Association between Mobile Phone Using and DNA Damage of Epithelial Cells of the Oral Mucosa
Article Information
Ahmad M Khalil*, Israa F Alemam, Khaled M Al-Qaoud
Department of Biological Sciences, Yarmouk University, Irbid, Jordan
*Corresponding Author: Ahmad Khalil, Department of Biological Sciences, Irbid, Jordan
Received: 09 May 2020; Accepted: 19 May 2020; Published: 29 May 2020
Citation:
Ahmad M Khalil, Israa F Alemam, Khaled M Al-Qaoud. Association between Mobile Phone Using and DNA Damage of Epithelial Cells of the Oral Mucosa. Journal of Biotechnology and Biomedicine 3 (2020): 50-66.
View / Download Pdf Share at FacebookAbstract
Exposure to high levels of radiofrequency radiation can potentially cause tissue damage characteristic of many diseases including cancer. This study explored relationship between use of mobile phone and DNA damage in oral mucosal cells. One hundred individuals completed a questionnaire were grouped according to frequency and duration of mobile usage. Comet and TUNEL assays were used to determine DNA damage and rate of apoptosis, respectively. Number of damaged cells in right cheek was significantly higher than in left cheek in right ear phone dominant users, but not in left ear dominant people. Years of phone use was not positively correlated to degree of DNA damage, however, damage increased with increased frequency of phone use. Apoptotic cells were highest in medium (30-60 min/day) phone users. Although no significant correlation was observed between degree of apoptosis and cumulative year of mobile phone use, phone use must be minimized to reduce health effects.
Keywords
Apoptosis, Cell phone, Comet assay, DNA damage, Oral mucosa, TUNEL assay
Apoptosis articles Apoptosis Research articles Apoptosis review articles Apoptosis PubMed articles Apoptosis PubMed Central articles Apoptosis 2023 articles Apoptosis 2024 articles Apoptosis Scopus articles Apoptosis impact factor journals Apoptosis Scopus journals Apoptosis PubMed journals Apoptosis medical journals Apoptosis free journals Apoptosis best journals Apoptosis top journals Apoptosis free medical journals Apoptosis famous journals Apoptosis Google Scholar indexed journals Cell phone articles Cell phone Research articles Cell phone review articles Cell phone PubMed articles Cell phone PubMed Central articles Cell phone 2023 articles Cell phone 2024 articles Cell phone Scopus articles Cell phone impact factor journals Cell phone Scopus journals Cell phone PubMed journals Cell phone medical journals Cell phone free journals Cell phone best journals Cell phone top journals Cell phone free medical journals Cell phone famous journals Cell phone Google Scholar indexed journals Comet assay articles Comet assay Research articles Comet assay review articles Comet assay PubMed articles Comet assay PubMed Central articles Comet assay 2023 articles Comet assay 2024 articles Comet assay Scopus articles Comet assay impact factor journals Comet assay Scopus journals Comet assay PubMed journals Comet assay medical journals Comet assay free journals Comet assay best journals Comet assay top journals Comet assay free medical journals Comet assay famous journals Comet assay Google Scholar indexed journals DNA damage articles DNA damage Research articles DNA damage review articles DNA damage PubMed articles DNA damage PubMed Central articles DNA damage 2023 articles DNA damage 2024 articles DNA damage Scopus articles DNA damage impact factor journals DNA damage Scopus journals DNA damage PubMed journals DNA damage medical journals DNA damage free journals DNA damage best journals DNA damage top journals DNA damage free medical journals DNA damage famous journals DNA damage Google Scholar indexed journals Oral mucosa articles Oral mucosa Research articles Oral mucosa review articles Oral mucosa PubMed articles Oral mucosa PubMed Central articles Oral mucosa 2023 articles Oral mucosa 2024 articles Oral mucosa Scopus articles Oral mucosa impact factor journals Oral mucosa Scopus journals Oral mucosa PubMed journals Oral mucosa medical journals Oral mucosa free journals Oral mucosa best journals Oral mucosa top journals Oral mucosa free medical journals Oral mucosa famous journals Oral mucosa Google Scholar indexed journals TUNEL assay articles TUNEL assay Research articles TUNEL assay review articles TUNEL assay PubMed articles TUNEL assay PubMed Central articles TUNEL assay 2023 articles TUNEL assay 2024 articles TUNEL assay Scopus articles TUNEL assay impact factor journals TUNEL assay Scopus journals TUNEL assay PubMed journals TUNEL assay medical journals TUNEL assay free journals TUNEL assay best journals TUNEL assay top journals TUNEL assay free medical journals TUNEL assay famous journals TUNEL assay Google Scholar indexed journals technologies articles technologies Research articles technologies review articles technologies PubMed articles technologies PubMed Central articles technologies 2023 articles technologies 2024 articles technologies Scopus articles technologies impact factor journals technologies Scopus journals technologies PubMed journals technologies medical journals technologies free journals technologies best journals technologies top journals technologies free medical journals technologies famous journals technologies Google Scholar indexed journals RFR-related articles RFR-related Research articles RFR-related review articles RFR-related PubMed articles RFR-related PubMed Central articles RFR-related 2023 articles RFR-related 2024 articles RFR-related Scopus articles RFR-related impact factor journals RFR-related Scopus journals RFR-related PubMed journals RFR-related medical journals RFR-related free journals RFR-related best journals RFR-related top journals RFR-related free medical journals RFR-related famous journals RFR-related Google Scholar indexed journals blood-brain barrier articles blood-brain barrier Research articles blood-brain barrier review articles blood-brain barrier PubMed articles blood-brain barrier PubMed Central articles blood-brain barrier 2023 articles blood-brain barrier 2024 articles blood-brain barrier Scopus articles blood-brain barrier impact factor journals blood-brain barrier Scopus journals blood-brain barrier PubMed journals blood-brain barrier medical journals blood-brain barrier free journals blood-brain barrier best journals blood-brain barrier top journals blood-brain barrier free medical journals blood-brain barrier famous journals blood-brain barrier Google Scholar indexed journals damaged cells articles damaged cells Research articles damaged cells review articles damaged cells PubMed articles damaged cells PubMed Central articles damaged cells 2023 articles damaged cells 2024 articles damaged cells Scopus articles damaged cells impact factor journals damaged cells Scopus journals damaged cells PubMed journals damaged cells medical journals damaged cells free journals damaged cells best journals damaged cells top journals damaged cells free medical journals damaged cells famous journals damaged cells Google Scholar indexed journals
