Effects of Cold Saline Solution Infusion on Cooling Performance with Endovascular Therapeutic Hypothermia
Article Information
Luis Augusto Palma Dallan1,2*, Michael Dae3, Natali Schiavo Giannetti1, Tathiane Facholi Polastri1, Carlos Eduardo Rochitte1, Ludhmila Abrahao Hajjar1, Claudia Yanet Bernoche San Martin1, Felipe Gallego Lima1, Jose Carlos Nicolau1, Mucio Tavares de Oliveira Jr1, Luis Alberto Oliveira Dallan1, Expedito Eustaquio Ribeiro da Silva1, Roberto Kalil Filho1, Pedro Alves Lemos Neto1, Sergio Timerman1
1InCor – Heart Institute, School of Medicine, University of Sao Paulo, Sao Paulo, Brazil
2University Hospitals Cleveland Medical Center, Case Western Reserve University, School of Medicine, Cleveland, USA
3University of California, San Francisco Medical Center, CA, USA
*Corresponding author: Luis Augusto Palma Dallan, Department of Interventional Cardiology, InCor – Heart Institute, School of Medicine, University of Sao Paulo, Av Dr Eneas de Carvalho Aguiar, 44 – 2 andar – sala 11, Sao Paulo, SP, Brazil
Received: 24 June 2020; Accepted: 01 July 2020; Published: 20 July 2020
Citation: Luis Augusto Palma Dallan, Michael Dae, Natali Schiavo Giannetti, Tathiane Facholi Polastri, Carlos Eduardo Rochitte, Ludhmila Abrahao Hajjar, Claudia Yanet Bernoche San Martin, Felipe Gallego Lima, Jose Carlos Nicolau, Mucio Tavares de Oliveira Jr, Luis Alberto Oliveira Dallan, Expedito Eustaquio Ribeiro da Silva, Roberto Kalil Filho, Pedro Alves Lemos Neto, Sergio Timerman. Effects of Cold Saline Solution Infusion on Cooling Performance with Endovascular Therapeutic Hypothermia. Cardiology and Cardiovascular Medicine 4 (2020): 334-345.
View / Download Pdf Share at FacebookAbstract
Background: Therapeutic hypothermia (TH) reduces the damage by ischemia/reperfusion cell syndrome in cardiac arrests, however, the role of cold saline as an adjuvant therapy to endovascular cooling in STEMI remains controversial. The aim was the evaluation of cold saline infusion versus no cold saline concomitant to endovascular cooling in the development of a TH protocol in STEMIs.
Methods: Patients within the 6h onset of chest pain, presenting anterior or inferior STEMIs. TH induced through the Proteus® Endovascular System implant, by cooling for 18 minutes before coronary recanalization at the target temperature of 33±1.0°C before PCI. Patients were randomized to the administration of 1L cold saline solution at 1-4°C (CSS) versus no cold saline solution (NCSS). The primary endpoint was lower temperature by the opening of the vessel through primary PCI.
Results: TH was successfully induced in 10 patients – 5 in the CSS group and 5 in the NCSS group. After 18 minutes of TH, all patients (100%) had already achieved core temperature <35ºC, and 90% of them within the pre-specified 33.0 ± 1.0ºC target temperature. In the CCS group, all five patients (100%) reached the target temperature, as compared with four patients (80%) in the NCCS group. The mean temperature reached was 33.0ºC (± 0.6) in the CCS group as compared to 33.3ºC (± 0.8) in the NCCS group (p=0.59), thus 0.3ºC lower temperature by the time of balloon angioplasty favoring the CSS group as compared with the NCSS group. After 30 min, the mean temperature was 32ºC (± 0.4ºC) in the CCS and 32.4ºC (± 0.5ºC) in the NCCS group (p=0.12). The MACE rates were similar between both groups (p=ns).
