Differentiation of Human Hematopoietic Progenitor Cells Into Dendritic Cells In Vitro Under Stimulation of PPAR-Gamma
Article Information
Angela Silvano1*, Sara Paccosi2, Maria Ida Bonetti1,3, Astrid Parenti2, Alberto Bosi1, Paolo Romagnoli1
1Department of Experimental and Clinical Medicine, University of Florence, Italy
2Department of Health Sciences, Clinical Pharmacology & Oncology Section, University of Florence, Italy
*Corresponding Author: Angela Silvano, Department of Experimental and Clinical Medicine, Section of Histology and Embryology, University of Florence, Viale Pieraccini 6, 50139 Florence, Italy
Received: 09 May 2019; Accepted: 01 June 2019; Published: 03 June 2019
Citation: Angela Silvano, Sara Paccosi, Maria Ida Bonetti, Astrid Parenti, Alberto Bosi, Paolo Romagnoli. Differentiation of Human Hematopoietic Progenitor Cells Into Dendritic Cells In Vitro Under Stimulation of PPAR-Gamma. Archives of Clinical and Biomedical Research 3 (2019): 222-240.
View / Download Pdf Share at FacebookAbstract
The differentiation of dendritic cells (DCs) in vitro is not yet under full control and although PPAR-γ stimulation may interfere with DC differentiation from cord blood progenitors, the expression of PPAR-γ by different precursors and the effect of PPAR-γ stimulation on DC differentiation are poorly known. To address these issues, CD14+ monocytes, CD34+ progenitors and CD133+ progenitors were isolated from adult healthy donors and cultured with cytokines; rosiglitazone (1 μmol/l) was used to stimulate PPAR-γ. All precursors generated large, HLA-DR+ DCs, a proportion of which, highest when starting from CD133+ precursors expressed the Langerhans cell marker CD207/langerin; many cells expressed the connective tissue DC marker CD209/DC-SIGN, even together with CD207, and some cells contained Birbeck granules. Only CD133+ precursors expressed PPAR-γ mRNA appreciably; the DCs from these precursors contained a higher proportion of Langerhans like cells and caused 25% less stimulation of lymphocyte proliferation when generated in the presence of rosiglitazone. In conclusion, the phenotype of DCs differentiated in vitro does not match exactly that of DCs in vivo; PPAR-γ is expressed by freshly isolated CD133+ precursors; PPAR-γ agonists can direct DC differentiation from CD133+ precursors towards Langerhans type cells and inhibit the lymphocyte stimulating activity of the generated DCs.
Keywords
Langerhans Cells; Rosiglitazone; CFSE; Immunophenotype; Electron Microscopy
classification text asfasdf
Article Details
Abbreviations
CD = cluster of differentiation
DC = dendritic cell
DC-SIGN = dendritic cell-specific intercellular adhesion molecule-3-grabbing non integrin
Flt3-L = fms-like tyrosine kinase-3 ligand
GM-CSF = granulocyte-macrophage colony-stimulating factor
HLA = human leukocyte antigen
ICAM = intercellular adhesion molecule
IFN = interferon
IL = interleukin
LC = Langerhans cell
M-CSF = macrophage colony-stimulating factor
MHC = major histocompatibility complex
PPAR = peroxisome proliferator-activated receptor
SCF = stem cell factor
TGF = transforming growth factor
TLR = Toll-like receptor
TNF = tumor necrosis factor
TPO = thrombopoietin
Introduction
Dendritic cells, the professional antigen presenting cells of the immune system, deserve attention because they can direct immune response to pathogenic organisms [1] and tumors [2] towards reaction anergy or active tolerance [3, 4] and play crucial roles in allergy, autoimmunity and transplant rejection [5]; they are also involved in HIV infection by hosting the virus and transferring it to lymphocytes [6]. To play their roles since primary response, dendritic cells express the major histocompatibility complex class I (MHC-I) and class II antigens (MHC-II) and a combination of costimulatory (CD80, CD86) and adhesion molecules (CD54) [7].
Dendritic cells originate from bone marrow hematopoietic stem cells and two main subsets have been identified: myeloid and plasmacytoid dendritric cells [7, 3, 8]. Only the former (abbreviated as DCs) will be dealt with here. Among them one recognizes interstitial DCs of connective tissue proper - which express CD1c, DC-SIGN/CD209, DEC205 and, sometimes, also CD1a - and Langerhans cells (LCs) in stratified squamous epithelia of the skin and mucosae. The latter express E-cadherin, CD1a and CD207 and contain a specific inclusion, Birbeck granules, visible by electron microscopy [9]. Upon antigen capture mDCs mature and come to express CD83 and the co-stimulatory molecules CD54, CD80 and CD86 together with increased amounts of MHC-II.
In vitro, DCs can differentiate from circulating CD14+ and CD14- monocytes [10-12], from CD34+ progenitors and from CD133+ precursors; the last cells have been used only in a few studies [13-15]. Attempts to drive the differentiation towards specific subtypes of DCs, in particular towards LCs, have given inconsistent results, the best ones have been obtained starting from CD14- cells of adult peripheral blood [11] and from CD34+ [16] or CD133+ precursors isolated from cord blood [15].
The lipid signal molecules receptor PPAR-γ has been included among the pathways possibly involved in immune system regulation, since it is expressed in humans by at least some hematopoietic cells [17] and some cells of the immune system [18-20], either spontaneously or upon culture with rosiglitazone, an agonist of this receptor [21, 22]. Differentiation of DCs in the presence of PPAR-γ ligands has an inhibiting effect on immunogenicity, possibly through inhibition of several signal pathways [23, 24] and of co-stimulatory molecules expression [18, 21, 25-27]. During differentiation of DCs from CD133+ haematopoietic precursors of the cord blood, rosiglitazone favoured the full differentiation of lymphocyte-stimulating LCs expressing CD207/langerin and containing Birbeck granules, but at the expense of the number of generated cells [15].
Because of their roles, dendritic cells are emerging as attractive targets and mediators of immune therapies designed to promote or attenuate the immune responses [28], however the possibility of influencing their behaviour is still limited, largely due to incomplete knowledge of their differentiation and its regulation, including the differentiation potential of different circulating precursors of adults into DCs and their subtypes and the influence of PPAR-γ on this process.
To address these issues, experiments were started with the selection of circulating precursors according to the expression of either CD14 or CD34 or CD133. The cells were cultivated with cytokines to generate immature DCs and then further cultivated with a different cytokine mixture to induce maturation, and the effect of rosiglitazone was tested by its addition throughout culture. The generated cells were analyzed for immunophenotype, morphology, and the ability to stimulate mixed lymphocyte reaction (MLR).
Materials and Methods
Isolation and culture of circulating precursor cells
All cells were isolated from buffy coats obtained from healthy donors in the respect of the Italian law and upon approval of the local ethical committee (authorization n° 0011762/2010). Each donor was used for one experiment.
