minutes [7]. Pellets were re-suspended in 1 ml of distilled water. The process was repeated 5 times to ensure removal of impurities from silver nanoparticles. This was used as 100% silver nanoparticles solution.
2.7 Phytochemical screening
The aqueous extract of Pittosporum senacia was screened both before and after addition of silver nitrate for the presence of alkaloids, tannins, steroids, carbohydrates using the biochemical method of [8], flavonoids [9], terpenoids [10], saponins [11], phenols and protein using Biuret test.
2.8 Antibacterial screening of silver nanoparticles
Antibacterial activity was assessed against Gram positive bacteria Listeria innocua (ATCC 33090), Bacillus cereus (ATCC 10876), Staphylococcus aureus (ATCC 29213) and Gram negative bacteria Pseudomonas fluorescens (ATCC 13525), Escherichia coli (ATCC 25922). Antifungal activity was tested against Candida albicans (ATCC 10231). Bacteria were cultured in Mueller Hinton broth while Potato Dextrose broth was used to grow the fungus. Agar-disc diffusion method was used as a preliminary screening. The plate was divided into quadrants and 100 µl of the bacterium inoculum was spread on the agar. The sterile disc was dipped into the silver nanoparticle solution, placed in the first quadrant. The second quadrant contained disc dipped into solution of silver nitrate, while the third consisted of disc dipped into solution containing equal volume of silver nanoparticles and chloramphenicol antibiotic (5 mg/ml). The last quadrant consisted of the positive control, chloramphenicol. Similar procedure was followed for the fungus and chemically synthesized nanoparticles, except that Nystatin (3 mg/ml) was used as positive control for fungus. The plates were incubated for 24 hours at 37°C. Inhibition zone was measured using a ruler in mm.
2.9 Minimum Inhibitory Concentration (MIC)
Extract showing clear inhibition zone were selected for the determination of MIC. Chloramphenicol (10 mg/ml) was used as positive control and distilled water as negative control. Samples to be tested included: 100% biologically synthesized silver nanoparticles, mixture containing equal volume of 100% biologically synthesized silver nanoparticle and 10 µg/ml chloramphenicol, 1 mM silver nitrate solution, 100% chemically synthesized nanoparticle. 100 µl extracts were two-fold serially diluted with 100 µl Mueller Hinton Broth (MHB). Bacterial inoculums were diluted to a concentration of 106 cfu/ml and for clear distinction, 40 µl of 0.2 mg/ml p-iodonitrotetrazolium violet (INT) dye indicator was added to each well.
3. Results and Discussion
3.1 UV-Visible spectra analysis and colour change
After addition of aqueous extract to silver nitrate solution, a color change to brownish yellow was observed while for the chemically synthesized silver nanoparticles, color changed from colourless to dark green. Colour change was due to the excitation of surface plasmon resonance (SPR) in the production of silver nanoparticles [12-14]. Maximum absorption of silver nanoparticles was observed at 400 nm which is indicative of spherical nanoparticles [6, 15]. Broaden peak at 300 nm was also discerned for 3.33 mg/ml plant extract, indicative of nanoparticle polydispersion [4]. Absorption peak observed at 300 nm can also show that there were electronic transitions of metallic silver, Ag0 [16].
As the diameter of silver nanoparticles increases, the absorbance spectra tends to shift over longer wavelength and become broader [17]. This may explain why absorption was obtained at 600 nm. Metal nanoparticle synthesis is mostly observed using UV-Vis spectroscopy as a result of its simplicity [4]. As extract concentration increased, the absorption peak at 400 nm and 600 nm would increase consequently. This would result from the increase number of biomolecule contributing to the reduction of silver ion reductive process [18].
Compared to the biologically synthesized nanoparticles, the characterisation of chemically synthesized one showed shorter and broader peaks. This indicated that smaller amount of silver nanoparticles have been produced since intensity of peaks are known to be proportional to the concentration. The broaden peak might also demonstrate that the diameter of chemically synthesized nanoparticle is larger than the biologically synthesized one.
