Ecological and Genetic Variation of the Distribution of Various Species of Amphibians at the Southern Border of their Distribution
Article Information
Gad Degani1,2*
1MIGAL – Galilee Research Institute, KiryatShmona, Israel
2Faculty of Science and Technology, Tel-Hai Academic College, KiryatShmona, Israel
*Corresponding autor: Gad Degani, Faculty of Science and Technology, Tel-Hai Academic College, KiryatShmona, Israel
Received: 25th Feb-2019; Revised: 25th Mar-2019; Accepted: 26th Mar-2019;
Copyright: ©2019 Gad Degani. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Citation: Gad Degani. Ecological and Genetic Variation of the Distribution of Various Species of Amphibians at the Southern Border of their Distribution. International Journal of Plant, Animal and Environmental Sciences 9 (2019): 37-53.
View / Download Pdf Share at FacebookAbstract
The current mini-review describes the distribution of amphibian species in terms of their adaptation from Mediterranean to desert climates. According to the data collected in this mini-review and from some unpublished data, it was found that the adaptation of amphibian species from arid to Mediterranean climates was highest for Bufovariabilis, followed by Triturusvittatus,Hylasavignyi, Pelobatessyriacus, Rana bedriagae and Latonia nigriventer. Many parameters affecting adaptation to different habitats have been described, including aquatic and terrestrial habitats. Among them, the most important parameters affecting semi-arid and arid habitats are the large number of tadpoles, the short growth and complete metamorphosis period, finding hiding places to prevent dehydration, and physiological adaptation to accumulate urea in the body fluid. A quality model is suggested to show the adaptation of various amphibian species to habitats at the southern border of its distribution.
Keywords
Bioindicator;Environmental pollution;Genetic markers; RAPD PCR; Amphibian populations
Article Details
INTRODUCTION
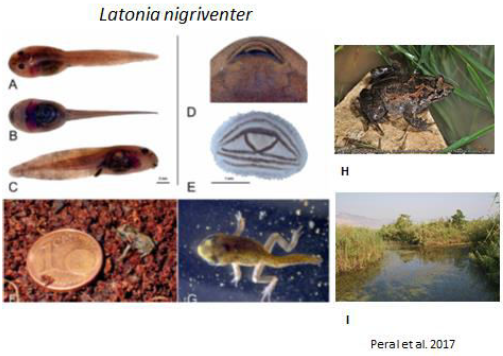
Amphibian species populations are important bioindicators for the environment affected by human development and pollution [1, 2]. Israel is developing very rapidly both in terms of agriculture and urban expansion. Seven amphibian species exist in Israel: two are Urodaela – banded newt, TriturusvittatusLitvinchuk et al. [3] synonym Ommatotritonvittatus, which is found in Israel, Lebanon, Jourdan, Syria, Turkey and Iraq, and fire salamander, Salamandrainfraimmaculata[4],which is distributed in Israel, Lebanon, Jordan, Syria, Turkey and Iran. Both of these species belong to the Salamandrinae family. Five Anuran species also survive in Israel: tree frog, Hylasavignyi(Hylidae family), synonym H. arborea[5, 6],which is found in Yemen, Jordan, southern Syria and extreme north-eastern Israel; green toad, Bufoviridis (Bufonidae family) synonym PseudepidaleaViridis[7, 8], which is found throughout many countries in Europe in different habitats, most of them relatively dry, as well as in Israel, including mountainous areas, semi-arid and arid habitats, and they have also penetrated into urban areas; water frog,Rana bedriagae (Ranidae family) synonym Rana ridibunda[9, 10]; spadefoot, Pelobatessyriacus (Pelobatidae family) [11-14], whose wide distribution belongs to the Pelobatidae family in part of Europe and East and West Asia, including North Syria, Israel, North Iraq, Iran, Asia Minor and Caucasus; and Hula painted frog, Latonia nigriventer (Alytidae family) [15, 16] belonging to the Alytidae family (this is an endemic species found in the Hula reserve).
All these amphibian species are found at the southern border of their distribution at relatively extreme conditions. In Israel and other places, amphibians breed in different aquatic habitats, including temporary ponds, semi-permanent ponds, spring and streams, where water is available all year round or part of the year [11, 12, 17-24]. Many factors affect the aquatic phase of amphibians [25].
Israel offers mainly xeric habitats, unusual for amphibians, and constitutes the southeastern range of distribution of these species at the extreme condition of these species [8, 11, 22, 26]. Hence, amphibian larvae occupy a very narrow and specific ecological niche in the region, and are under severe pressure from predators and other biotic and abiotic factors [12, 27]. To define the range of water quality in which tadpoles of various species can grow and complete metamorphosis, it is not only important to gain knowledge about those species for their protection but it also may serve as a bioindicator for environmental pollution. The adaptation of amphibians to various habitats at the southern border of their distribution in Israel in a relatively small area is examined by genetic variation in different areas. Genetic markers have been used to study the variation among different populations. All of the seven amphibian species in Israel belong to a different genus, and some belong to different families and orders [11, 21, 28]. Various methods are used to study the genetic variation of these species and their adaptation to different habitats or geographical distribution. A relatively large number of studies on genetic variation have been carried out for S. infraimmaculata using the enzyme system loci [29] DNA polymerase chain reaction (RAPD PCR) [30], mitochondrial DNA analysis [13, 31-33]. Amplified Fragment Length Polymorphism (AFLP) [34], gene expression microarrays [35], microsatellites [36, 37] and RNA excretion [38]. Some of these methods can be applied to other species in order to study the genetic variation among various habitats in Israel, but not all of them. The variation among populations in Israel was studied for T. vittatusvittatus[26, 33, 39], PearlsonH. savignyi[5], B. viridis (P. Viridis) [8, 42], R. bedriagae[43] and Latonia nigriventer[44].
The purpose of this mini-review and unpublished data is to examine the different adaptations of seven species of amphibians in semi-arid habitats at the southern border of their distribution in both aquatic and terrestrial habitats. The hypothesis examined in the present study is that the adaptation to different areas of amphibians at the southern border of distribution is mainly by metamorphosis phases and not aquatic phases.