Article Details
Introduction
Advances in radiofrequency radiation RFR-related technologies have been and continue to be rapid. In recent years, most of the global populations (especially college and university students), use mobile phones due to their wide range of applications [1]. It has been noted that the average person spends 90 min a day on his/her phone [2]. Mobile phones have many perceived benefits, including increased accessibility and social connection, efficiency in the workplace. However, the way mobile phone are held close to the head as well as the duration and frequency of calls have raised serious health concerns. An extensive review of the recent published literature confirms non-thermally induced effects people’s health (e.g., male infertility, oxidative stress, DNA damage, alteration of gene expression, breakdown of the blood-brain barrier, and induction or promotion of cancer) from exposure to RFR [3-8]. Furthermore, the increasing use of mobile phones in children has been associated with emotional and behavioral disorders [5] and high frequency of hearing loss [9]. The 5G mobile networking technology (which is projected to use mainly the higher microwave frequencies part of the spectrum in the highest performance mode) will affect not only the skin and eyes, but will have adverse systemic effects as well [10]. The frequency of electromagnetic waves emitted from mobile phones significantly increased the human response to stimulus time i.e. slower reaction of the subjects [7]. The loss of mental attention [2] and accidents caused by distracted driving [11]have been highlighted as a public health concern. The inconsistent results between similar studies and the same research groups have made it very diffcult to make any comprehensive interpretation [12].
The limit of mobile phone use is the SAR of 2 W/kg for the human head [13].Depending on the different type of mobile phones, the maximum local SAR values ranged between 0.2 and 1.5 W/kg on an average for 10 grams of tissue [14].40% of which is absorbed in the head and neck region [15]. The oral cavity and other extraoral structures including the salivary glands, dental appliances and dental restorations are the closest organs and tissues of the body for mobile phone use. Consequently, they are expected to be the most exposed to mobile phone emitted radiations during the conversation period [14, 16].
The knowledge of the effects of RFR on oral mucosal cells may give a perspective about what kind of effects of the radiation could have on other organs. Several studies have confirmed the genotoxic effect of mobile phone radiation on oral mucosa [15, 16], but others have apparently denied such effects [17, 18]. In the light of the contradictory results obtained from previous studies, the reevaluation of the effect of mobile radiation on the oral epithelium has been kindled. The aim of the present cross-sectional study was comparing high-, medium-, and low-RFR exposure persons to generate information about association of this exposure with DNA damage and cell death in oral mucosal cells of people exposed to RFR from mobile phones.
2. Materials and Methods
2.1 Subjects
This cross-sectional study was approved by a specialized Human Research Ethics Committee of Yarmouk University. A written informed consent was obtained from each participant. A sample of 100 university student volunteers in the age range of 18-30 years was randomly recruited. Before providing the oral mucosal cells, each participant was interviewed by the same research staff to standardize data collection data regarding age, gender, locality of residence, type of mobile used, duration of daily mobile phone usage (min a day), the overall period of exposure (number of years) and the of use of headsets by completing a specially constructed detailed questionnaire. The study excluded subjects with smoking habit, or receiving drug therapy in the last three months, suffering any illness including cancer, subjected to radiotherapy including dental procedures, dietary supplements, and regular mouthwash users. Nobody dropped out of the study and was ready to answer any query that may arise at any time during the study.
Participants were randomly stratified into three groups based on the frequency and intensity of phone use: Group 1: Low mobile phone users (less than 30 min/day), Group 2: Medium mobile phone users (30-60min/day) and Group 3: Heavy mobile phone users (more than 60 min/day). The participants were further divided into three categories on the basis of the history of phone use; short-, mediate-, and long- duration users for those who used their phones for periods less than 5 years, 5-10 years and longer than 10 years, respectively. Data collection and results analysis of all experimental work were performed under blind code.
2.2 Collecting cells
Before collecting the oral mucosal cells from the subject, he/she was asked not to eat and drink an hour. Then, the oral cavity was cleansed by using drinking water. Two samples were obtained from the inner surface of both sides of the donor cheeks using a sterile, small headed plastic toothbrush. Separate brushes were used for each cheek. Cell samples with the preferential side (right or left) used during phone calls were taken to analyze the effect sidedness. The samples were collected daily in the morning between 10 and 12. The brushes were placed in their respective buffer containers and rotated repeatedly to dislodge the cells and release them into the buffer medium. The material collected was centrifuged for 10 min at 50 × g, the pellet resuspended and smeared with the aid of two drops of the physiological saline.