CD14+ precursors
Human buffy coats were stored at +4 °C for 24 h before use and then subjected to density gradient centrifugation on Ficoll (Lymphoprep, Euroclone, Pero, Italy) followed by immunomagnetic separation of CD14+ cells, following a previously published protocol [12, 29]. In detail, the buffy coat was suspended in 2,5% dextran (Amersham Pharmacia Biotech, Sweden) in 0.9% NaCl and stabilized at 37 °C for 30 min. Upon dilution with phosphate buffered saline 0.1 mol/litre, pH 7.4 (PBS), the suspension was stratified over Ficoll and centrifuged for 15 min at 800 x g. Upon washing in PBS with 1%, heat inactivated, foetal bovine serum (FBS; Sigma-Aldrich, St Louis, Mo, USA), colloidal superparamagnetic microbeads conjugated with mouse anti- human CD14 monoclonal antibody (Microbeads UltraPure, human, Miltenyi Biotec, Bergisch Gladbach, Germany) were added to the cell suspension (20 µl beads per 107 total cells) and let incubate at 4 °C for 15 min. After washing with PBS the cell suspension was loaded onto a MACS column (Miltenyi Biotec) which was placed in a magnetic field MACS Separator (Miltenyi Biotec). After elution of unlabelled cells, the column was extracted from the magnet and CD14+ cells were recovered with PBS.
The cells were seeded at mean concentration of 1x106 cells/ml in RPMI 1640 with 10% FBS, 100 U/ml penicillin and 0.1 ng/ml streptomycin (all from Sigma-Aldrich), GM-CSF (10 ng/ml), IL-4 (10 ng/ml), TNF-α (10 ng/ml) and TGF-β (10 ng/ml) for 7 days; final maturation was induced with the same cytokines plus IL-1β (10 ng/ml), IL-6 (1000 U/ml) and TGF-β (20 ng/ml) for additional 24 h. All cytokines were purchased from PeproTech. For TGF-β the isoform 3 was used: the three isoforms of TGF-β signal through the same receptor and elicit similar biological responses [30].
CD34+ precursors
To obtain human CD34+ cells, freshly recovered buffy coats were used. The mononuclear cells were isolated by Ficoll density gradient centrifugation as indicated above and up to 108 cells were resuspended in a final volume of 300 µl PBS and labelled with superparamagnetic microbeads conjugated with mouse anti-human CD34 monoclonal antibodies for 30 min at 4° C, according to the producer instructions (Microbeads UltraPure, human, Miltenyi Biotec). The cell suspension was loaded onto a column and subjected to magnetic field separation as indicated above. After removing the column from the magnetic field, the retained CD34+ cells were eluted and counted.
Purified CD34+ cells (500,000 cells/ml) were seeded in RPMI 1640 with FBS, penicillin and streptomycin as above indicated and cultivated for 7 days with SCF (20 ng/ml), TPO (10 ng/ml), Flt3-L (25 ng/ml), GM-CSF (10 ng/ml), IL-4 (10 ng/ml), TGF-β (10 ng/ml; all cytokines from Peprotech). The culture was further continued for 7 more days with GM-CSF, IL-4, TNF-α and TGF-β, at the same concentrations indicated above for CD14+ cells. For last additional four days, i.e. from day 14 to day 18 of culture, IL-1β (10 ng/ml) and IL-6 (1000 U/ml; both from Peprotech) were also added and TGF-β concentration was raised to 20 ng/ml, while GM-CSF, IL-4 and TNF-α were maintained at the same concentration indicated above.
CD133+ precursors
To obtain human CD133+ cells, freshly recovered buffy coats were used. The mononuclear cells were isolated by Ficoll density gradient centrifugation as described above, then resuspended in a final volume of 200 µl PBS for up to 5x107 cells and subjected to two-step immuno-magnetic purification with Diamond human CD133 isolation kit (Miltenyi Biotec; the kit includes all primary antibodies and two types of microbead-linked secondary antibodies) following the indications of the producer. In a first step the cells were incubated with a cocktail of biotin-conjugated mouse monoclonal antibodies against human CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD61 and CD253a, for 10 min at 4 °C, washed in PBS and resuspended in 400 µl PBS. Anti-biotin microbeads were added for 15 min at 4 °C, then the cell suspension was loaded onto a column and subjected to magnetic field separation. In a second step the unlabelled, effluent cells were washed and resuspended in 200 µl PBS (up to 5x107 per sample), adding 50 µl of micro beads conjugated with monoclonal CD133 antibodies for 30 min at 4 °C. Upon subsequent magnetic separation, the CD133+ cells were eluted and placed in cult0ure with the same cytokines and for the same times indicated for CD34+ cell culture.
Rosiglitazone challenge
The cells isolated from each donor - whichever precursor had been isolated - were split in two and rosiglitazone (Selleckchem, Munich, Germany; 1 µmol/l, i.e. 0.36 µg/ml) was added to half cells since the start of culture, while the other half cells were cultured without rosiglitazone as control. The drug was obtained from the producer as a powder and was dissolved in dimethyl sulfoxide at a concentration of 100 mmol/l for storage. It was then diluted in 0.1% bovine serum albumin (BSA) in PBS down to 100 µmol/l before adding to the culture medium at the indicated final concentration. Generated cells were analysed at the end of culture.
Immunofluorescence
Double immunofluorescence analyses were performed on cytospins of mature DCs, that were fixed with cold acetone for 3-5 min at room temperature. After blocking non-specific binding sites with 10 mg/mL BSA (Sigma-Aldrich) in PBS with the addition of 0.5% triton X-100 (Sigma-Aldrich) for 30 min at room temperature, primary antibodies (anti-human) were applied over night at 4 °C at the indicated dilutions. The following antigens were tagged first: ICAM-1 (CD54; Merck Millipore, Darmstadt, Germany; monoclonal mouse IgG1, 1:50), langerin (CD207; Dendritics, Lyon, France; mouse monoclonal IgG1, 1:50), DC-SIGN (CD209; Sigma-Aldrich; rabbit polyclonal, 1:50). In each case, secondary goat anti-mouse or anti-rabbit polyclonal antibodies - as appropriate - conjugated with Alexa Fluor AF594 (red fluorescence), all from Life Technology (Thermo Fisher Scientific, Waltham, MA), were applied for 2 hours at room temperature. Afterwards, fluorescein isothiocyanate (FITC) conjugated anti-human HLA-DR antibody (Miltenyi Biotec, mouse monoclonal IgG2aκ, 1:20) was added overnight at 4°C. The signal was amplified with anti-FITC goat polyclonal antibody conjugated with AF488 (green fluorescence; Thermo Fisher Scientific; 1:100). Nuclei were labelled with Hoechst 33342 (20 µg/mL; Sigma-Aldrich). Omission of primary antibodies or substitution with irrelevant ones were used as negative controls.
The slides were mounted with Gel/Mount (Fluoromount, Diagnostic BioSystems, Pleasanton, CA), observed in an Axioskop microscope equipped for epifluorescence (Zeiss, Oberkochen, Germany) and captured with an Axio Vision 4 system, consisting of a digital multichannel fluorescence module and dedicated software (Zeiss), or observed in a DMR HC microscope equipped for epifluorescence (Leica Microsystems GmbH, Wetzlar, Germany).