MATERIALS AND METHODS
Sampling
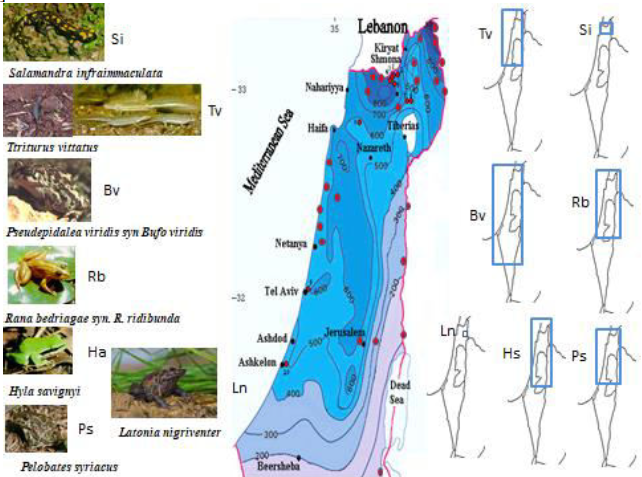
The breeding sites of amphibian populations in Israel (Figure1) at both terrestrial and aquatic phases from north to south were examined in different samplings, namely, near various water bodies including winter pools, pits, springs and streams, and the locations and various amphibian larvae of these places were defined. Tadpoles were collected with a net (pore size 450 μm) from a depth of approximately 10-40 cm every two weeks during larvae growth and after complete metamorphosis [11, 12], and their species were identified as previously described [17]. The metamorphosed animals were collected mainly during the winter and spring near the breeding places as previously described [45-48].
Water quality, including pH, ammonium concentration, electrical conductivity (EC), temperature, relative oxygen content (O2) and pH [49], genetic variation [13, 40, 44], were measured. The evolutionary history tree was derived using the Neighbor-Joining method(Saitou and Nei, 1987). The phylogenetic tree was done as previously described [51]. Phylogenetic analyses were conducted using MEGA4 [51].
RESULTS
The seven species of amphibians were found in various habitats in Israel. Two belong to Urodela – Triturusvittatus and fire salamander,Salamandrainfraimmaculata,and five are Anuran species, including the tree frog, Hylasavignyi, the green toad, Bufoviridis (Pseudepidaleaviridis), the water frog,Rana bedriagae, the spadefoot, Pelobatessyriacus and the Hula painted frog (Latonia nigriventer) (Figure 1). Latonia nigriventerand S. infraimmaculata are distributed in small areas compared to all the other amphibian species. B. viridis and T. vittatusare distributed in large areas. B. viridis adapted to a wide range of areas in Israel and also survived in desert environments. In the ecological conditions of the examined larvae in the breeding sites shown in Figures 2 to 8, the conditions are not extreme different between species, and some species adapted to different types of breeding sites (ponds, spring and streams) in the same area [1, 5, 18, 52-60]. However, there are different conditions among breeding sites in the area of the species distribution of larvae habitats. Some species were studied in more detail, e.g., S. infraimmaculata, which are bred in all kinds of water bodies such as springs, streams, winter ponds and rock poles (Figure2) [61].
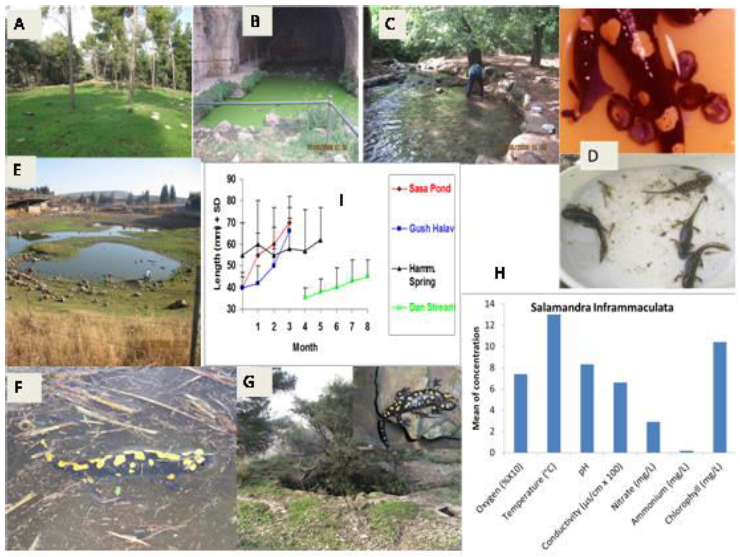
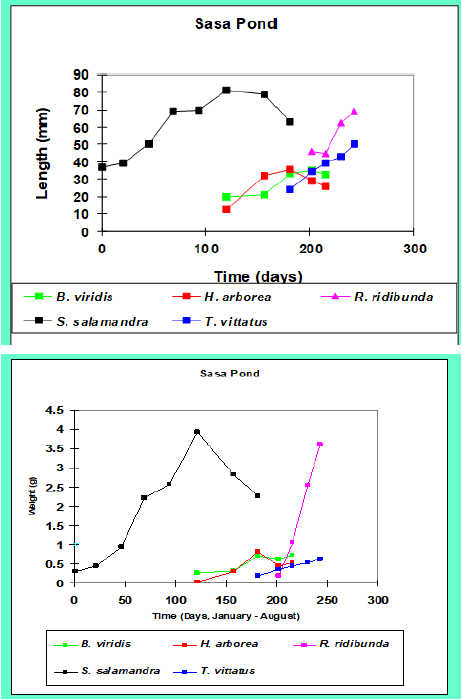
Figure 2: Presentation of ecological terrestrial and aquatic habitats of S. infraimmaculata including niches of larvae in northern Israel with respect to five ecological characteristics – oxygen (mg/L), ammonium ([mg/L]/2), temperature (°C), conductivity ([uS/cm] ×100) and pH. The different areas in Israel (see Figure1) are: A, terrestrial habitats, Western Galilee; B, breeding place, Nimrod pit, Harmon mountain; C, breeding place, Tel-Dan stream, northern Israel; D, oviposition and large development of larvae S. infraimmaculata; E, Raihaniya pond, Lower Galilee; F, female S. infraimmaculataoviposition in Sasa pond, Upper Galilee; and G, Maalot pit, Western Galilee. H, ecological niches of water where S. infraimmaculata tadpoles are found in northern Israel.I, measurement of tadpoles grown in different breeding sites in the Upper Galilee.
The oviposition of larvae in most populations took time, from November to December, and totaled 90-130; the oviposition in permanent springs totaled 68-72 [62]. On the other hand, some species such as T. vittatus (Figure3)
- and viridis (Figure4) Degani et al. [40] used only relatively specific breeding sites and unpredictable breeding places in winter (ponds, rock pools and streams). Both these species are part of a wider distribution; they adapted to relatively extreme conditions and were also found in all areas of other amphibian distributions (Figure1)
- Both vittatus and B. viridislay eggs and have external fertilization [20].T. vittatus deposit between 18-68 eggs on plants or rock surfaces [59] and the number of eggs per female of B. viridis spawning is much greater (5,687-17,602) [8, 63].
Both species have a relatively short period of larval growth, and complete metamorphosis takes place over a short period [11, 12, 16, 64]. It is very difficult to compare larval growth in natural habitats so it is suggested examining habitats having extensive larval growth and development [16]. The only differences in this habitat are the temperature in the ponds and the growth period of B. viridis in winter, and the time and cold water of T. vittatus in spring and summer [16].