2.3 DNA damage assay (Comet assay)
Cellular DNA damage was investigated by using OxiSelectTM Comet Assay Kit (Cell Biolabs INC, San Diego, CA, USA) according to manufacturer’s instructions. The DNA damage was assessed in representative fields of view of a fluorescent microscope (Nikon 400, Tokyo, Japan) under 400× magnification. The images of 100 randomly chosen nuclei were analyzed by the CometScore Version 2.0.0.38 TriTek documentation. To quantify the DNA damage, seven parameters were calculated: head length, tail length, %head DNA, and %tail DNA, tail moment (TM), Olive tail moment comet (OTM) (Figure 1).
2.4 Apoptosis assay (TUNEL assay)
The DeadEnd TM colorimetric TUNEL kit (Promega, Madison, WI, USA) was used to measure apoptosis-induced nuclear fragmentation via a colorimetric fluorescence assay according to the manufacturer’s instructions. The exfoliated buccal mucosal cells were examined under light microscope. The number of apoptotic cells among 100 cells/sample in five randomly selected fields was counted. Only clearly defined cells were considered, excluding the clumped or folded cells and unusually distorted nuclei and cells. The presence of clear positive brown nuclear staining (BAB chromogen) was indicative of apoptotic cells (Figure 2).
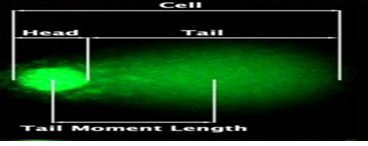
Figure 1: Typical comet: Cell consisting of a head and a tail. DNA damage is calculated as the %DNA tail area and the comet tail length (from the posterior end of the DNA head to the end the DNA tail). The larger the %DNA tail area or the longer the DNA tail length, the more significant the DNA damage. Tail moment length is the distance from the center of the head to the center of the tail. In a negative comet, the nucleus appears intact head without a tail (Magnification 400 ×).
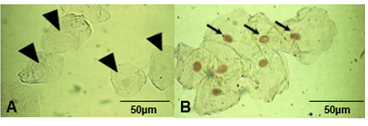
Figure 2: Representative photographs of TUNEL staining examined under light microscope. (A) Arrow heads indicate negative TUNEL test (healthy cells); (B) Arrows indicate strong positive TUNEL test (apoptotic cells) (Magnification 400×).
2.5 Statistical method
Data analysis was performed using SPSS (Statistical package for Social Sciences) Version 16.0 Software (Chicago, IL, USA). The values were represented in Mean ± SEM. Differences within groups were analyzed using ANOVA test. Differences between groups were evaluated by paired t-test. A difference at p<0.05 was accepted as statistically significant.
3. Results
The study group included 100 subjects. All of mobile phones used by participants were within the most common SAR levels (in the range between 0.244 to 1.552 W / kg) which have been built based on official data for most popular mobile phones brands (LG, iPhone, Motorola, Nokia, Samsung, Siemens, Sony Ericson, etc.), taken from the official websites of their manufacturers [19]. Out of the 100 participants 55 individuals were females and 45 were males. Table 1 depicts summary of the results of questionnaire analysis according to age, gender and habituation characteristics of mobile phone use. Most of the participants (69%) belonged to the youngest group (18-21 years); 63.63% (35/55) of females and 75.55% (34/45) of males. It appeared that most of the investigated group (57%), regardless of the sex, were short time callers (<30 min); 30 out of 55 (54.54%) for females and 25 out 45 (55.55%) for males. About half (47%) of the total respondents had used the mobile phone between 5 and 10 years; 22/55 or 40%, and 20/45 or 44.44% among females and males, respectively. Because there were no statistically significant differences in the records of the males and females users the results were pooled. Simlarly, 90 out of the 100 subjects (90%) preferrably used their right ear during calls; 49 out of 55 (89.09%) for females and 41 out of 45 (91.11%). Only 6 out of 55 females (10.91%) and 4 out of 45 males (8.89%) kept their phones against their left ear. Because there were no statistical difference between males and females in respect to ear dominance, the data pooled for further calculatoins.
Analysis of the pooled data from both sexes (Table 2) showed that in general, among the three groups of total daily calling times there were no statistically significant differences in the percentage of damaged cells, estimated by comet assay, neither on the left- nor on the right- ear preferring mobile phone users (P-values equal 0.46 and 0.96, respectively). However, the average percent was slightly higher on the right side relative to the left side; 7.57 ± 0.635% and 6.17 ± 0.503%, respectively. A similar trend was noted regarding the duration of use (Table 3).
The percentage of damaged cells on the right side was significantly higher than on the left side for the participants who preferred to use phone on the right ear (P-value 0.02). Whereas, no significant differences were noticed between the two cheeks for the left ear dominant users (P-value 0.76). DNA damage in 100 cells exposed to RF fields detected by comet assay and estimated by measuring the DNA extent of the migration toward anode pole is summarized in Figure 3. The results of comet analysis are summarized in Tables 4 and 5. In respect to time of calls and within the users of the ear, comet length increased with increasing time of daily calls. Significant differences between increases in comet length between the left (P-value=0.05) and right (P-value=0.04) users were observed (Table 4). While the 30-60 min group showed more obvious effect on the right side, the effect was greater on left ear of the >60 min group. In contrast, no significant differences (P-value>0.05) in the decrease of the head length were noticed when the groups of calling times were compared. However, the most clear and significant decrease in the head length was seen in the >60 min right side users relative to the left side (184.71 ± 14.605 μm versus 224.30 ± 29.206 μm, respectively, Table 4). In regard to relation between increases in time of calls and increase in tail length, significant correlations (P- value=0.04) were recorded on both right side and the left side. The mean tail length was 224.27 ± 25.725 μm compared to 175.54 ± 21.267 μm on the right and left sides, respectively (Table4).