3.2 Phytochemical screening
Phytochemical screening revealed the presence of saponins, carbohydrates and phenols in both the plant extract and the biosynthesized silver nanoparticles. The latter could be responsible for the reduction and stabilisation of nanoparticles [6]. Tannins and terpenoids was also recorded in the plant extract.
3.3 Antibacterial screening
For both the chemically and biologically synthesized silver nanoparticles, no activity was denoted against either Gram positive bacteria B. cereus and S. aureus or fungus C. albicans. In this study, Gram negative bacteria were more susceptible to the silver nanoparticle reporting at average of 21.67 ± 1.15 mm and 20.0 ± 0.0 mm against E.coli using biologically and chemically synthesized silver nanoparticle respectively (Table 1). Maximum activity against E.coli was also recorded by [18], 16 mm zone of inhibition using C. spinosa leaf extract. Antibacterial activity of nanoparticles is greatly dependent on its size and shape [18]. It is hypothesized that due to its increase surface area, the silver nanoparticle would be able to better interact with the bacterium’s cell wall [6, 19]. Likewise, it might also be possible that silver ions inhibit DNA replication, reduce cell viability and lead to apoptosis by directly interacting with the basic component that makes up DNA and proteins such as sulphur and phosphorus [6, 20]. Other possible mechanism would include the adverse reaction that silver ions have on functional group of enzymes and their inductive oxidative stress [6, 20, 21].
Lowest MIC value obtained was for P. fluorescens; 12.5% using biologically synthesized silver nanoparticles alone and 6.3% using a combination of biologically synthesized silver nanoparticles and chloramphenicol. Unfortunately, no significant synergistic interaction (p>0.05) was observed with chloramphenicol alone yielding an MIC value of 0.6%.
3.4 Synergistic effect of silver nanoparticle with antibiotic
There were no significant difference in inhibition zone of biosynthesized nanoparticle alone and combination of biosynthesized nanoparticle with 10 µg/ml of chloramphenicol (p>0.05). Compared to other studies such as [6], no synergistic interaction was observed between silver nanoparticle and chloramphenicol antibiotic.
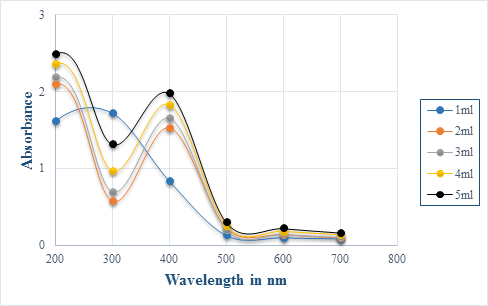
Figure 1: UV- Spectra illustrating silver nanoparticles synthesised by P. senacia extract at different concentration indicated by the legend.
|
Microorganism |
Silver nanoparticles |
Antibiotic & Biosynthesized nanoparticle |
Control |
||
|
Biologically |
Chemically |
Silver nitrate |
Positive |
||
|
B. cereus |
- |
- |
26.67 ± 1.15 |
- |
27.33 ± 0.58 |
|
S. aureus |
- |
- |
19.33 ± .15 |
- |
33.0 ± 1.00 |
|
L. innocua |
11.0 ± 0.0 |
12.67 ± 1.15 |
25.67 ± 0.58 |
24.67 ± 0.58 |
23.33 ± 0.58 |
|
E.coli |
21.67 ± 1.15 |
20.0 ± 0.0 |
12.0 ± 0.0 |
24.33 ± 0.58 |
27.0 ± 0.58 |
|
P. fluorescens |
13.33 ± 1.57 |
15.33 ± 0.58 |
22.67 ± 0.58 |
13.33 ± 0.58 |
30.0 ± 0.0 |
|
P. mirabilis |
17.67 ± 0.58 |
17.33 ± 0.58 |
19.0 ± 0.0 |
10.0 ± 0.0 |
24.0 ± 0.0 |
|
C. albicans |
- |
- |
18.0 ± 0.0 |
- |
15.67 ± 0.58 |
Table 1: Zone of inhibition zone (in mm) using silver nanoparticles.