Conclusions: We conclude that when using more powerful endovascular cooling
Keywords
Therapeutic hypothermia; ST elevation
Therapeutic hypothermia articles, ST elevation myocardial infarction (STEMI) articles, Percutaneous coronary intervention (PCI) articles, Acute coronary syndrome (ACS) articles, Coronary disease articles
Article Details
1. Introduction
Endovascular therapeutic hypothermia (ETH) is performed to reduce ischemia/reperfusion cell syndrome damage in cardiac arrests [1], however its role in ST-segment elevation myocardial infarction (STEMI) patients still remains controversial [2-9]. Experimental studies showed that mild hypothermia, if rapidly induced before the reperfusion of acute coronary occlusion, can reduce infarct size (IS) [11, 12]. So a fast cooling prior to reperfusion may be effective adjunct to primary percutaneous coronary intervention (PCI) in STEMI patients to reduce IS and to improve cardiac outcomes [13, 14]. The development of powerful endovascular cooling systems, which are able to cool down the patient to low temperatures in a few minutes [15], created the concerning regarding a possible futility of the traditional endovascular cold saline infusion in the ETH protocol, and the role of cold saline as an adjuvant therapy to endovascular cooling in STEMI remains unclear. It is difficult to estimate the amount of fluid required to achieve hypothermia merely on the basis of body weight, and there is no standardized load management regarding the administration of cold saline [16]. It is still unknown if with the more powerful ETH systems, saline infusion still contributes to cooling dose, so we ought to determinate the current role of cold saline in the ETH era. The aim of this study was to evaluate if cold saline endovascular infusion concomitant to endovascular cooling is better than no cold saline infusion in the current protocol for ETH in STEMIs.
2. Methods
This was a single-center, prospective, interventional, randomized controlled, two-arm trial, performed at InCor – Heart Institute – Clinical Hospital, University of Sao Paulo. Patients admitted to the emergency department within up to 6 hours of the chest pain onset, presenting anterior or inferior STEMIs, and eligible for PCI were included. All procedures were carried out in accordance with the Declaration of Helsinki and the local/national ethics committees approved the study protocol. All patients gave written informed consent prior to inclusion in the study. An independent Data and Safety Monitoring Board, consisting of independent physicians unrelated to the trial sponsor and operational leadership, monitored the safety of the study based on access to unblinded data.
The study enrolled patients ≥ 18 years of age with a duration of symptoms of ≤ 6 hours presenting with an anterior or inferior STEMI with persistent ST-segment elevation of > 0.2 mV in two contiguous leads at arrival to the catheterization laboratory and before randomization. Patients with resuscitated cardiac arrest, previous acute myocardial infarction, PCI or coronary artery bypass grafting, Killip class II-IV at presentation, atrial fibrillation, end-stage kidney disease or hepatic failure, recent stroke, coagulopathy and pregnancy were excluded. Eligible patients were randomized electronically 1:1 using a computer generating system (online website Sealed Envelope™) [17] to the forced infusion of 1L of cold saline solution infusion at 1-4°C using pressure bags (CSS group) versus no cold saline solution (NCSS group). All the patients were submitted to ETH using ZOLL® Proteus™ Intravascular Temperature Management System™ (ZOLL Medical Corporation, Chelmsford, MA, USA). According to technical specifications, it is significantly more powerful than devices used in previous trials, with up to four times the power as compared the current commercial available devices (430 watts, cooling rate 9.6°C/hour).
All the patients were submitted to primary percutaneous coronary intervention (PCI) plus standard of care. All patients received acetylsalicylic acid, heparin and P2Y12 receptor blockade. Glycoprotein IIb/IIIa inhibitors were administered at the discretion of the treating physician. Initially it was administered 60 mg of oral buspirone and pethidine (meperidine) as an intravenous loading dose of 1 mg/Kg (maximum 100 mg) or 0.5 mg/Kg if the patient had already received morphine. After 15 minutes, an additional dose of 0.5 mg/Kg was given and continued as an infusion at 25 mg/hour (up to 80 kg patient) or 35 mg/hour (>80 kg patient) for the duration of the device deployment. Patients were placed on a Bair Hugger™ (3M, Maplewood, MN, USA) which covered the catheterization table for skin counter warming. Patients were supposed to remain awaken and comfortable all over the procedure, without shivering. Respiratory rate and pulse oximetry were monitored with targets of >10 breaths/minute and arterial oxygen saturation of ≥90%. In the CCS group, cooling was initiated with a forced infusion of 1L of cold saline (1-4°C) using pressure bags and continued by the ZOLL Proteus Intravascular Temperature Management System. In the NCCS group, cooling was initiated directly by the ETH system. The cooling catheter was inserted via the femoral vein into the inferior vena cava with the tip positioned at the level of the diaphragm.
The Proteus temperature probe (X-Probe; ZOLL) was put through the catheter lumen to the right atrium for continuous measurement of core temperature. The console temperature was set to 32.0°C and cooling at maximum power started. Following placement and activation of the cooling catheter, arterial puncture was performed and coronary angiography/PCI conducted in a standard way. An interval of at least 18 minutes of endovascular cooling from catheter activation to coronary guidewire passing across the acute occlusion was performed. Cooling was maintained for three hours, followed by active rewarming at the rate of 1.0°C/hour to attain 36.0°C. The catheter was then removed by manual compression. Shivering was continuously assessed by the bedside shivering assessment scale (BSAS) using the following categories:
- No shivering on palpation of the masseter, neck or chest wall.