Flow cytometry
In all analyses, the indicated amounts of the following monoclonal antibody solutions were added to 100 µl of cell suspension containing 10,000-60,000 cells, as suggested by the producer: 5 µl HLA-DR-Horizon violet (HV450) or 7.5 µl HLA-DR-FITC (BD Biosciences, Franklin Lakes, NJ), 6.5 µl CD1a-phycoerythrin (PE) (BD Biosciences), 6.5 µl CD11c-allophycocyanin (APC)-H7 or CD11c-PE (BD Bioscience), 5 µl CD14-APC (BD Biosciences), 5 µl CD33-phycoerythrin-cyanine dye Cy7 (PE-Cy7), 5 µl CD34-peridinin chlorophyll protein with the cyanine dye Cy5.5 (PerCP-Cy 5.5) (BD Biosciences), 7.5 µl CD45-FITC or 5 µl CD45-HV450 (BD Bioscience), 7.5 µl CD80-FITC (Miltenyi Biotec), 6.5 µl CD83-PE, 6.5 µl CD86-PE or 5 µl CD86-APC (BD Bioscience), 10 µl CD207-APC (Miltenyi Biotec). The cells were immuno-labelled for 15 min at room temperature, protected from light. 7-amino-actinomicin D (AAD; BD Biosciences) was used to recognize dead cells and exclude them from analysis. Isotype-matched antibodies were used as negative controls.
For some samples fixation and permeabilization were performed to evaluate intracytoplasmic langerin/CD207 expression, using the cell permeabilization kit of Nordic-Mubio (Susteren, Netherlands) according to the directions of the manufacturer.
Flow cytometry was performed by collecting more than 10,000 events on a FACSCanto II (BD Biosciences ) and data were analysed with Infinicyt 1.7 (Citognos, Salamanca, Spain).
Electron microscopy
Cell pellets were fixed in 2% formaldehyde and 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.4, osmicated and embedded in epoxy resin. Sections were stained with gadolinium acetate (Electron Microscopy Sciences, Hatfield, PA) [31] and either lead citrate or bismuth subnitrate, and observed in a Jeol JEM 1010 electron microscope (Tokyo, Japan) at 80 kV. Photomicrographs were taken with a digital camera MegaView III (Soft Imaging System, Muenster, Germany) connected with a personal computer (Dell, Round Rock, TX) with dedicated software (AnalySIS, Soft Imaging Software, Muenster, Germany).
Mixed lymphocyte reaction
Allogeneic lymphocytes were recovered from buffy coats - obtained from healthy donors and stored at +4 °C for 24 h before use - through density gradient centrifugation on Ficoll, as above indicated. The cells were washed in PBS and seeded in RPMI with 10% FBS (both from Sigma-Aldrich) for 45 min. Non-adherent cells were collected and centrifuged at 160 x g, for 10 min at 20 °C, counted and used for MLR.
Lymphocytes were loaded with the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE) following manufacturer instructions. 2x105 lymphocytes were cultured 5 days in RPMI with 10% FCS (Sigma-Aldrich) with 4x104 DCs generated from CD133+ precursors. Lymphocytes stimulated with 5 µg/ml phytohaemoagglutinin (PHA, Biochrom, MA) were used as positive controls. At the end of co-culture lymphocytes were recovered and stained with fluorescent mouse monoclonal antibodies following manufacturer’s instructions: anti-CD3-PerCP-Cy5.5, anti-CD4-PE and anti-CD8-APC (BD Bioscience). Freshly isolated lymphocytes were also loaded with CFSE, cultured 24 h without DCs and immunolabelled as above described, as a baseline. The lymphocytes were subjected to flow cytometry on a FACSCanto II cell analyzer (BD Biosciences) and data were analysed with FACs Diva software (BD Biosciences).
Gene expression
The expression of PPAR-γ was evaluated by quantitative qRT-PCR using Cells-to-CT 1-Step TaqMan Kit (Thermo Fisher Scientific), that allows to measure relative gene expression by qRT-PCR analysis directly from cultured cells, without preliminary RNA purification and amplification. The technique is designed for 10,000-100,000 cultured cells per sample, thus for each assay ~80,000 cells were washed in PBS, counted and lysed for 5 min at room temperature; genomic DNA was simultaneously removed with DNase. Lysis was terminated at room temperature by 2 min incubation with Stop Solution. The lysate was mixed with TaqMan® 1-Step qRT-PCR Mix (Thermo Fisher Scientific) and with TaqMan® Gene Expression Assays for PPAR-γ and GAPDH housekeeping gene (Applied Biosystems for Thermo Fisher Scientific), at the volumes indicated by the producer. The results were read in a Rotor-Gene Q (Qiagen, Germany) with the following settings: 1 cycle of reverse trascription at 50 °C for 5 min, 1 cycle of reverse trascription inactivation/initial denaturation at 95 °C for 20 seconds, 40 cycles of amplification at 95 °C for 15 seconds and at 60 °C for 1 minute. Rotor-Gene Q series software was used for acquisition. A lysate of human pre-adipocytes was used as positive control for PPAR-γ
Statistics
Quantitative data were expressed as mean ± standard error (SE) and analyzed as appropriate by ANOVA or Student's t-test for paired data. Values of p<0.05, p<0.01 and p<0.001 were recorded separately and assumed as significant.
Results
Cell culture
From buffy coats, on the average 2.3x107 CD14+ cells (range 1x107-3.9x107), 1.26x106 CD34+ cells (range 0.60x106-2.0x106) and 1.13x106 CD133+ cells (range 0.60x106-1.6x106) were isolated. The cells harvested at the end of culture were less than those seeded: ~75% of CD14+ precursors, ~60% of CD34+ and CD133+ precursors. Cell morphology and vitality were not affected by the addition of rosiglitazone to the culture medium.
Cells differentiated from CD14+ monocytes
Most cells generated from CD14+ precursors showed a dendritic morphology after 7 days. The latter cells were homogeneous for both forward and side scatter and virtually all (98%) were HLA-DR+. Fifty-eight per cent cells expressed CD1a.
The percentage of HLA-DR+ DCs generated from CD14+ monocytes which expressed CD207 ranged between 4 and 6% and did not vary appreciably when cells were labelled after permeabilization. The results were not influenced by the addition of rosiglitazone. Immunohistochemistry confirmed the very low expression of CD207 by differentiated cells while ~95% expressed CD209 (not shown).