Table 4 also indicates that the mean% head DNA significantly (P-value = 0.01) decreased with increase in total time/ a day both in the right ear dominant callers and the left ear callers (P-value=0.04). Regardless of the ear use, the %tail DNA significantly (p-value < 0.05) increased with increased daily time use of mobile phone (Table 4). Similar to the %head DNA, no difference in the mean %tail DNA was noticed between the right and left ear dominance. Moreover, significant elevations in values of tail moment were calculated as min calls increased on the left (P-value = 0.01) and on right ear (P-value = 0.03) (Table 4). Mean differences peaked at 30-60 min/day; 104.11 ± 19.124 and 74.46 ± 14.793, for the right and left ears, respectively. Increases in the Olive tail moment (Table 4) both on left (P-value = 0.002) and on the right ear (P-value = 0.01) were positively associated with longer daily times on the phone. The most prominent difference between the right and left ear groups was recorded among the mediate (30-60 min/day) call times; 51.50 ± 7.959 and 35.20 ± 5.998, respectively.
In general, the length of the history of phone usage did not seem to be positively correlated to the degree of DNA damage (Tables 5). The two comet parameters; comet length and tail length reached a maximum in the < 5 years group. These decreases were significant only on the left side among low (< 5 years, P-value = 0.04) and heavy users (>10 years, P-value = 0.01). The readings of the %Head DNA and the %tail DNA were not different (P-value > 0.05) on either side of the cheek. Within groups there were no significant difference in tail moment neither on the left side (P- value = 0.08) nor on the right side (P-value = 0.59). However, a clear difference in tail moment showed up between those who used the phone for a period between 5 and 10 years on the right side and those who used it on the left side (Table 5). A similar trend was repeated with respect to Olive tail moment.
Lack of TUNEL expression (staining level=0) represented normal cells (Figure 2A), while the presence of deep brown nuclear staining was indicative of dead (apoptotic) cells (Figure 2B). Cell death reflecting the genotoxic effects of exposure to RFR was demonstrated by occurrence of degenerative nuclei (Figure 4). There were no obvious differences between the percent of TUNEL positive cheek cells from the right- and the left- ear irrespective of the gender of the phone user. Therefore, the extents of cell death caused by exposure to RF fields estimated by TUNEL assay were pooled (Tables 6 and 7). The cell samples obtained from the cheeks of students whose were medium in their daily phone use (30-60 min) had a slightly higher, but not significant, percentage of apoptosis than those collected from cheeks of low and heavy users; P-values 0.21 and 0.10 for the left and right cheeks, respectively (Table 6). Within a dominant side, the percent of apoptotic cells peaked in the medium (30-60 min/day) phone users. Cells from the right side had higher nonsignificant proportions of apoptosis than those examine from the left side. Similarly and regardless of the dominant ear, no significant correlation was observed between the percent of TUNEL staining nuclei (apoptotic cells) and the cumulative year of mobile phone usage (Table 7).
|
Parameter |
Frequency (%) |
|
|
Gender |
Females |
55 |
|
Males |
45 |
|
|
Age Group (Years) |
18-21 |
69 |
|
22-25 |
19 |
|
|
26-29 |
9 |
|
|
30-33 |
3 |
|
|
Dominance Ear of Phone Use |
Right |
90 |
|
Left |
10 |
|
|
History of Phone Use (Years) |
<5 |
37 |
|
5-10 |
47 |
|
|
>10 |
16 |
|
|
Daily Use of Phone (Minutes) |
<30 |
57 |
|
30-60 |
27 |
|
|
>60 |
16 |
|
Table 1: Characteristics and habituation of the investigated population (N=100).
|
Side of Phone Use |
Number of Participants |
Total Time of Phone Use (Minute/day |
%Damaged Cells Mean ± SEM |
p-value* |
|
Left |
57 |
<30 |
5.68 ± 0.711 |
0.46 |
|
27 |
30-60 |
6.48 ± 0.851 |
||
|
16 |
>60 |
7.38 ± 1.193 |
||
|
Right |
57 |
<30 |
7.42 ± 0.921 |
0.96 |
|
27 |
30-60 |
7.78 ± 1.146 |
||
|
16 |
>60 |
7.75 ± 1.226 |
*ANOVA test at 95% confidence interval
Table 2: Percentage of damaged exfoliated buccal mucosal cells estimated from comet assay parameters, in reference to ear dominance, and daily use of phone. A total of 100 cells from each cheek were screened.
|
Side of Phone Use |
Number of Participants |
Duration of Phone Use (Years) |
%Damaged Cells Mean ± SEM |
p-value* |
|
Left |
37 |
<5 |
6.49 ± 0.970 |
0.73 |
|
47 |
5-10 |
5.74 ± 0.664 |
||
|
16 |
>10 |
6.69 ± 1.083 |
||
|
Right |
37 |
<5 |
8.57 ± 1.055 |
0.25 |
|
47 |
5-10 |
6.45 ± 0.812 |
||
|
16 |
>10 |
8.56 ± 2.012 |
*ANOVA test at 95% confidence interval.
Table 3: Estimation of the percentage of damaged exfoliated buccal mucosal cells by comet assay, in reference to ear dominance, and duration of phone use. A total of 100 cells from each cheek were screened.
Table 4: DNA damage observed in exfoliated buccal mucosal cells estimated by comet parameters in relation to the frequency (min/day) of phone use.
*ANOVA test at 95% confidence interval
Table 5: DNA damage observed in exfoliated buccal mucosal cells estimated by comet parameters in relation to duration of mobile phone use (Years).