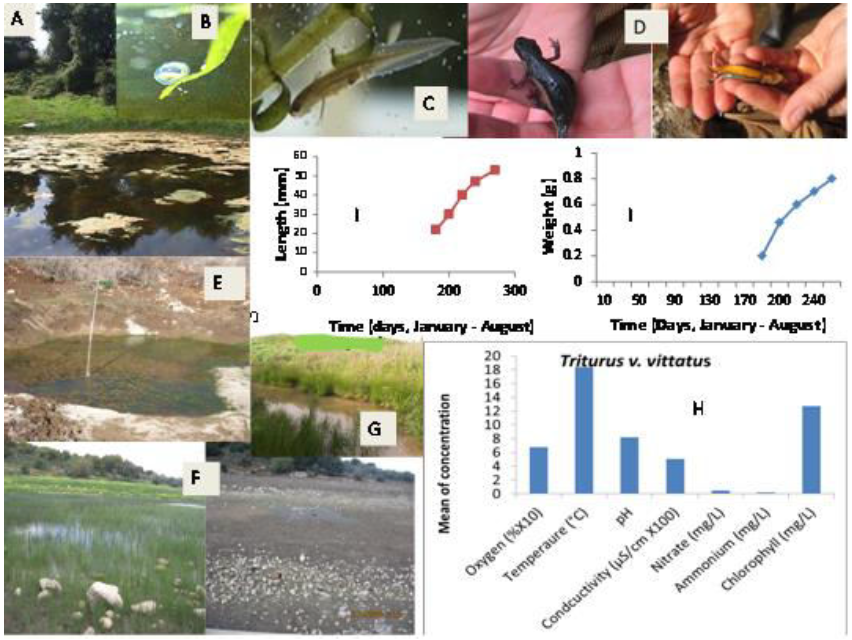
Figure 3:Presentation of ecological terrestrial and aquatic habitats of Triturusvittatus including niches of larvae in northern Israel with respect to five ecological characteristics – oxygen (mg/L), ammonium ([mg/L]/2), temperature (°C), conductivity ([uS/cm] ×100) and pH.A, winter pond in Upper Galilee. B, eggs. C, larvae newt. D, adult female’s terrestrial newt. E and F (Sasa in winter and summer) winter ponds in Upper Galilee.H, water qualities of Triturusvittatus tadpoles in northern Israel.
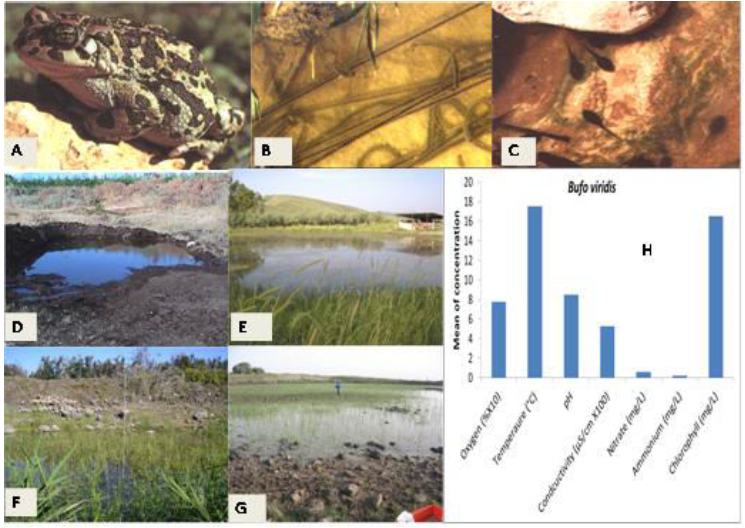
Figure 4: Presentation of ecological terrestrial and aquatic habitats of Bufoviridis (Syn.PseudepidaleaViridis) including niches of larvae in northern Israel with respect to five ecological characteristics – oxygen (mg/L), ammonium ([mg/L]/2), temperature (°C), conductivity ([uS/cm] ×100) and pH. Larval growth (weight and length) and complete metamorphosis in winter ponds in the Upper Galilee (Sasa pond) are presented. A, Adult Bufoviridis. B, eggs of Bufoviridis. C, tadpoles of Bufoviridis.D, winter pond before drying up.E, winter pond in Upper Galilee.F, Fara pond.G, Kash pond (Hula valley). H, ecological niches of Bufoviridis larvae in northern Israel. I, measurement of tadpoles grown in winter ponds in Upper Galilee.
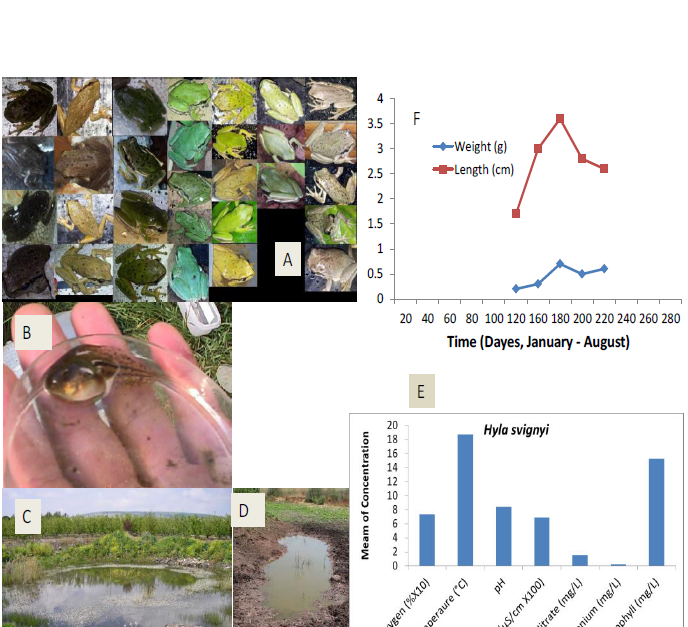
savignyi larvae are also found in unpredictable habitats, many of which are winter ponds (Figure5). Like in the larvae of T. vittatusand B. viridis, a short growth period and complete metamorphosis occurs between 1-2 months [11, 12, 16, 64], and they can use the same breeding places as T. vittatusand B. viridisif the water conditions are suitable from winter to summer.
Figure 5: Presentation of ecological terrestrial and aquatic habitats of Hylasavignyi include niches of larvae in northern Israel with respect to five ecological characteristics – oxygen (mg/L), ammonium ([mg/L]/2), temperature (°C), conductivity ([uS/cm] ×100) and pH.A, adult Hylasavignyi with different colors as recorded in northern Israel.B, tadpoles of Hylasavignyi, C and D, Lahavot pond. E, ecological niches of Rana bedriagae larvae in northern Israel. F, measurements of tadpoles grown in a winter pond (Sasa) in Upper Galilee.