- Shivering localized to the neck and/or thorax only.
- Shivering with gross movement of the neck, thorax and upper extremities.
- Shivering involving gross movements of the trunk, upper and lower extremities.
If BSAS was ≥2, additional boluses of pethidine (25 mg) were used and infusion increased to a maximum of 35 mg/hour. If shivering persisted, the Proteus target temperature was raised stepwise by 0.5°C until shivering disappeared.
2.1 Study endpoints
The primary endpoint was lower core temperature at 18 minutes of cooling with ETH Proteus system, with the pre-specified target temperature < 33.0 ± 1.0ºC. The secondary endpoints were death, major adverse cardiac events (MACE), severe arrhythmias, stent thrombosis, major bleeding, infection and severe complications at 30 days. Time of reperfusion was defined by the guidewire crossing the culprit lesion. Door to balloon time (DTB) was defined from the electrocardiogram (EKG) to guidewire crossing the lesion. If there was no lesion, it was defined from EKG to the beginning in the angiogram. The delta-temperature was defined by the difference between the core baseline temperature and the core temperature after 18 minutes of ETH using ZOLL® Proteus™ Intravascular Temperature Management System™. Major adverse cardiac events (MACE) was defined as the composite of death, myocardial infarct or need for revascularization.
2.2 Statistical analysis
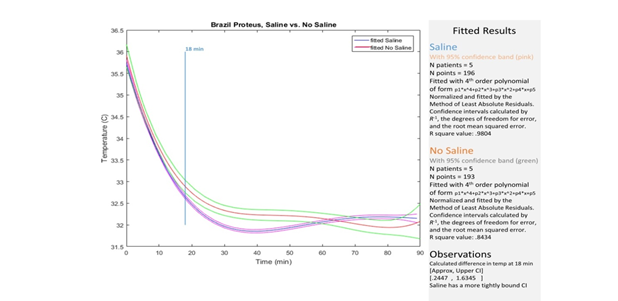
In this study, evaluation of variables was calculated with number and proportion with exact 95% confidence interval. For all clinical, angiographic, periprocedural characteristics and safety outcomes, mean, standard deviation, median, range or frequency and proportion were reported. For categorical variables, Fisher’s exact test or the chi-square test was used to compare between the two treatment groups. For continuous variables, the Wilcoxon rank-sum test and t-test were used to compare between the two treatment groups as appropriate. No imputation was carried out for missing data. All tests were two-sided. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 17.0 (IBM SPSS Inc., Chicago, IL, USA). ClinicalTrials.gov identification: NCT02664194. The graphic construction was fitted with 4th order polynomial of form p1*x^4+p2*x^3+p3*x^2+p4*x+p5. Normalized and fitted by the Method of Least Absolute Residuals. Confidence intervals were calculated by R-1, the degrees of freedom for error, and the root mean squared error. R square value was calculated in each group.
3. Results
From January 2017 to July 2017, among 103 screened patients with anterior and inferior STEMI, 10 patients (9.7%) were enrolled and randomized to either the CSS group (n=5) or the control group (n=5). The clinical characteristics of each group are shown in Table 1. The mean age was 63.6 years, predominantly male (80%), type 2 diabetes (90%), hypertension (80%) and dyslipidemia (90%). There were high risk patients with high risk outcome scores, including average TIMI risk score 4.6, Grace risk score 131.3 and ROXANA bleeding risk score 17.6. We observed predominantly anterior wall involvement (70%), and the anterior descending coronary artery (LAD) was the most predominant culprit vessel (70%), followed by the right coronary artery (RCA) in 30% of cases. The initial TIMI flow was 0 in 90% of cases, because 1 patient already presented TIMI III flow by the time of the procedure.