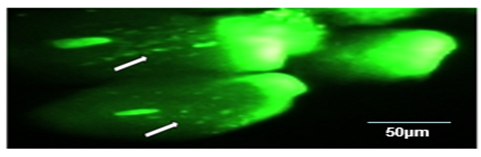
Figure 3: A representative photograph of a nucleus showing a comet: Head on right and tail on left. DNA fragments appear as brightly fluorescinated threads in the tail of comet (arrows). (Magnification 400×).
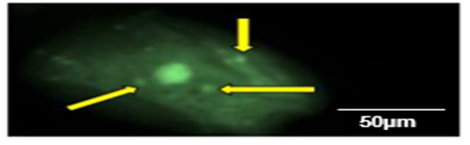
Figure 4: Cell presenting karyorrhexis with condensed and fragmented chromatin (arrows) as occurs during later stages of the cell death (apoptotic) process (Magnification 400×).
|
Side of Phone Use |
Number of Participants |
Total Time of Phone Use (Minute/day) |
%Damaged Cells Mean ± SEM |
p-value* |
|
Left |
57 |
<30 |
57.1 ± 2.47 |
0.21 |
|
27 |
30-60 |
61.9 ± 3.35 |
||
|
16 |
>60 |
52.1 ± 3.60 |
||
|
Right |
57 |
<30 |
57.1 ± 2.13 |
0.10 |
|
27 |
30-60 |
65.1 ± 3.015 |
||
|
16 |
>60 |
60.4 ± 3.98 |
* ANOVA test at 95% confidence interval
Table 6: Percent cell death (apoptosis) estimated by the TUNEL staining of nuclei as related to frequency (min/day) of phone use. (Total number of examined cells from a subject is 100).
|
Side of Phone Use
|
Number of Participants
|
Duration of Phone Use (Years) |
%Apoptotic Cells Mean ± SEM |
p-value* |
|
Left |
37 |
<5 |
59.5 ± 2.43 |
0.66 |
|
47 |
5-10 |
57.0 ± 2.87 |
||
|
16 |
>10 |
54.8 ± 4.73 |
||
|
Right |
37 |
<5 |
60.4 ± 2.25 |
0.79 |
|
47 |
5-10 |
60.2 ± 2.56 |
||
|
16 |
>10 |
57.3 ± 4.45 |
*ANOVA test at 95% confidence interval
Table 7: Percent cell death (apoptosis) estimated by the TUNEL staining of nuclei as related to ear dominance and duration of phone use (Total number of examined cells from a subject is 100).
4. Discussion
The principal finding of the present study was the absence of significant difference in the effect of RFR fields between the two cheeks. Another major finding was the increase in tail moment (tail length × tail density) and in tail density (DNA% in tail) as well as Olive tail moment, which are the most frequently used values of the comet parameters, and daily durations of exposure to radiation were positively correlated (P-values <0.05) to one another. Therefore, more intense daily mobile phone radiation was expected to be the immediate and possible cause for increased DNA breaks.
Since damaged DNA is characteristic of cancer cells, indications of damage due to RFR field exposure are important. In vitro risk testing prior to in vivo evaluation has several significant limitations including extrapolation of the results to in vivo. When animal studies are carried out several biological (dose, species, sex, etc.) and physicochemical properties must be addressed beforehand. Epidemiological studies such as cross-sectional studies that analyzes data from a population, or a representative subset, at a specific point in time may indicate relative or absolute risk. Cross-sectional studies are often unable to include data on confounding factors, other variables which affect the relationship between the putative cause and effect [20]. For example, data only on present RFR exposure and cytotoxicity would not allow the role of past exposure, or of other causes, to be explored. In the cross-sectional studies, investigated factors aren’t controlled, repetition of events aren’t generally possible and randomization facilities are limited. However, the results of the studies are largely consistent with real life. Confounding bias is potentially present in all epidemiological studies. In our study, however, all confounding factors that could cause cytogenetic toxicity were excluded (tobacco, alcohol, recent medication, systemic factor etc.). Therefore, more intense daily mobile phone radiation was expected to be the immediate and possible cause for increased DNA breaks.
Several apoptosis, genotoxic and carcinogenic studies have shown positive results after exposure to mobile radiation, but an equal or greater number have shown no effect. A search of the PubMed database from 1989 to 2016 for “radiofrequency radiation” and “genotoxicity tests” [21] concluded that 19 of 53 studies indicated genotoxic effects (35.8%) and 34 of total studies reported no significant effect (64.2%). The contradictory results may arise from the cellular and molecular alterations resulting from RFR, which vary with the duration of exposure of the tissue, penetration, the healing regeneration of the tissue and some exposure parameters. Toxicity to the genome can lead to a change in gene expression, disruption of normal function of cells, cancer, and cell death.