The larval growth period of R. bedriagae (Figure6) and P. syriacus (Figure7) is relatively long, and complete metamorphosis lasts for over four months from winter to summer; tadpoles are found at these breeding places [16]. The studies on amphibian larvae interactions regarding growth and complete metamorphosis may show specific niches for each species. The studies were carried out in winter ponds in the Upper Galilee (Figure 9) [16, 62] and in many breeding sites in northern Israel [11, 12].
Based on this information, the tadpoles are found in specific niches. For example, the tadpoles of T. vittatusand fire salamander, S. infraimmaculata, which followed the same diet, were found at different times in the ponds, and water temperatures differed during the growth periods: S. infraimmaculata during the winter and T. vittatus during the spring and beginning of summer (Figure 9). The tadpoles of S. infraimmaculata, T. vittatus and Rana bedriagae were found at the bottom of the pond most of the time; H. savignyi were found throughout the pond and were usually sedentary compared to P. syriacus,which moved up and down constantly [16].
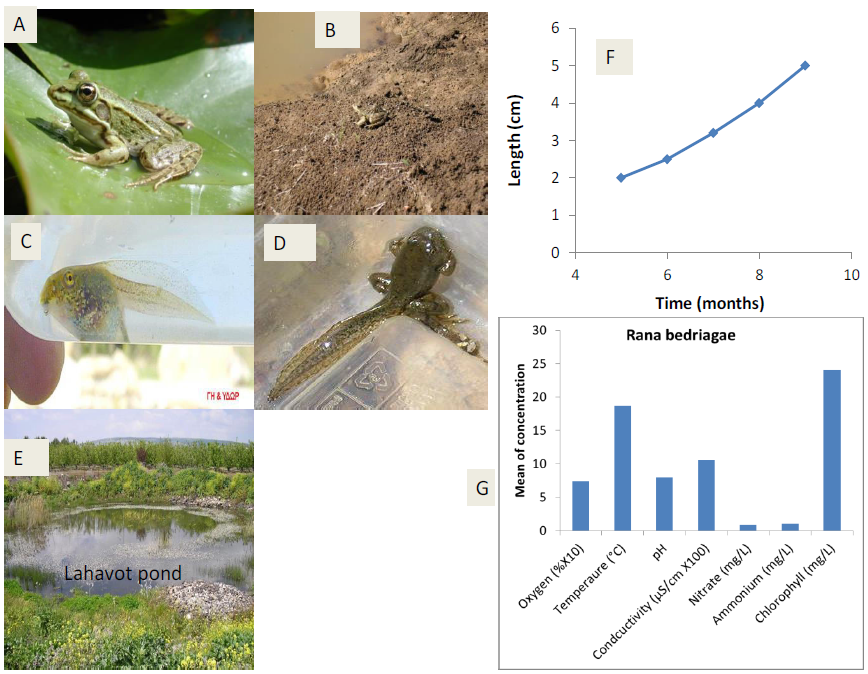
Figure 6: Presentation of ecological terrestrial and aquatic habitats of Rana bedriagaeincluding niches of larvae in northern Israel with respect to five ecological characteristics – oxygen (mg/L), ammonium ([mg/L]/2), temperature (°C), conductivity ([uS/cm] ×100) and pH.A, adult Rana bedriagae.B, juvenile R. bedriagae, C, R. bedriagae tadpoles.D, R. bedriagae during metamorphosis.E, Lahavot pond in Hula valley.F, larval growth in Lahavot pond. G, ecological niches of R. bedriagae tadpoles in northern Israel.
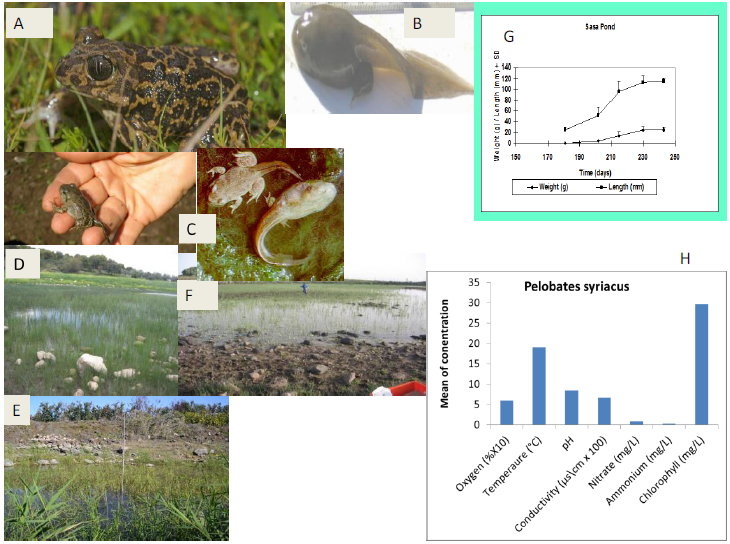
Figure 7: Presentation of ecological terrestrial and aquatic habitats of Pelobatessyriacus include niches of tadpoles in northern Israel with respect to five ecological characteristics – oxygen (mg/L), ammonium ([mg/L]/2), temperature (°C), conductivity ([uS/cm] ×100) and pH.A, adult P. syriacus, B. P. syriacus tadpole.C, tadpole under metamorphosis.D, E and F, breeding sites (northern Israel) in ponds of P. syriacus.G, larval growth in a pond (Sasa) from spring and summer in Upper Galilee.H, ecological niches of tadpoles in northern Israel.
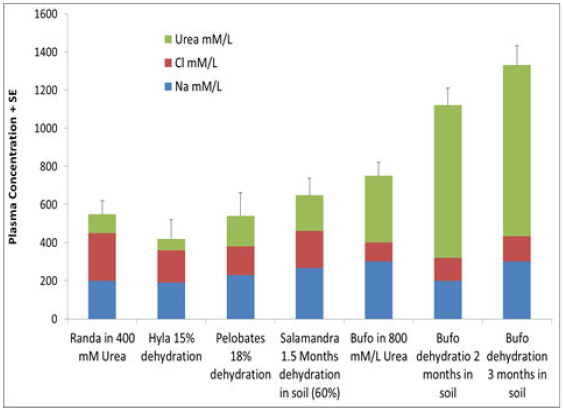
Relatively fewer studies were carried out to examine amphibian adaptation after metamorphosis to terrestrial life compared to tadpoles in Israel.B. viridis, which has the largest distribution (Figure1), penetrated into desert and arid environments and showed very high adaptation [65-69] through their ability to store water in the urine bladder and accumulate urea in the plasma compared to other species such as T. vittatus, P. syriacus[70, 71], S. infraimmaculata[53, 72, 73], R. bedriagae[65] and H. savignyi (Figure10) [74].