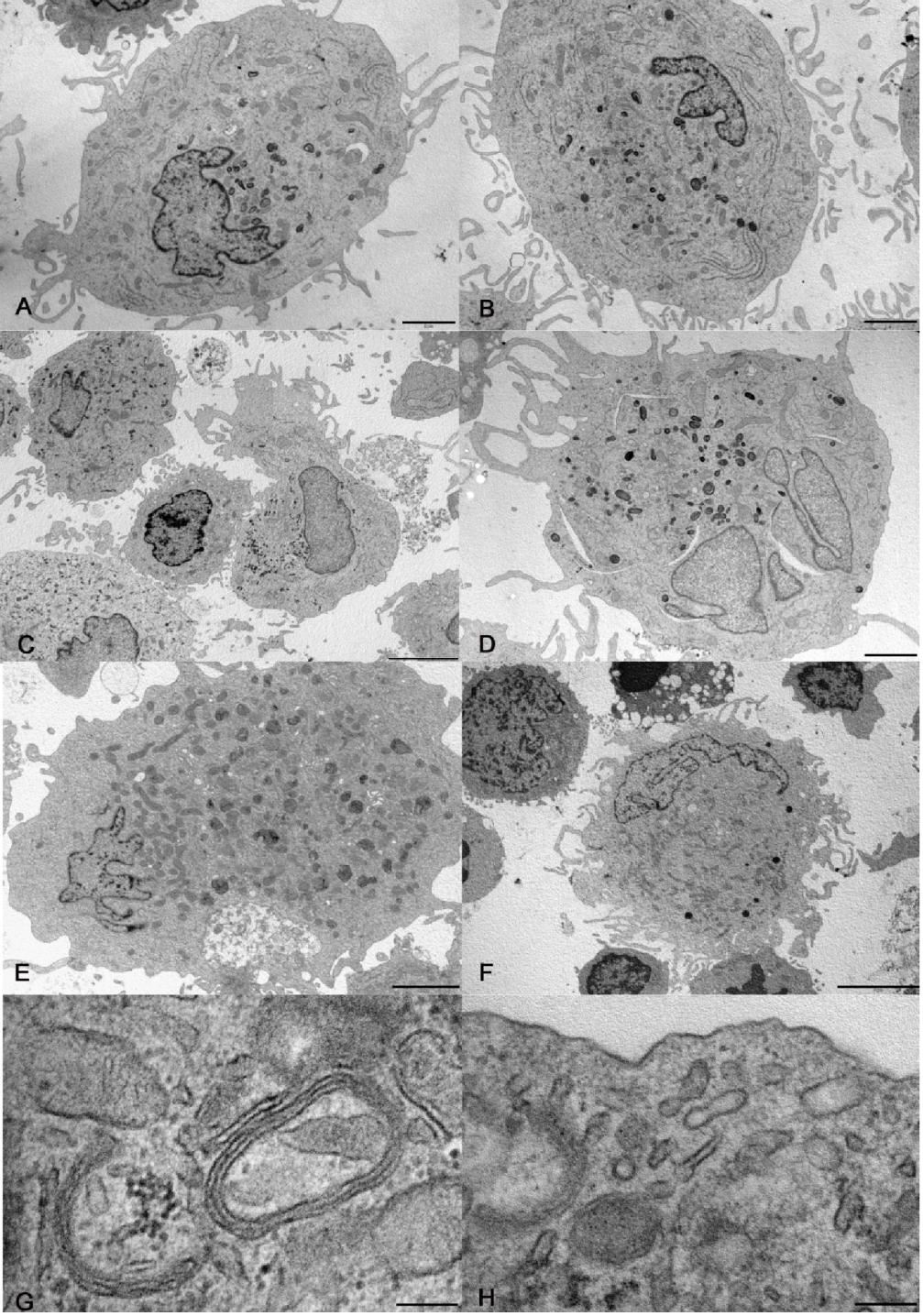
At electron microscopy, some DCs differentiated from CD14+ monocytes contained electron dense lysosomes and thin curved cisternae, presumably cup-shaped, with electron dense content (Figure 1A, B, G).
The expression of PPAR-by CD14+ monocytes was negligible (0.57±0.360 copies/cell, N=3).
Cells differentiated from CD34+ precursors
The cells obtained from CD34+ precursors could be divided into two populations, one CD33+ CD45+ and the other CD33-CD45+. This latter population was excluded from analysis, because the lack of CD33 prevented to identify those cells as DCs; it represented up to 35% cells at the end of culture, as estimated by flow cytometry. Two distinct populations were observed among CD33+ CD45+ cells, which differed in size (as estimated by forward scatter) and "granularity" (as estimated by side scatter).
Figure 1: Electron microscopy of cells differentiated in vitro. A, B) Large dendritic cells generated from CD14+ monocytes without (A) and with rosiglitazone (B). C, D) Dendritic cells generated from CD34+ precursors without (C) and with rosiglitazone (D). Cholesterol clefts, as in panel D, were occasional finding in culture. E, F) Dendritic cells generated from CD133+ precursors without (E) and with rosiglitazone (F). G, H) details of dendritic cells generated from CD14+ monocytes (G) and from CD133+ precursors (H), showing respectively curved cisternae with parallel membranes and electron dense content, and a rudimentary Birbeck granule. Bar= 2 µm for panels A, B, D, E; 5 µm for panels C, F; 200 nm for panels G, H.
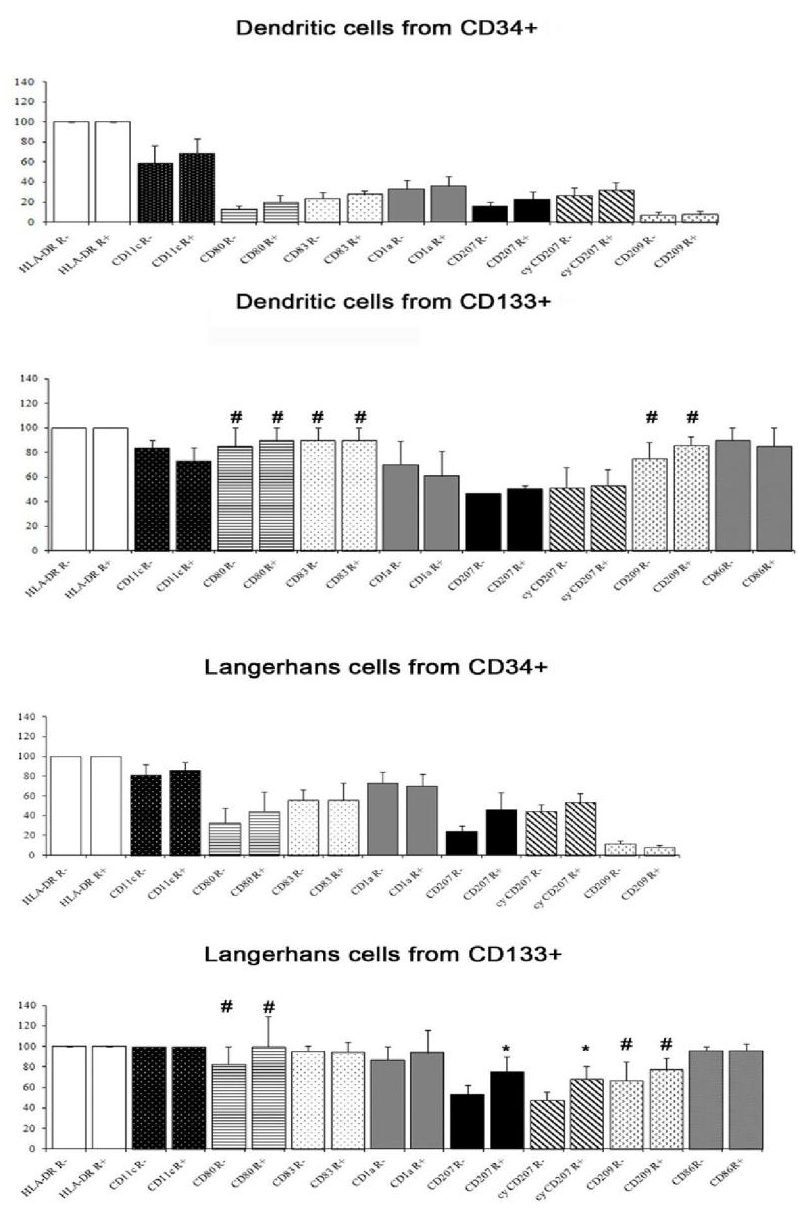
The antigen expression of DCs differentiated from CD34+ precursors is reported in Figure 2; it varied among donors for several antigens. From any donor, all DCs obtained from CD34+ cells were HLA-DR+. Between 50% and 70% of these cells were classified as LC-like on the basis of scatter parameters (high forward scatter and high side scatter). The majority of the latter cells were CD1a+ and almost a half were also CD207 positive. A high percentage of LC-like cells expressed CD11c, lower percentages were found for the maturation marker CD83 and the co-stimulatory molecule CD80. A part of non-LC-like DCs and of LC-like cells expressed CD209. The results were not influenced significantly by the addition of rosiglitazone.