Following tukey’s test, it was found that there was no significant difference between the antimicrobial activity of both biosynthesized and chemically synthesized nanoparticles, (p>0.05). Hence, biosynthesized nanoparticles would exert the same antibacterial activity as chemically synthesized one and can be employed instead, considering its numerous benefits.
Acknowledgement
The authors wished to thank the University of Mauritius, for providing the necessary facilities and consumable as well as the Mauritius Herbarium for assisting in the correct identification of the plant sample.
References
- Abou ENM, Eftaiha A, Al-Warthan A, et al. Synthesis and application of silver nanoparticles. Arab J Chem 3 (2010): 135-140.
- Oliveira MM, Ugarte D, Zanchet D et al. Influence of synthetic parameters on the size, structure and stability of dodecanethiol-stabilized silver nanoparticles. Journal of Colloid and Interface Science 292 (2005): 429-435.
- Firdhouse JM, Lalitha P. Biosynthesis of Silver Nanoparticles and Its Applications. Journal of Nanotechnology 1-18 (2015).
- Sumi Maria B, Devadiga A, Shetty Kodialbial V, et al. Synthesis of silver nanoparticle using medicinal Zizyphus xylopyrus bark extract. Applied nanoscience 5 (2015): 755-762.
- Rashid MU, Bhuiyan KMH, Quayum EM. Synthesis of silver nanoparticles (AgNP) and their uses for quantitative analysis of vitamin C tablets. Dhaka University Journal of Pharmaceutical Sciences 12 (2013): 312-319.
- Jyoti K, Baunthiyal M, Singh A. Characterization of silver nano particles synthesized using Urtica dioca Linn. leaves and their synergistic effects with antibiotics. Journal of Radiation and Applied Sciences 9 (2016): 217-227.
- Krithiga N, Rajalakshmi A, Jayachitra A. Green synthesis of silver nanoparticles using leaf extracts of Clitoria ternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. Journal of Nanoscience 1-8 (2015).
- Kodangala C, Saha S, Kodangala P. Phytochemical studies of aerial parts of the plant Leucas lavandulaefolia. Der Pharma Chemica 2 (2010): 434-437.
- Hossain MA, Al-Raqmi KA, Al-Mijizy ZH, et al. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed 3 (2013): 705-710.
- Ayoola GA, Coker HAB, Adeseguun SA, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for Malaria Therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research 7 (2008): 1019-1024.
- Kareru P, Keriko J, Gachanja A, et al. Direct detection of triterpenoid saponins in medicinal plants. African Journal of Traditional Complementary and Alternative Medicines 5 (2008): 56-60.
- Narayanan K, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett 62 (2008): 4588-4590.
- Xiaoming S, Liming Z, Songhua H. Amplified immune response by ginsenoside-based nanoparticles (ginsomes). Vaccine 27 (2009): 2306-2311.
- Medda S, Hajra A, Dey U, et al. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Applied Nanoscience 5 (2015): 875-880.
- Zaheer Z, Rafiuddin. Silver nanoparticles to self-assembled films: green synthesis and characterization. Colloids and Surfaces B. Biointerfaces 90 (2012): 48-52.
- Baia L, Simon S. UV-Vis and TEM assessment of morphological features of silver nanoparticles from phosphate glass matrices. Modern Research and Educational Topics in MIcroscopy (2007): 576-583.
- Amendola V, Bakr OM, Stellacci F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: effect of shape, size, structure and assembly. Plasmonics 5 (2010): 85-97.
- Benakashani F, Allafchian AR, Jalali SAH. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala International Journal of Modern Science 2 (2016): 251-258.
- Kvitek L, Panacek A, Soukupova J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles. Journal of Physical Chemistry C 112 (2008): 5825-5834.
- Matsumura Y, Yoshikata K, Kunisaki S, et al. Mode of bactericidal action of silver zeolite and its composition with that of silver nitrate. Applied Environmental Microbiology 69 (2003): 4278-4281.
- Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticlevs. Nanomedicine 3 (2007): 95-101.


 Impact Factor: * 5.3
Impact Factor: * 5.3 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 