The amphibian skin does not prevent dehydration, and the ability to survive in terrestrial life depends on physiological adaptation ability, e.g., plasma concentration. Based on the information collected regarding the ability to survive, high plasma concentrations might be related to the ability to accumulate urea and water in the bladder (Figure10). The ability to accumulate urea is related to a high adaptation to dry conditions, as was found in B. viridis[66].
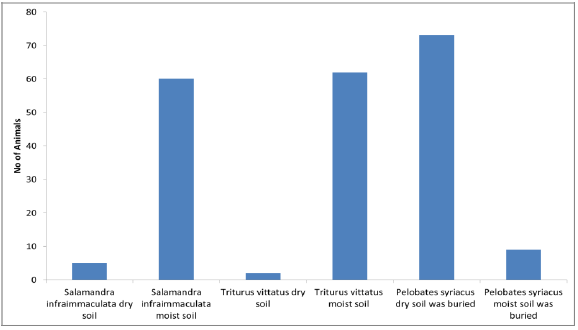
Another ability to adapt to terrestrial life is environmental behavior, involving finding hiding places and getting past relatively long dry seasons. Most amphibian species in Israel, except for R. bedriagae, are active at night, and they find hiding places at a relatively high soil humidity and negative photo taxis.
Once metamorphosis is complete, the amphibians become terrestrial animals and are in danger of dehydration. The environmental behavior in finding a hiding place is an important adaptation to prevent dehydration and survive. The subject of finding hiding places was studied in various species and it was based on moisture, light and temperature, or even digging in the soil, as was described in P. syriacus[7, 75] (Figure11). Environmental behavior was studied in various amphibian species at the southern border of their distribution: S. infraimmaculata[76-78], T. vittatus[19, 36, 79] and P. syriacus[7, 75] (Figure11). In all of these species, the effected to hiding places is to seek high humidity in the soil and dark.
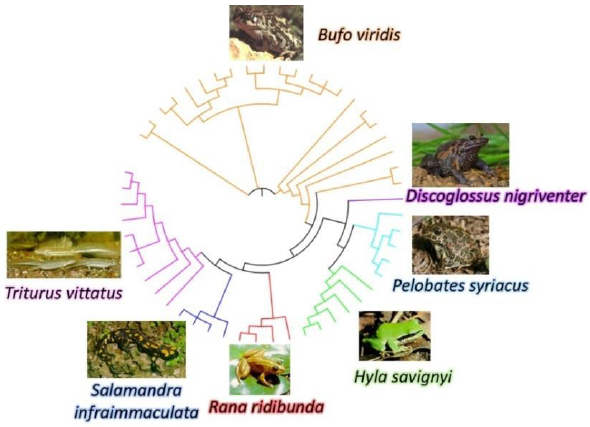
Thephylogenetic tree of amphibian species based on the Cytochrome b (Cyt b) sequence is presented in Figure 12. A maximum probability calculated accordingly [47]shows differences among various species whereby all the Anura species belong to different families and the two Urodela species belong to one Salamandrinae family. The phylogenetic tree (Figure12)is support the this bring closer between those species. The variation among individuals in the same species is higher in B. viridis and T. vittatus than in other species found in Israel. Those species seem to have very high adaptation to terrestrial life and extreme variation among habitats.
Table 1:The source of Cyt b sequences used for alignment and the phylogenetic tree.
|
GenBank accession no. and references |
Name |
Location |
|
(Goldberg et al., 2011a) |
Bufoviridis |
Matityahu rev comp |
|
= |
Bufoviridis |
Fara |
|
= |
Bufoviridis |
Herzliya |
|
= |
Bufoviridis |
Hadera |
|
= |
Bufoviridis |
Bet Zayit |
|
= |
Bufoviridis |
Hulon B |
|
= |
Bufoviridis |
Shafdan |
|
= |
Bufoviridis |
Kziv rev comp |
|
= |
Bufoviridis |
Jerusalem |
|
= |
Bufoviridis |
Ga’ash |
|
= |
Bufoviridis |
Naaran |
|
= |
Bufoviridis |
Orvim |
|
= |
Bufoviridis |
Raihaniya |
|
= |
Bufoviridis |
Nahalit |
|
= |
Bufoviridis |
Hulon A |
|
= |
Bufoviridis |
Kash |
|
= |
Bufoviridis |
Manof |
|
= |
Bufoviridis |
Bika |
|
= |
Bufoviridis |
Afeka |
|
= |
Bufoviridis |
Hermon |
|
= |
Bufoviridis |
Hazeva |
|
= |
Bufoviridis |
Masade |
|
KC867706.1 |
Discoglossusnigriventer |
Lake Hula |
|
FJ595201.1 |
Pelobatessyriacus |
Kash pond |
|
FJ595200.1 |
Pelobatessyriacus |
Fara pond |
|
FJ595202.1 |
Pelobatessyriacus |
Raihaniya pond |
|
FJ595203.1 |
Pelobatessyriacus |
Sasa pond |
|
FJ595199.1 |
Pelobatessyriacus |
Elrom pond |
|
FJ595189.1 |
Hylasavignyi |
Dir-Hanna pond |
|
FJ595193.1 |
Hylasavignyi |
Jauda spring |
|
FJ595195.1 |
Hylasavignyi |
Matityahu pond |
|
FJ595191.1 |
Hylasavignyi |
Fara pond |
|
FJ595194.1 |
Hylasavignyi |
Leshem pond |
|
FJ595197.1 |
Hylasavignyi |
Sasa pond |
|
DQ474161 |
Rana ridibunda |
Greece, Thrace (Nestos R.) |
|
DQ474160 |
Rana ridibunda |
Greece, Thrace (Kotili) |
|
DQ474162 |
Rana ridibunda |
Greece, Thrace (Therma) |
|
DQ474163 |
Rana ridibunda |
Greece, Thrace (Dadia) |
|
EU852738.1 |
Salamandrainfraimmaculata |
Tel Dan |
|
EU852731.1 |
Salamandrainfraimmaculata |
Balad |
|
EU852736.1 |
Salamandrainfraimmaculata |
Matityahu |
|
EU852735.1 |
Salamandrainfraimmaculata |
Manof |
|
(Pearlson et al., 2010) |
Triturusvittatus |
Berekhya |
|
= |
Triturusvittatus |
Afeka |
|
= |
Triturusvittatus |
Nahalit |
|
= |
Triturusvittatus |
Pharaa |
|
= |
Triturusvittatus |
Matityahu Q. |
|
= |
Triturusvittatus |
Amiad |
|
= |
Triturusvittatus |
Leshem |
|
= |
Triturusvittatus |
Jaudha |
|
= |
Triturusvittatus |
Dovev |
|
= |
Triturusvittatus |
Kash |
DISCUSSION AND CONCLUSION
The adaptation of seven amphibian species in various areas in Israel changes from Mediterranean to desert climates. According to these results, a hypothesis is suggested that characteristicsamong the various parameters affect amphibian adaptation to semi-arid environments. In conclusion, all the data show that B. variabilis adapted most to the terrestrial habitat and extreme conditions, and after it, T. vittatus, H. savignyi, P. syriacus, S. infraimmaculata, R. ridibunda and D. nigriventer, respectively. However, considerably more studies must be carried out in order to support this hypothesis due to the fact that many parameters are involved in amphibian adaptation to arid and semi-arid habitats [5, 7, 8, 35, 36, 40, 57, 61, 68, 75, 77, 82, 83, 84]. When trying to summarize the adaptation to arid and semi-arid habitats of amphibians based on those species, the idea is supported that adaptation must take place in both terrestrial and aquatic phases. Two species, B. variabilis and T. vittatus, may show high adaptation to arid environments. In both these species, the breeding places are winter pools and other unpredictable habitats, and the growth period and complete metamorphosis are relatively short compared to other amphibian species [8, 23, 58,71]. The physiological adaptation of B. variabilis to arid environments [9, 68, 69, 85], requires considerably more studies compared to T. vittatus[86, 87]. It seems that the physiological adaptation of B. variabilis to extreme conditions is by urea accumulation in the blood [74].