Consistent with our results are those reported earlier on the effect of mobile phone on oral mucosa using MN assay [15, 16]. Our data also are a long with previous reports that an 1800-MHz RFR caused DNA damage in human lens epithelial cells [22, 23], lymphocytes [24] and hair root stem cells located around the ear [25, 26] as evidenced by comet technique. It was observed that leucocytes in those living in close proximity to base stations had a significant increase in their DNA migration length, damage frequency, and damage indexes. These findings are parallel with previous studies [26, 27] which reported that the DNA damage measured as tail DNA intensity increased with higher dose of radiation. DNA damage, in terms of tail moment of comet in peripheral blood lymphocytes and MN formation in buccal cells, was significantly increased in human populations exposed to radiation from mobile towers [3]. Residing within a perimeter of 80 meters of mobile base stations showed significantly higher frequency of MN when compared to the control group, residing 300 meters away from the mobile base stations [28]. It has been reported that phone radiation is capable of disturbing the DNA repair mechanism, and this can continue for several hours after the phone use [5]. It is known that DNA damage that can occur is related to the cell type and experimental setup (Exposure time, RF frequency, SAR, continuous or pulsed wave, exposure as mobile phone user, etc.) [29, 30]. Mobile phone radiation may increase oxidative stress indices [3, 28, 31, 32, 33] and increase oxidative DNA damage (8-Oxo-7, 8- dihydro-2'-deoxyguanosine) formation [34, 35]. These free radicals could cause membrane and macromolecule damage by three basic mechanisms: lipid peroxidation, DNA fragmentation, and protein oxidation. When DNA damage reaches an unrepairable level or it is not repaired in the right way, it might lead to apoptosis mutation, aging, and cancer [31, 36]. Contradictory results were obtained, where mobile phone-associated electromagnetic fields did not induce significant increase in MN formation in the human oral cavity’s mucosa cells [18, 37, 38] and in cells of the human hematopoietic system [39] at the observed exposure levels.
It should be mentioned that statistical comparison of percent damaged cells on the left and right side regarding the total time call revealed that no significant difference between the right and the left cheek. Furthermore, the cumulative duration of previous history of exposure to RFR did not significantly influence the rate of DNA damage measured by comet. Both the daily frequency of calls and duration of exposure to RFR did not significantly influence the levels of cell death estimated by TUNEL assay. In this regard, radiofrequency electromagnetic fields did not affect apoptosis rate in the human hematopoietic stem cells [39]. However, it should be kept in mind that DNA damage is not a unique feature of apoptosis and TUNEL in situ technique for the detection of apoptosis is not completely specific, as overlap between apoptotic and necrotic cell death has been reported, which may result in the fact that some of the apoptotic cells do not stain [40, 41]. Moreover, DNA damage occurs not only during apoptosis but also is associated with necrosis and is caused by toxic compounds or other insults. DNA damage from other sources can thus cause false positive TUNEL assay results [42, 43]. In addition, it was reported [44] that TUNEL could not distinguish between apoptotic, autolytic and necrotic cells, strengthens this point. On the other hand, early DNA fragmentation may not be readily detected by the TUNEL assay and this may lead to underestimation of the apoptotic values. Moreover, the margin of error in scoring TUNEL between cases is too narrow to allow for definitive categorization. In rat liver and intestine, inclusion of an incubation step with diethyl pyrocarbonate enhanced specificity of TUNEL assay by inactivating endogenous endonucleases that can give false positive results in the assay [43]. This modification allowed a better differentiation between death by necrosis and apoptosis. In addition, it is necessary to use another independent method, such as Western blotting, caspase-3 activity assay, or other methods, along with the TUNEL assay, to validate and characterize apoptosis [45].
Several reports found statistically significant increases in cellular pathological manifestations such as the number of heterochromatin granules, condensation of chromatin and increase in membrane permeability in human buccal epithelium cells following exposure to mobile phone radiation [14, 46-49]. Two groups of researchers [37, 46] considered the increased nuclear fragmentation as a parameter which leads to a lower transcription activity or eventual disintegration of the nucleus.
To our knowledge, the present investigation is the first study in which putative effects (e.g., influence on apoptosis rate) of RFR were investigated in oral mucosal cells. One limitation of the study is that it depended on the student’s response to health problems rather than making sure if they are already affecting the results or not. The limitation is due to the nature of the study and its basic drawings of the problem. Cluster sampling from a wider population base could have provided a more clear idea regarding the topic of interest. Epidemiological studies with adequate statistical power need to be based based upon large numbers of subjects with suffcient latency and intensity of exposure to specific technologies. Although limited by a convenience sample, the present results highlight a correlation between mobile phone use (exposure to RFR) and genetic damage and require interim public health actions in the wake of widespread use of mobile phone. They provide valuable insights to the design, analysis, and interpretation of future epidemiological studies concerning the health effects of exposure resulting from cellular phone use in young people. The expression of pro-apoptosis genes (e.g. bax, cytochrome c and caspase-3) and anti-apoptosis (e.g. Bcl-2) genes in buccal cells of exposed people is being examined by real-time reverse transcription polymerase chain reaction (Real-Time RT- PCR) in our laboratory to find out the effect of chronic exposure to RFR on apoptosis.
5. Conclusion
The present findings indicate that current RFR wavelengths we are exposed to appear to may act as a toxin to biological systems. Our data did not predict any statistically significant differences in cellular damage induced between males and females or in the dominant ear compared to nondominant ear. However, a lack of epidemiological evidence does not necessarily indicate an absence of effect, but rather an inability to study an exposure for the length of time necessary, with an adequate sample size and unexposed comparators, to draw clear conclusions. The information provided by this research may be used to design strategies to minimize RF exposure. We feel that the public health authorities, and physicians/allied health professionals must educate the younger generation about the adverse effects of prolonged use of mobile phones. Mobile phone users must follow methods to minimize such hazards; putting mobile away from the body, decreasing mobile using frequency and increasing the usage of hand free mode.
Acknowledgements
Authors would like to thank the Deanship of Scientific Research and Graduate Studies at Yarmouk University/Jordan for financially supporting this research (Grant number: 35/2018). Special thanks are extended to Dr. Mohamad Al Qaderi and Dr. Ayman Rawashdeh for their assistance in statistical analysis.
Conflict of Interest
None Declared.