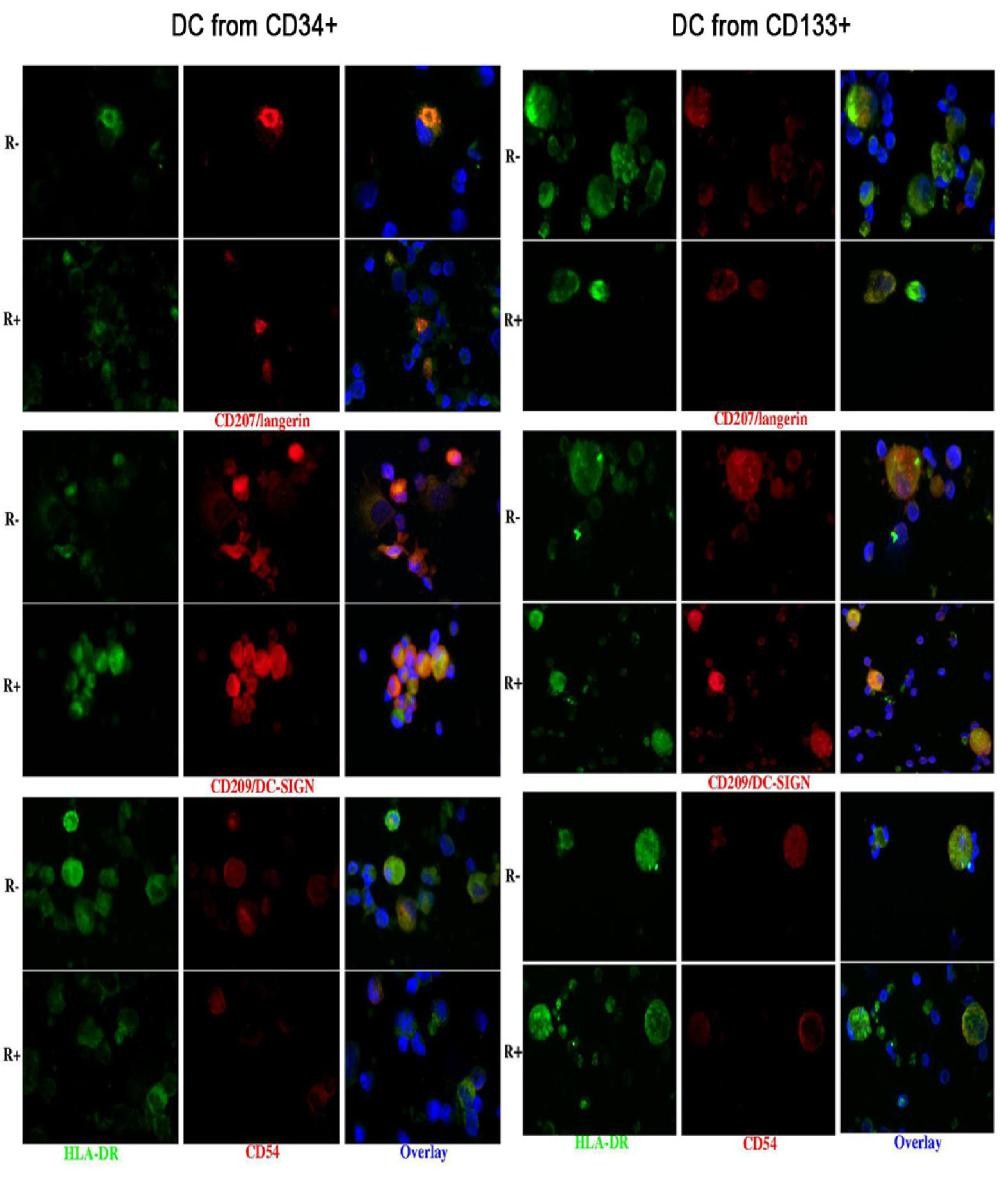
Immunohistochemistry confirmed the co-expression of CD207 and CD209, and showed that of CD54 (Figure 3).
At electron microscopy, the harvested cells included large and medium size DCs and small, roundish cells. The largest cells were highly dendritic and rich in mitochondria, rough and smooth endoplasmic reticulum, Golgi apparatus and lysosomes. The medium size cells had shorter and poorly branched dendrites and less abundant organelles than large cells. The smallest cells had a smooth surface and were poor in organelles, except for many free ribosomes (Figure 1C, D). Curved cisternae were present in DCs and occasional straight, rudimentary Birbeck granules (Figure 1H) were recognized in large DCs. The expression of PPAR-by CD34+ precursors was negligible (0.01±0.004 copies/cell, N=3).
Cells differentiated from CD133+ precursors
Also the DCs obtained from CD133+ precursors could be divided into a CD33+ CD45+ and a CD33- CD45+ population and only the first one (about 60-70% of harvested cells) was subjected to further analysis. They were all HLA-DR+; 53-94% cells were LC-like, both with and without rosiglitazone, as appreciated by scatter parameters and the expression of CD1a and CD207. The percentage of cells expressing CD207 was significantly higher among cells grown with than without rosiglitazone (Figure 2); this was true for both membrane and total (i.e. including intracytoplasmic) labelling. All LC-like cells expressed CD11c and the vast majority expressed the maturation marker CD83 and the co-stimulatory molecule CD80; a considerable percentage of non-LC-like DCs and LCs expresses CD209 (Figure 2). As for cells differentiated from CD34+ precursors, immunohistochemistry confirmed the co-expression of CD207 and CD209 and showed that of CD54 (Figure 3).