The other amphibians, H. savignyi, P. syriacus, S. infraimmaculata, R. ridibunda and D. nigriventer,adapt less to extreme conditions, however, only a few studies on physiological adaptation have been carried out for those species and it is very difficult to compare between them. In S. infraimmaculata, a different physiological adaptation between the populations in semi-arid habitats was found compared to moist habitats where water is available all year round [17, 53, 72, 73, 87]. Another Anuran species might exhibit a different strategy of adaptation to terrestrial life that could help them survive in semi-arid habitats. Among them, environmental behavior is very important. P. syriacusdig into the soil to prevent dehydration [8, 75, 88] and H. savignyimight find hiding places among plants or in the ground and change their color according to the substrate [5, 89].
In comparing the phylogenetic tree of Cyt sequences, the high genetic variation found in the species B. variabilis and T. vittatus (Figure12) enabled them to adapt better to extreme conditions than the other species. These species are distributed in wider areas in Israel than the other species.
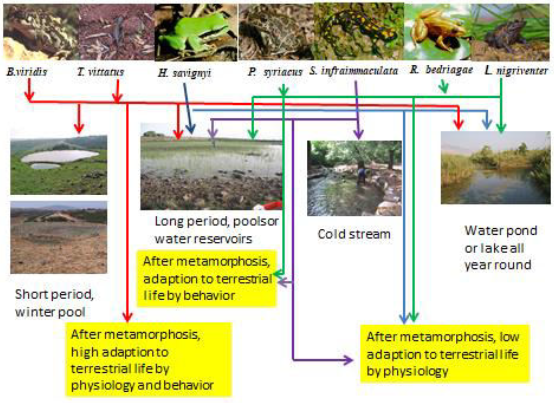
In summary, a quality model is suggested to show the adaptation of various amphibian species to habitats at the southern border of their distribution(Figure13).
REFERENCES
- Blaustein AR, Johnson PTJ. 2003. The complexity of deformed amphibians. Frontiers in Ecology and the Environment 1: 87-94.
- McCallum ML. 2007. Amphibian decline or extinction? Current Declines Dwarf Background Extinction Rate Journal of Herpetology 41: 483-491.
- Litvinchuk SN, Zuiderwijk A, Borkin LJ, 2005. Taxonomic status of Triturus vittatus (Amphibia: Salamandridae) in western Turkey: trunk vertebrae count, genome size and allozyme data. Amphibia-Reptilia 26: 305-323.
- Steinfartz S, Veith M, Tautz D. 2000. Mitochondrial sequence analysis of Salamandra taxa suggests old splits of major lineages and postglacial recolonizations of central Europe from distinct source populations of Salamandra salamandra. Molecular Ecology 9: 397-410.
- Degani G. 2016. Life cycle of tree frogs (Hyla savygnyi) in semi-arid habitats in northern Israel. International Journal of Biology 8: 17-26.
- Gvozdik V, Moravec J, Klutsch C, 2010. Phylogeography of the Middle Eastern tree frogs (Hyla, Hylidae, Amphibia) as inferred from nuclear and mitochondrial DNA variation, with a description of a new species. Molecular Phylogenetics and Evolution 55:1146-1166.
- Degani G. 2015. The habitats, burrowing behavior, physiology adaptation and life cycle of Spadefoot toads (Pelobates syriacus, Boettger, 1869) at the southern limit of its distribution in Israel. Open Journal of Animal Sciences 5: 249-257.
- Degani G. 2016. The habitats, life cycle and physiological adaptation of green toad (Pseudepidalea viridis) in northern Israel. Internal Journal of Scientific Research 5: 113-117.
- Degani G, Mendelssohn H. 1984. Amphibia plants and animals of the Land of Israel Alon A. Misrad Habitachon Pub, Tel Aviv: 190-221.
- Schneider H, Sinsch U, Nevo E. 1993. The lake frog in Israel represent a new species. Zoologischer Anzeiger: 97-106.
- Degani G,Kaplan D. 1999. Distribution of amphibian larvae in Israeli habitats with changeable water availability. Hydrobiologia 405: 49-56.
- Goldberg T, Nevo E, Degani G. 2009. Breeding site selection according to suitability for amphibian larval growth under various ecological conditions in the semi-arid zone of northern Israel. Ecologia Mediterranea 35: 65-74.
- Tarkhnishvili D, Serbinova I, Gavashelishvili A. 2009. Modelling the range of Syrian spadefoot toad (Pelobates syriacus) with combination of GIS-based approaches Amphibia-Reptilia 30: 401-412.
- Mendelssohn H, Steinitz H. 1943. A new frog from Palestine. Copeia 4: 231-233.
- Perl BRG, Gafny S, Malka Y, 2017. Natural history and conservation of the rediscovered Hula painted frog, Latonia igriventer. Contribu. Zool 86: 11-37.
- Degani G. 1982. Amphibian tadpole interaction in a winter pond. Hydrobiologia 96: 3-8.
- Degani G. 1996. The Salamander at the Southern Limit of its Distribution. Laser Pages Publ. Ltd, Jerusalem.
- Degani G, 1982. Mendelssohn H. Seasonal activity of Salamandra salamandra (L.) (Amphibia, Urodela) in headwaters of the Jordan River. Israel Journal of Zoology 31: 77-85.