References
- Parasuraman S, Sam AT, Yee SW, et al. Smartphone Usage and Increased Risk of Mobile Phone Addiction: A concurrent Study. International Journal of Pharmaceutical Investigation 7 (2017): 125-131.
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Conclusions on Mobile Phones and Radio Frequency Fields (2015).
- Gulati S, Yadav A, Kumar N, et al. Phenotypic and Genotypic Characterization of Antioxidant Enzyme System in Human Population Exposed to Radiation from Mobile Towers. Molecular and Cellular Biochemistry 440 (2017) :1-9.
- Nasser S, Amer NM, Ghobashi MM, et al. Knowledge, Attitude, and Practices (KAP) Study and Antioxidant Status among Mobile Phone Users. Bioscience Research 15 (2018): 3658-3664.
- Nath A. Comprehensive Study on Negative Effects of Mobile Phone/ Smart Phone on Human Health. International Journal of Computer and Communication Engineering 6 (2018): 575-581.
- Miller AB, Sears ME, Morgan LL, et al. Risks to Health and Well-being from Radio-frequency Radiation Emitted by Cell Phones and Other Wireless Devices. Frontiers in Public Health (2019).
- Movahedi MM, Golpaygani AT, Safari A, et al. Effects of Short-term Exposure to Electromagnetic Fields Emitted by 3G and 4G Mobile Phones on Reaction Time and Short-term Memory. Iranian Journal of Medical Physics 16 (2019): 250-254.
- Soffritti M, Giuliani L. The Carcinogenic Potential of Non-ionizing Radiations: The Cases of S-50Hz MF and 1.8GHz GSM RFR. Basic and Clinical Pharmacology and Toxicology 125 (2019): 58-69.
- Philip P, Bhandary SK, Aroor R, et al. The Effect of Mobile Phone Usage on Hearing in Adult Population. Indian Journal of Otology 23 (2017): 1-6.
- Kostoff RN, Heroux P, Aschner M, et al. Adverse Health Effects of 5G Mobile Networking Technology Under Real-life Conditions. Toxicology Letters 323 (2020): 35-40.
- McCartt AT, Hellinga LA, Bratiman KA. Cell Phones and Driving: Review of Research. Traffic Injury Prevention 7 (2006): 89-106.
- Panagopoulos DJ. Comparing DNA Damage Induced by Mobile Telephony and Other Types of Man-made Electromagnetic Fields. Mutation Research 781 (2019): 53-62.
- ICNIRP (International Commission on Nonionizing Radiation Protection). Guidelines for Limiting Exposure to Time Varying Electric, Magnetic, and Electromagnetic Fields (up to 300 GHz). Health Physics 97 (2009): 257-258.
- Mishra SK, Chowdhary R, Kumari S, et al. Effect of Cell Phone Radiations on Orofacial Structures: A Systematic Review. Journal of Clinical and Diagnostic Research 11 (2017): 01-05.
- Gandhi G, Singh P, Kaur G. Perspectives Revisited - The Buccal Cytome Assay in Mobile Phone Users. International Journal of Human Genetics 15 (2017): 173-182.
- Banerjee S, Singh NN, Sreedhar G, et al. Analysis of the Genotoxic Effects of Mobile Phone Radiation Using Buccal Micronucleus Assay: A Comparative Evaluation. Journal of Clinical and Diagnostic Research 10 (2016): 82-85.
- McNamee JP, Bellier PV, Gajda GB, et al. DNA Damage and Micronucleus Induction in Human Leukocytes After Acute In Vitro Exposure to a 1.9 GHz Continuous-wave Radiofrequency Field. Radiation Research 158 (2002): 523-533.
- Hintzsche H, Stopper H. Micronucleus Frequency in Buccal Mucosa Cells of Mobile Phone Users. Toxicology Letters 193 (2010): 124-130.
- SAR Values & Radiation Levels Of Smartphones In 2020. EMF Advice (2020).
- Sut N. Study Designs in Medicine. Balkan Medical Journal 31 (2014): 273-277.
- Gurbuz N, Sirav B, Seyhan N. Genotoxic Studies Performed After Radiofrequency Radiation Exposure. Gazi Medical Journal 29 (2018): 87-93.
- Lixia S, Kaujin W, Deqiang L, et al. Effects of 1.8 GHz Radiofrequency Field on DNA Damage and Expression of Heat Shock Protein 70 in Human Lens Epithelial Cells. Mutation Research 602 (2006): 135-142.
- Yao K, Wu W, Wang K, et al. Electromagnetic Noise Inhibits Radiofrequency Radiation-induced DNA Damage and Reactive Oxygen Species Increase in Human Lens Epithelial Cells. Molecular Vision 14 (2008): 964-969.
- El-Abd SF, Eltoweissy MY. Cytogenetic Alterations in Human Lymphocyte Culture Following Exposure to Radiofrequency Field of Mobile Phone. Journal of Applied Pharmaceutical Science 2 (2012): 16-20.
- Çam ST, Seyhan N. Single-strand DNA Breaks in Human Hair Root Cells Exposed to Mobile Phone Radiation. International Journal of Radiation Biology 88 (2012): 420-424.
- Akdag M, Dasdag S, Canturk F, et al. Exposure to Non-ionizing Electromagnetic Fields Emitted from Mobile Phones Induced DNA in Human Ear Canal Hair Cells. Electromagnetic Biology and Medicine 37 (2018): 66-75.
- Brunborg G, Rolstadaas L, Gutzkow KB. Electrophoresis in the Comet Assay, Electrophoresis - Life Sciences Practical Applications, Oana-Maria Boldura and Cornel Balta, IntechOpen (2018).