The difference in the expression of CD80, CD83 and CD209 between cells obtained from CD34+ and from CD133+ precursors was significant, the higher expression being by cells obtained from CD133+ cells (Figure 2). At electron microscopy, the cells generated from CD133+ precursors (Figure 1E, F, H) resembled those harvested upon culture of CD34+ precursors, including the finding of rudimentary Birbeck granules in large DCs. An appreciable number of copies of PPAR-γ mRNA was found in CD133+ precursors: 12.21±7.635 (N=5).
Figure 2: Antigen expression, evaluated by flow cytometry, of non-Langerhans-like dendritic cells and of Langerhans-like dendritic cells (as identified from scatter parameters) differentiated from CD34+ precursors and from CD133+ precursors. The percentage of cells CD80, CD83 and CD209 positive was significantly higher in cells obtained from CD133+ precursors than in those obtained from CD34+ precursors, both with and without rosiglitazone (p<0.01; hashtag). The percentage of cells CD207 and cyCD207 (respectively, on membrane and on membrane plus cytoplasm) positive among cells obtained from CD133+ precursors was significantly higher with than without rosiglitazone (p<0.05; asterisk). A significant difference was found in the expression of CD80 and of CD209 between Langerhans-like cells obtained from CD34+ and those obtained from CD133+ precursors, with or without rosiglitazone (p<0.01; hashtag). Mean and standard error; N = 4 except for CD209 in cells differentiated from CD133+ precursors, where N =3.
Mixed lymphocyte reaction
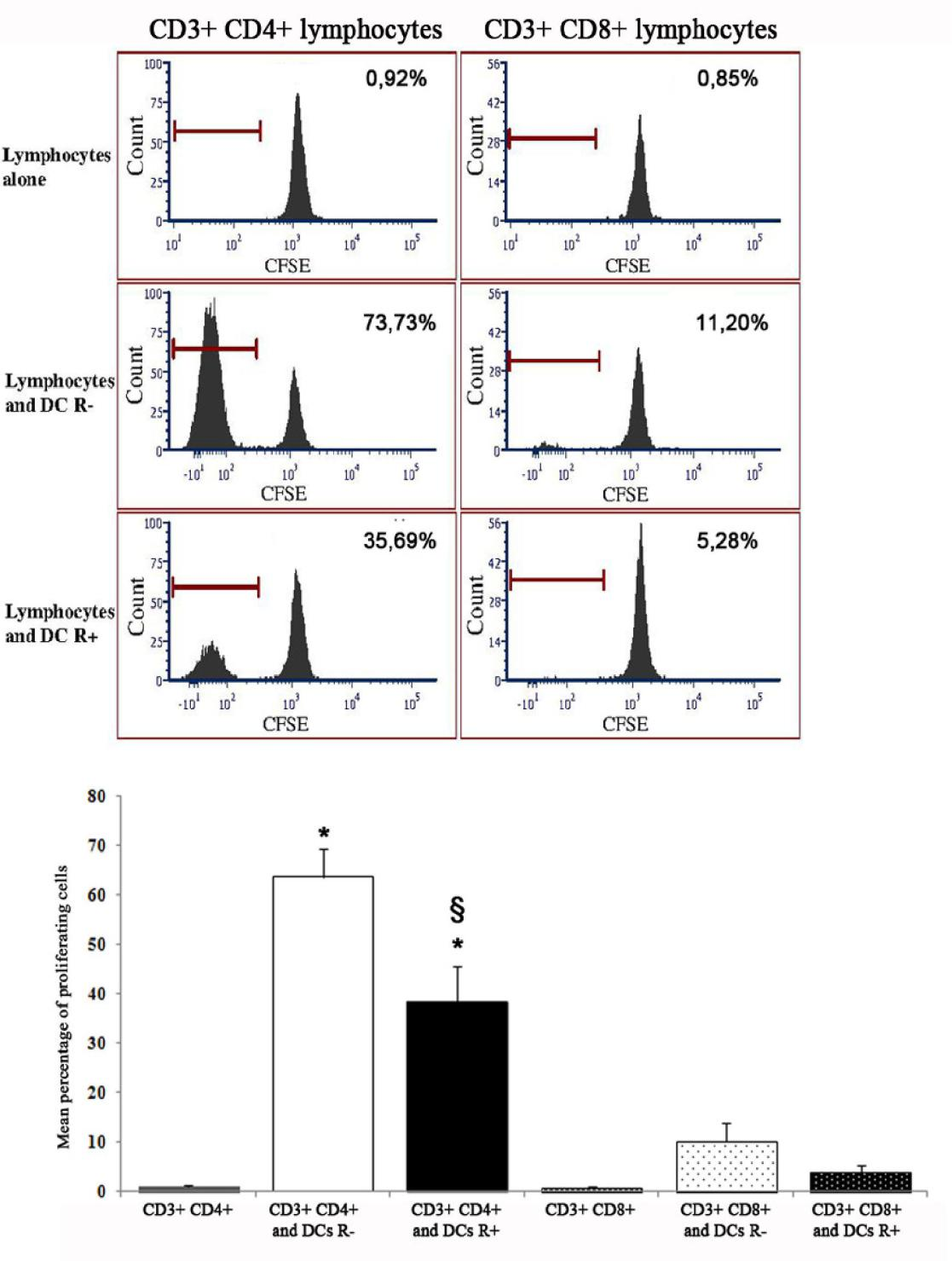
The proliferation of CD4+ lymphocytes after 5 d co-culture with DCs derived from CD133+ precursors was significantly higher than that of lymphocytes alone. The use of CFSE dilution as a measure of proliferation allowed to recognize that the proliferative response of CD8+ lymphocytes to DCs was much lower than that of CD4+ lymphocytes; both appeared decreased when DCs had been differentiated in the presence of rosiglitazone, the difference being significant for CD4+ lymphocytes (Figure 4).
Figure 4: Stimulating activity, in mixed lymphocyte reaction, of dendritic cells (unfractionated) derived from CD133+ precursors generated without (R-) or with rosiglitazone (R+). Upper panels: results of a representative experiment. The peak on the right in all graphs indicates the population of non-proliferating lymphocytes after 5 days of culture. The peak on the left (absent if dendritic cells had not been added to culture) indicates the lymphocytes that have proliferated after 5 days of culture and therefore have undergone dilution of the label. The percentages indicate the fraction of proliferating lymphocytes for each experimental condition. Lower panel: percentage of proliferating cells in mixed lymphocyte reaction (mean and standard error of 3 experiments). The percentage of CD4+ lymphocytes and CD8+ lymphocytes proliferating was higher if they were in co-culture with dendritic cells than alone, the difference was significant for CD4+ lymphocytes (p<0,001; asterisk) and was reduced if dendritic cells had been cultured with rosiglitazone (p<0.01; double S). The proliferative response of CD8+ lymphocytes was much lower than that of CD4+ lymphocytes (p not significant versus lymphocytes alone).