- Degani G, Mendelssohn H. 1983. The habitats, distribution and life history of Triturus vittatus vittatus (Jenyns) in the Mount Meron area (Upper Galilee, Israel). British Journal of Herpetology 6: 317-319.
- Degani G, Mendelssohn H. 1990. Reptiles and amphibians, in: A. Arbel (ed.), Plant and Animals of Land of Israel, 2nd Society for Protection of Nature, Israel: 190-221.
- Degani G. 2013. Mitochondrial DNA sequence analysis in Anabantoidei fish. Advances in Biological Chemistry 3: 347-355.
- Degani G, Sharon R, Warburg M. 1997. Ovarian steroid levels in Salamandra salamandrainfraimmaculata during the reproductive cycle. General and Comparative Endocrinology 106: 356-360.
- Pearlson O, Degani G. 2011. Water and ecological conditions of striped newt, Triturus v. vittatus (Urodela), breeding sites at various altitudes near the southern limit of its distribution. Herpetol. Romanica 5: 27-42.
- Laurila A. 1998. Breeding habitat selection and larval performance of two anurans in freshwater rockpools. Ecography 21: 484-494.
- Pearlson O. 2012. Ecology and genetic variance of the banded newt Triturus vittatus vittatus in northern Israel. Doctor of Philosophy, University of Haifa, Supervised by Prof. Gad Degani, Hebrew and English summary: 1-135.
- Perl R, Gafny S, Malka Y, 2017. Natural history and conservation of the rediscovered Hula painted frog, Latonia nigriventer. Contributions to Zoology Bijdragen tot de dierkunde 86: 11-37.
- Veith M. 1994. Morphological, molecular and life history variations in Salamandra salamandra(L.). Mertensiella 4: 355-397.
- Goldberg T, Degani G, Gazith A, 2011. Sequence variation in the mitochondrial DNA of Pseudepidalea Viridis (Syn. Bufo Viridis) in Israel, Bulletin UASVM Animal Science and Biotechnologies: 51-57.
- Goldberg T, Nevo E, Degani G. 2010. Genetic variation in Salamandra infraimmaculata from various breeding sites - a model for habitat selection. Asian Herpetological Research 1:1-9.
- Goldberg T,Pearlson O, Nevo E. 2007. Mitochondrial DNA analysis of Salamandra infraimmaculata larvae from habitats in northern Israel, Progrese ?i Perspective in Medicina Veterinar?” - Lucr?ri ?tiin?ifice: 23-31.
- Goedbloed DJ, Czypionka T, Altmüller J, 2017. Parallel habitat acclimatization is realized by the expression of different genes in two closely related salamander species (genus Salamandra). Heredity 119: 1-9.
- Blank L, Blaustein L. 2012. Using ecological niche modeling to predict the distributions of two endangered amphibian species in aquatic breeding sites. Hydrobiologia 693: 157-167.
- Blank L, Blaustein L. 2014. A multi-scale analysis of breeding site characteristics of the endangered fire salamander (Salamandra infraimmaculata) at its extreme southern range limit. Hydrobiologia 726: 229-244.
- Arntzen JW, Beukema W,Galis F, 2015. Vertebral number of highly evolvable salamanders and newts (family Salamandrae) variably associated with climatic parameters. Contributions to Zoology 84: 85-113.
- Degani G. 2018. Genetic variation in xeric habitats of Triturus vittatus vittatus (Urodela) using mitochondrial DNA of 12S and16S, and nuclear gene, rhodopsin, on the southern border of its distribution. Intern. J. of Zool. Invest 4: 31-40.
- Degani G. 2017. Ecological, biological, behavioral and genetic adaptation to xeric habitats of Triturus vittatus vittatus (Urodela) on the southern border of its distribution. J Marine Sci. Res. Dev 7:9910-2155.
- Pearlson O, Bluestein L, Snir S, 2010. Molecular variation in Triturus vittatus vittatus (Urodela) from breeding sites near the southern extremity of its distribution revealed by DNA sequencing of mitochondrial cytochrome b gene and control region. Current Herpetology 29: 11-22.
- Pearlson O, Degani G. 2007. Molecular DNA variations among Triturus vittatus vittatus (Urodela) from different breeding sites at the southern limit of its distribution. Acta Herpetologica 2: 69-77.
- Degani G, Goldberg T, Gazith A, 2013. The DNA variation of Pseudepidalea viridis (Syn. Bufo viridis) from various habitats. Zoological Studies 52: 1-15.
- Degani G. 2016. Cannibalism, among other solutions of adaptation, in habitats where food is not available for salamandra infraimmaculata larvae diet in breeding places in xeric habitats. Open Journal of Animal Sciences 6: 31-41.
- Degani G, Goldberg T. 2013. Aquatic invertebrates in different bodies of water in a semi-arid zone. American Open Animal Science Journal 1: 1-15.
- Renan S, Gafny S, Bina Perl, 2017. Living quarters of a living fossil - uncovering the current distribution pattern of the rediscovered Hula painted frog (Latonia nigriventer) using environmental DNA. Molecular Ecology 26: 6801-6812.
- Degani G, Warburg MR. 1978. Population structure and seasonal activity of the adult Salamandra salamandra (L.) (Amphibia Urodela Salamandridae) in Israel. Journal of Herpetology 12: 437-444.
- Dessauer HC, Nevo E, Chuang KC. 1975. High genetic variability in an ecologically variable vertebrate, Bufo viridis. Biochemical Genetics 13:651-661.
- Warburg M. 2007. Longevity in Salamandra infraimmaculata from Israel with a partial review of life expectancy in urodeles. Salamandra 43: 21-34.
- Warburg MR. 1993. Long-term studies on population structure and reproductive strategies in a Salamandra salamandra population on Mt. Carmel. Israel Journal of Zoology 39: 77.
- Goldberg T, Pearlson O, Nevo E, 2009. Sequence analysis of mitochondrial DNA in Salamandra infraimmaculata larvae from populations in northern Israel. South American Journal of Herpetology 4: 268-274.
- Saitou N, Nei M. 1987.The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406-425.
- Tamura K, Dudley J, Nei M, 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution : 10.1093/Molbev/Msm092.
- Blaustein AR, Belden LK, Olson DH, 2001. Amphibian breeding and and climate change. Conservation Biology 15:1804-1809.
- Blaustein AR, Hokit DG, O’Hara RK, 1994. Pathogenic fungus contributes to amphibian losses in the Pacific north-west. Biological Conservation 67:251-254.
- Blaustein L, Friedman J, Fahima T, 1996. Larval salamandra drive temporary pool community dynamics: evidence from an artificial pool experiment. Oikos 76:392-402.
- Degani G. 1994. Ecophysiology of Salamandra salamandra at the southern limit of its distribution. Mertensiella 4:111-124.