- Zothansiama, Zosangzuali M, Lalramdinpuii M, et al. Impact of Radiofrequency Radiation on DNA Damage and Antioxidants in Peripheral Blood Lymphocytes of Humans Residing in the Vicinity of Mobile Phone Base Stations. Electromagnetic Biology and Medicine 4 (2017): 1-11.
- Desai NR, Kesari KK, Agarwal A. Pathophysiology of Cell Phone Radiation: Oxidative Stress and Carcinogenesis with Focus on Male Reproductive System. Reprod Biol Endocrinol 7 (2009): 114.
- Vijayalaxmi, Prihoda TJ. Genetic Damage in Human Cells Exposed to Non-ionizing Radiofrequency Fields: A Meta-analysis of the Data from 88 Publications (1990–2011). Mutation Research 749 (2012): 1-16.
- Hamzany Y, Feinmesser R, Shpitzer T, et al. Is Human Saliva An Indicator of the Adverse Health Effects of Using Mobile Phones? Antioxidants and Redox Signaling 18 (2013): 622-627.
- Carlberg M, Hardell L. Evaluation of Mobile Phone and Cordless Phone Use and Glioma Risk Using the Bradford Hill Viewpoints from 1965 on Association or Causation. BioMed Research International 2017 (2017): 9218486.
- Khan A, Naheed S, Alam M, et al. Saliva- A New Horizon for Estimating Antioxidant Profile of Mobile Phone User. Journal of Biological Research and Applied Sciences 10 (2019): 1-7.
- Khalil AM, Gagaa MH, Alshamali AM. 8-Oxo-7, 8- dihydro-2'-deoxyguanosine as A Biomarker of DNA Damage by Mobile Phone Radiation. Human and Experimental Toxicology 31 (2012): 734-740.
- Gürler G, Ozgur E, Keles H, et al. The Neurodegenerative Changes and Apoptosis Induced by Intrauterine and Extrauterine Exposure of Radiofrequency Radiation. Journal of Chemical Neuroanatomy 75 (2016): 128-133.
- Ragy MM. Effect of Exposure and Withdrawal of 900-MHz Electromagnetic Waves on Brain, Kidney and Liver Oxidative Stress and Some Biochemical Parameters in Male Rats. Electromagnetic Biology and Medicine 34 (2015): 279-284.
- Ros-Llor I, Sanchez-Siles M, Camacho-Alonso F, et al. Effect of mobile phones on micronucleus frequency in human exfoliated oral mucosal cells. Oral Dis 18 (2012): 786-792.
- Oliveira FM, Carmona AM, Ladeira C. Is Mobile Phone Radiation Genotoxic? An Analysis of Micronucleus Frequency in Exfoliated Buccal Cells. Mutation Research 822 (2017): 41-46.
- Gläser K, Rohland M, Kleine-Ostmann T, et al. Effect of Radiofrequency Radiation on Human Hematopoietic Stem Cells. Radiation Research 186 (2016): 455-465.
- Finlay CA, Hinds PW, Tan TH, et al. Activating Mutations for Transformation by p53 Produce A Gene Product that Forms an hsc70-p53 Complex with An Altered Half-life. Molecular and Cellular Biology 8 (1988): 531-539.
- Wyllie AH, Bellamy CO, Bubb VJ, et al. Apoptosis and Carcinogenesis. British Journal of Cancer 80 (1999): 34-37.
- Ansari B, Coates PJ, Greenstein BD, et al. In Situ End-labelling Detects DNA Strand Breaks in Apoptosis and Other Physiological and Pathological States. Journal of Pathology 170 (1993): 1-8.
- Stähelin BJ, Marti U, Solioz M, et al. False Positive Staining in the TUNEL Assay to Detect Apoptosis in Liver and Intestine is Caused by Endogenous Nucleases and Inhibited by Diethyl Pyrocarbonate. Journal of Clinical and Molecular Pathology 51 (1998): 204-208.
- Grast-Kraupp B, Ruttkay-Nedecky B, Koudelka H, et al. In Situ Detection of Fragmented DNA (TUNEL Assay) Fails to Discriminate among Apoptosis, Necrosis, and Autolytic Cell Death: A Cautionary Note. Hepatology 21 (1995): 1465-1468.
- Farhadi F, Jahanpour S, Hazem K, et al. Garlic (Allium sativum) Fresh Juice Induces Apoptosis in Human Oral Squamous Cell Carcinoma: The Involvement of Caspase-3, Bax and Bcl-2. Journal of Dental Research, Dental Clinics, Dental Prospects 9 (2015): 267-273.
- Skamrova GB, Lantushenko AO, Shckorbatov YG, et al. Influence of Mobile Phone Radiation on Membrane Permeability and Chromatin State of Human Buccal Epithelium Cells. Biochemistry and Biophysics 1 (2013): 22-28.
- Souza Lda C, Cerqueira Ede M, Meireles JR. Assessment of Nuclear Abnormalities in Exfoliated Cells from the Oral Epithelium of Mobile Phone Users. Electromagnetic Biology and Medicine 33 (2014): 98-102.
- Daroit NB, Visioli F, Magnusson AS, et al. Cell Phone Radiation Effects on Cytogenetic Abnormalities of Oral Mucosal Cells. Brazilian Oral Research 29 (2015): 1-8.
- Ayrapetyan S. The Intracellular Signaling System Controlling Cell Hydration as A Biomarker for EMF Dosimetry. In: Markov M, ed. Dosimetry in Bioelectromagnetics. Boca Raton, Florida, USA: CRC Press (2017): 339-366.


 Impact Factor: * 5.3
Impact Factor: * 5.3 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 