Discussion
The results of this study have shown that DCs can be generated from different precursors obtained from adult human blood and that they include cells with features of a specific DC type, i.e. LCs; that the percentage of this last cell type and more in general the phenotype and functional ability of the generated DCs depend on the precursor; that the phenotype of generated cells does not correspond entirely to that of either LCs nor connective tissue DCs in situ, in particular CD207 expression can coexist with that of CD209; that among freshly isolated cells only highly immature, CD133+ precursors express an appreciable amount of PPAR-γ; and that PPAR-γ stimulation by means of rosiglitazone influences the immunophenotype and lymphocyte stimulating capacity of the cells derived from CD133+ precursors, enhancing the expression of langerin/CD207 and reducing the capacity to stimulate CD4+ lymphocyte proliferation in MLR.
This study moreover confirmed previous results on precursors from cord blood [15], that the cells that can be harvested upon culture in conditions useful to generate DCs are less than the seeded cells, i.e. there is only limited proliferation, except in the first days of culture.
At variance with previous results of this laboratory on CD133+ cord blood cells [15], culture of CD133+ precursors from adult blood with the PPAR-γ agonist rosiglitazone did not restrict the generated cells to a small number of cells with features of LCs, although such cells were indeed increased in those conditions as compared with cultures without the drug. It cannot be excluded that this depends on technical reasons because in the meantime from previous research the cytokines and the isolation kit have been bought new and were from different lots and in some cases different producers than in the study on cord blood cells, and also rosiglitazone had to be obtained new and from a different producer. However, it is reasonable to conceive that the differentiation potential and response to PPAR-γ agonists differ between foetus and adult.
Large DCs with mature features at electron microscopy, i.e. rich in organelles and especially lysosomes, contained inclusions resembling Birbeck granules. Moreover, they contained flat, curved cisternae that were delimited by parallel membranes and had a central density over most of there extension; the images resemble those of the inclusions elicited by anti-CD1a treatment of human epidermal LCs [32].
At variance with what is usually observed in vivo, the cells with LC features, namely a typical scatter pattern at flow cytometry and the expression of CD207 and CD1a at immunophenotypical analysis, also expressed CD209 which instead is typical of connective tissue, non-Langerhans DCs [33]. This was true with either CD133+ or CD34+ cells as starting precursors and suggests that the culture conditions cannot reproduce completely those occurring in vivo. The expression of CD209 had not been addressed in previous studies on cells generated from CD133+ precursors. This issue was not addressed here for the cells generated from CD14+ precursors because of the extremely low number of CD207 cells in those cultures.
Most if not all cells identifiable as LCs, or at least LC-like, expressed CD207 at the cell surface as well as in the cytoplasm; immunocytochemistry confirmed the presence of a labelled granular compartment within cytoplasm of CD207 expressing cells, as expected [34]. The results upon rosiglitazone indicate that this drug has significant effects on the expression of CD207 only in cells generated from CD133+ precursors, where it stimulates the expression of CD207, both in the whole cell and at the cell surface. It is conceivable that this depends on the expression of PPAR-γ being appreciable in CD133+ cells and negligeable in the other cellular precursors considered in this study; the results also suggest that the observed effects of PPAR-γ stimulation on the differentiation of DCs depend on an action on very early stages of the process. The very small number of cells with LC-like features starting from CD14+ precursors is in substantial agreement with literature reports [35, 36]. A previous study by another laboratory had shown that rosiglitazone can induce the expression in culture of PPAR-γ when starting from CD14+ cells [28]; in the present study, however, such a treatment on cultures of CD14+ monocytes did not have an effect on immunophenotype.
The results upon rosiglitazone may have counterparts in vitro, because both connective tissue cells and keratinocytes can secrete molecules acting as PPAR-γ stimulants and so regulate the final differentiation and function of DCs [37-39]. The effect of rosiglitazone on the differentiation of DCs from CD133+ precursors was a stimulation of the expression of LC markers and a partial inhibition of the lymphocyte proliferation stimulating ability of the generated cells. The possibility that this depended on piggyback transfer of rosiglitazone to lymphocytes by DCs is highly improbable because DCs were extensively washed before transferring into co-culture wells for MLR. Also, it had been demonstrated by Nencioni et al. [18] and Appel et al. [23] that DCs generated from CD14+ precursors in the presence of PPAR-γ agonists had impaired T cell stimulating activity. It may be correlated with this effect of PPAR-γ stimulation the fact that in a mouse model of atopic dermatitis PPAR-γ agonists led to decreased severity of the disease and to selective inhibition of the maturation in vitro of DCs derived from untreated animals [40].
The use of CFSE for evaluating the proliferation of the lymphocytes in MLR allowed to recognize a different effect of DCs generated in vitro from CD133+ precursors on CD4+ and CD8+ lymphocytes: these cells stimulated CD4+ lymphocytes, but were very poorly effective on CD8+ lymphocytes. Correspondingly, the negative influence of rosiglitazone on the lymphocyte stimulating capacity of DCs generated from CD133+ precursors was higher towards CD4+ than CD8+ lymphocytes. The varying capacity of DCs to stimulate different lymphocyte subsets and the varying influence of PPAR-γ agonists on those subset should be taken into account both for the interpretation of results on a mixed lymphocyte population (as most often occurs) and for the planning of strategies for the clinical use of in vitro generated and manipulated DCs and of drugs acting on those cells in vivo.
During this study the results varied among experiments. For each experiments a single donor was used and each donor contributed to only one experiment. On account of the Italian law on privacy and personal data protection no information was available on the donors (not even sex or age), the only information being that they were healthy subjects acceptable as blood donors for transfusion. This drawback is intrinsic to studies with human cells and could not be avoided in this research either.
The findings of this research may be relevant to the question whether in order to induce, enhance or alternatively lower an immune response it would be better to differentiate DCs from haematopoietic precursors in vitro and inject them into a patient upon appropriate treatment, or find ways to influence the DCs of a patient in vivo, as also proposed in the clinics
The latter strategy would obviate the imperfect control of the differentiation of DCs in vitro and the risk of raising cells prone to induce responses inappropriate to the single case which they would be supposed to cure.
Acknowledgements
The authors gratefully acknowledge Dr. Grazia Gentilini for help with buffy coat harvesting; Dr Stefano Bacci, Dr. Paola Di Gennaro and Dr. Sergio Fabbri for advice on cell and molecular biology techniques; Mrs. Laura Calosi, Mr. Daniele Guasti and Mr. Stefano Catarinicchia for technical help in microscopic analyses; Associazione Italiana contro le Leucemie-Linfomi e Mieloma, Foemina Foundation, and the University of Florence for financial support.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Van Panhuys N Studying dendritic cell-T cell interactions under in vivo conditions. Methods Mol Biol 1584 (2017): 569-583.