- Degani G, Goldberg T. 2015. Effect of human activity in creating a death trap for Salamandra infraimmaculata seeking breeding places during colonization of new breeding sites. American Open Animal Science Journal 2:1-11.
- Degani G, Goldenberg S, Warburg MR. 1980. Cannibalistic phenomena in Salamandra-salamandra larvae in certain water bodies and under experimental conditions. Hydrobiologia 75: 123-128.
- Degani G, Mendelssohn H. 1978. The food of Salamandra salamandra (L.) tadpoles in Israel in different habitats. The Israel Journal of Ecology C:19-45.
- Degani G, Mendelssohn H. 1981. Interaction of amphibian larvae in a winter rain pond. Israel Journal of Zoology 30:99-100.
- Pearlson O, Degani G. 2007. Triturus v. vittatus (Urodela) larvae at various breeding sites in Israel. Progrese?i Perspective in Medicina Veterinar? - ucr?ri ?tiin?ifice 50:214-226.
- Pearlson O, Degani G. 2008. The life history of Triturus v. vittatus (Urodela) in various habitats. Asiatic Herpetological Research 11:91-95.
- Warburg MR, Degani G, Warburg I. 1979. Growth and population structure of Salamandra-salamandra larvae in different limnological conditions. Hydrobiologia 64:147-156.
- Degani G, Grosman U, Goldberg T, 2019. Damage to Salamandra infraimmaculata populations by human activity in creating water pits is a death trap in semi-arid habitats. Open J. Anim. Sci 9: 23-34.
- Degani G, Warburg M. 1995. Variation in brood size and birth rates of Salamandra salamandra (L.) (Amphibia, Urodela) from different habitats in northern Israel. Amphibia-Reptilia 16:341-349.
- Castellano S, Cucco M, Giacoma C. 2004. Reproductive investment of female green toads (Bufo viridis). Copeia 3:659-664.
- Geffen E, Gafny S, Gasith A. 1987. Contribution to the knowledge of the biology of the banded newt, Triturus vittatus vittatus, in rainpools in Israel. Israel Journal of Zoology 34:213-223.
- Degani G. 1985. Urea tolerance and osmoregulation in Bufo viridis and Rana ridibunda. Comparative Biochemistry and Physiology A 82:833-836.
- Degani G, Silanikove N, Shkolnik A. 1984. Adaptation of green toad (Bufo viridis) to terrestrial life by urea accumulation. Comparative Biochemistry and Physiology 77A:585-587.
- Hoffman J. Physiological response of Bufo viridis (Laurenti, 1768) populations across an aridity gradient Intern. J. Batracol 30 (2014):33-41.
- Katz U, Degani G, Gabbay S. 1984. Acclimation of the euryhaline toad Bufo viridis to hyperosmotic solution (NaCl, urea and mannitol). Journal of Experimental Biology 108:403-409.
- Katz U. 1973. Studies on the adaptation of the toad (Bufo viridis) to high salinities, oxygen consumption, plasma concentration and water content of the tissue. Journal of Experimental Biology 58: 785-796.
- Degani G, Goldenberg S, Warburg MR. 1983. Changes in ion, urea concentrations and blood plasma osmolarity of Pelobates syriacus juveniles under varying conditions. Comp. Biochem. Physiol 75A: 619-623.
- Degani G. 1986. Osmotic stress and osmoregulation of tadpoles and juveniles Pelobates syriacus. Comp. Biochem. Physiol 83A:365-370.
- Degani G. 1981. The adaptation of Salamandra salamandra (L.) from different habitats to terrestrial life. British Journal of Herpetology 6:169-172.
- Degani G. 1981. Salinity tolerance and osmoregulation in Salamandra salamandra (L.) from different populations. Journal of Comparative Physiology 145:133-137.
- Degani G, Warburg MR. 1984. Changes in concentrations of ions and urea in both plasma and muscle tissue in dehydrated Hylid anura. Comp. Biochem. Physiol 77A: 357 360.
- Degani G, Carmali D. 1988. Burrowing behavior of Pelobates syriacus. Bil. Behav 13: 22-29.
- Degani G, 1984. Temperature selection in Salamandra-salamandra larvae and juveniles from different habitats. Biology of Behaviour 9:175-183.
- Degani G, Mendelssohn H. 1981. Moisture as a factor influencing selection of hiding places by juvenile Salamandra-salamandra from semi arid habitats, in: H.I. Shuval (Ed.), International meeting of the 12th scientific meeting of the Israel Ecological Society. Balaban International Science Services, Philadelphia, PA, USA, Jerusalem, Israel: 49-56.
- Degani G, Warburg MR. 1980. The response to substrate moisture of juvenile and adult Salamandra salamandra (L.) (Amphibia; Urodela). Biol. Behav 5:281- 290.
- Degani G. 1982. The response to substrate moisture of Triturus v. vittatus (Jenys) (Amphibia, Urodela). Biol. Behav 3: 215-220.
- Degani G. 1984. The selection of hiding places by Triturus vittatus vittatus (Jenys). Biol. Behav 9:235-242.
- Degani G. 1987. Light color and moisture selection in hiding places of juvenile Tritutus vittatus (L.) Proceed. Fourth Ord. Gen. Meet. S.EH. Nijmegen: 111-114.
- Degani G, Goldberg T, Yom-Din S. 2013. The ecology and variation in DNA of Rana bedreagae from various breeding sites in North Israel. Res. Open. J. Anim. Sci 1: 1-14.
- Degani G. 2015. The effect of light and soil moisture on the environmental behavior of newts (Triturus vittatus vittatus, Urodela). Open Journal of Animal Sciences 5: 411-417.
- Pabijan M, Brown JL, Chan LM, 2015. Phylogeography of the arid-adapted Malagasy bullfrog, Laliostoma labrosum, influenced by past connectivity and habitat stability. Mol. Phylogenet. Evol 92:11-24.
- Degani G, Meltzer A. 1988. Oxygen consumption of a terrestrial toad (Bufo viridis) and semi-aquatic frog (Rana ridibunda). Comparative Biochemistry and Physiology A 89: 347-349.
- Warburg M. 1997. Ecophysiology of amphibians inhabiting xeric environments. Adaptations of desert organisms. Berlin: Springer-Verlag.
- Degani G. 1982. Water balance of salamanders Salamandra-salamandra from different habitats. Amphibia-Reptilia 2:309-314.
- Degani G. 1985. Water balance and body fluids of Salamandra-salamandra in their natural habitats in summer and winter. Comparative Biochemistry and Physiology A 82:479-482.
- Degani G, Pearlson O, Goldberg T. 2013. Impermanent breeding site selection - fitness of gonadal cycle and larval growth of Triturus vittatus vittatus (Urodela) from the southern limit of its distribution. Amer. Open. Anim. J 1:16-30.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 