- Gerlini G, Di Gennaro P, Borgognoni L Enhancing anti-melanoma immunity by electrochemotherapy and in vivo dendritic-cell activation. Oncoimmunology 1 (2012): 1655- 1657.
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392 (1998): 245-252.
- Probst HC, Muth S, Schild H Regulation of the tolerogenic function of steady-state DCs. Eur J Immunol 44 (2014): 927-993.
- Phillips BE, Garciafigueroa Y, Trucco M, Giannoukakis N Clinical tolerogenic dendritic cells: exploring therapeutic impact on human autoimmune disease. Front Immunol 8 (2017): 1279.
- Woodham AW, Skeate JG, Sanna AM, Taylor JR, Da Silva DM, Cannon PM, Kast WM Human immunodeficiency virus immune cell receptors, coreceptors, and cofactors: Implications for prevention and treatment. AIDS Patient Care STDS 30 (2016): 291-306.
- Steinman R.M Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30 (2012): 1-22.
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 165 (2000): 6037-6046.
- Birbeck MS, Breathnach AS, Everall JD An electron microscopic study of basal melanocytes and high level clear cells (Langerhans cell) in vitiligo. J Invest Dermatol 37 (1961): 51-64.
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol 9 (1997): 10-16.
- Ito T, Inaba M, Inaba K, Toki J, Sogo S, Iguchi T, Adachi Y, Yamaguchi K, Amakawa R, Valladeau J, Saeland S, Fukuhara S, Ikehara S A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol 163 (1999): 1409-1419.
- Paccosi S, Musilli C, Caporale R, Gelli AM. Guasti D, Clemente AM, Torcia MG, Filippelli A, Romagnoli P, Parenti A Stimulatory interactions between human coronary smooth muscle cells and dendritic cells. PLoS One 9 (2014): e99652.
- Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, Desaintvis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34(+) hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med 184 (1996): 695-706.
- Goussetis E, Theodosaki M, Paterakis G, Tsecoura C, Graphakos S In vitro identification of a cord blood CD133+CD34-Lin+ cell subset that gives rise to myeloid dendritic precursors. Stem Cells 24 (2006): 1137-1140.
- Bonetti MI, Bacci S, Santosuosso M, Mazzanti B, Aldinucci A, Ballerini C, Guasti D, Calosi L, Bosi A, Romagnoli P Rosiglitazone promotes the differentiation of Langerhans cells and inhibits that of other dendritic cell types from CD133 positive hematopoietic precursors. Histol Histopathol 29 (2014): 323-332.
- Hubert P, Bousarghin L, Greimers R, Franzen-Detrooz E, Boniver J, Delvenne P Production of large numbers of Langerhans' cells with intraepithelial migration ability in vitro. Exp Dermatol 14 (2005): 469-477.
- Greene ME, Blumberg B, McBride OW, Yi HF, Kronquist K, Kwan K, Hsieh L, Greene G, Nimer SD Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr 4 (1995): 281-299.
- Nencioni A, Grunebach F, Zobywlaski A, Denzlinger C, Brugger W, Brossart P Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J Immunol 169 (2002): 1228-1235.
- Asada K, Sasaki S, Suda T, Chida K, Nakamura H Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am J Respir Crit Care Med 169 (2004): 195-200.
- Szatmari I, Gogolak P, Im JS, Dezso B, Rajnavolgyi E, Nagy L Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity 21 (2004): 95-106.
- Szatmari I, Rajnavolgyi E, Nagy L PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann NY Acad Sci 1088 (2006): 207-218.
- Varga T, Nagy L Nuclear receptors, transcription factors linking lipid metabolism and immunity: the case of peroxisome proliferator-activated receptor gamma. Eur J Clin Invest 38 (2008): 695-707.
- Appel S, Mirakaj V, Bringmann A, Weck MM, Grunebach F, Brossart P PPAR-gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood 106 (2005): 3888-3894.
- Wei-guo Z, Hui Y, Shan L, Yun Z, Wen-cheng N, Fu-lin Y, Fang-yan F, Jun-hua G, Jian- hua Z PPAR-gamma agonist inhibits Ang II-induced activation of dendritic cells via the MAPK and NF-kappaB pathways. Immunol Cell Biol 88 (2010): 305-312.
- Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptors α and γ down-regulate allergic inflammation and eosinophil activation. J Exp Med 198 (2003): 411-421.
- Hammad H, de Heer HJ, Soullié T, Angeli V, Trottein F, Hoogsteden HC, Lambrecht BN Activation of peroxisome proliferator-activated receptor-γ in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol 164 (2004): 263-271.
- Szatmari I, Töröcsik D, Agostini M, Nagy T, Gurnell M, Barta E, Chatterjee K, Nagy L PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood 110 (2007): 3271-3280.
- Hargadon KM Murine and human model systems for the study of dendritic cell immunobiology. Int Rev Immunol 35 (2016): 85-115.
- Miltenyi S, Müller W, Weichel W, Radbruch A High gradient magnetic cell separation with MACS. Cytometry 11 (1990): 231-238.
- Sporn MB, Roberts AB Transforming growth factor-beta: recent progress and new challenges. Cell Biol 119 (1992): 1017-1021.
- Nakakoshi M, Nishioka H, Katayama E New versatile staining reagents for biological transmission electron microscopy that substitute for uranyl acetate. J Electron Microsc (Tokyo) 60 (2011): 401-407.
- Hanau D, Fabre M, Schmitt DA, Garaud JC, Pauly G, Cazenave JP Appearance of Birbeck granule-like structures in anti-T6 antibody-treated human epidermal Langerhans cells. J Invest Dermatol 90 (1998): 298-304.
- Klechevsky E, Banchereau J Human dendritic cells subsets as targets and vectors for therapy. Ann N Y Acad Sci 1284 (2013): 24-30.
- Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P Langerhans cells - dendritic cells of the epidermis. APMIS 111 (2003): 725-740.
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med 187 (1998): 961-966.
- Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med 194 (2001): 1013-1020.
- Mashima R, Okuyama T The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol 6 (2015): 297-310.
- Han H, Liang X, Ekberg M, Kritikou JS, Brunnström Å, Pelcman B, Matl M, Miao X, Andersson M, Yuan X, Schain F, Parvin S, Melin E, Sjöberg J, Xu D, Westerberg LS, Björkholm M, Claesson HE Human 15-lipoxygenase-1 is a regulator of dendritic-cell spreading and podosome formation. FASEB J 31 (2017): 491-504.
- Moore GY, Pidgeon GP Cross-talk between cancer cells and the tumour microenvironment: the role of the 5-lipoxygenase pathway. Int J Mol Sci 18 (2017): E236.
- Jung K, Tanaka A, Fujita H, Matsuda A, Oida K, Karasawa K, Okamoto N, Ohmori K, Jee Y, Shin T, Matsuda H Peroxisome proliferator-activated receptor γ-mediated suppression of dendritic cell function prevents the onset of atopic dermatitis in NC/Tnd mice. J Allergy Clin Immunol 127 (2011): 420-429.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 