The average door-to-balloon (DTB) time was 77.2, and all patients (100%) underwent primary percutaneous coronary intervention (PCI), 9 of them (90%) had DTB time under 90 minutes. The mean weight was 75 Kg (± 9) in the CCS group versus 80Kg (± 14) in the NCCS group (p = ns). The mean body-mass index was 75 Kg (± 9) in the CCS group versus 80Kg (± 14) in the NCCS group (p = ns). The median door-to-balloon time was 81.2 (± 18.4) in the CCS group as compared to 74.1 (± 25.1) in the NCCS group (p = ns), so there was no cooling-related time delay to reperfusion regarding cold saline infusion. None of the results were statistically significant. The baseline and 18 minutes’ core temperatures are described in Table 2. After 18 minutes of ETH, 10 patients (100%) already had core temperature < 35ºC, and 90% of them within the pre-specified 33.0 ± 1.0ºC target temperature. In the CCS group, all five patients (100%) reached the target temperature, as compared with four patients (80%) in the NCCS group. The variations among groups are shown in Table 3. The mean baseline core temperature was 36ºC (± 0.6ºC) in the CSS group versus 36.3ºC (± 0.5ºC) in the NCCS group (p=0.27). The mean temperature reached at 18min ETH was 33.0ºC (± 0.6ºC) in the CCS group as compared to 33.3ºC (± 0.8ºC) in the NCCS group (p=0.59). The mean delta temperature (difference between temperature reached after 18 minutes and the baseline temperature) was the same between the two groups (3.0ºC, p = ns) After 30 minutes, The mean temperature reached was 32ºC (± 0.4ºC) in the CCS group as compared to 32.4ºC (± 0.5ºC) in the NCCS group (p=0.12), as shown in Figure 1. There were no significant differences in adverse events between the two groups, including death, paroxysmal atrial fibrillation, ventricular fibrillation, renal impairment, stent thrombosis, infection and major bleeding (p=ns) (Table 4). From all 10 patients, only one died in the NCCS group (from sepsis shock after 10 days) and none in the CCS group (p=ns). Two patients (40%) in the NCCS group presented ventricular fibrillation, as compared to none patients in the CCS group (p=ns), but all those malign arrhythmias were solved immediately by the proper approach and no patient died from it. The atrial fibrillation resolved spontaneously to sinus rhythm in all patients during the rewarming phase. In the NCCS group, two patients (40%) had stent thrombosis, as compared to none in the CCS group (p = ns). There were no vascular complications, significant bleeding or infection related to the access site.
|
Cold Saline Solution Group |
Non-Cold Saline Solution Group |
Total (p= ns) |
|
|
Number of patients |
5 (50%) |
5 (50%) |
10 (100%) |
|
Average age (years) |
64.2 (± 11.1) |
63 (± 10.7) |
63.6 (± 10.2) |
|
Male gender |
4 (80%) |
4 (80%) |
8 (80%) |
|
Type 2 Diabetes |
5 (100%) |
4 (80%) |
9 (90%) |
|
Hypertension |
5 (100%) |
3 (60%) |
8 (80%) |
|
Dyslipidemia |
4 (80%) |
5 (100%) |
9 (90%) |
|
Tabagism |
0 |
2 (40%) |
2 (20%) |
|
Weight (Kg) |
75 (± 9) |
80 (± 14) |
77.4 (± 10) |
|
Body-Mass index (BMI) |
25.6 (± 0.7) |
28.5 (± 4.8) |
27.1 (± 3.5) |
|
GRACE risk score |
124.2 (± 22.5) |
138.4 (± 25.3) |
131.3 (± 23.8) |
|
TIMI risk score |
3.8 (± 2.1) |
5.4 (± 2.4) |
4.6 (± 2.3) |
|
ROXANA risk score |
18.2 (± 7.7) |
17.1 (± 6.9) |
17.6 (± 6.9) |
|
Door-to-balloon time |
81.2 (± 18.4) |
74.1 (± 25.1) |
77.2 (± 28.9) |
|
Door-to-balloon time <90 min |
4 (80%) |
5 (100%) |
9 (90%) |
|
Anterior wall myocardial infarct |
3 (60%) |
4 (80%) |
7 (70%) |
|
Inferior wall myocardial infarct |
2 (40%) |
1 (20%) |
3 (30%) |
|
Infarct culprit artery |
|||
|
Left descending artery (LAD) |
3 (60%) |
4 (80%) |
7 (70%) |
|
Right cororany artery (RCA) |
2 (40%) |
1 (20%) |
3 (30%) |
|
Initial TIMI 0 flow |
5 (100%) |
4 (80%) |
9 (90%) |
|
Final TIMI 3 flow |
5 (100%) |
5 (100%) |
10 (100%) |
|
Left ventricle ejection fraction (LVEF %) |
45% (± 14.1) |
37% (± 6.7) |
41% (± 11.2) |
Table 1: Clinical, angiographic and peri-procedure characteristics.
|
Subject |
Weight (Kg) |
Temp Start (ºC) |
Temp at 18 min (ºC) |
Cold Saline |
|
1 |
65 |
36.63 |
33.25 |
1L |
|
2 |
100 |
36.76 |
34.26 |
No |
|
3 |
70 |
35.98 |
32.55 |
No |
|
4 |
70 |
36.16 |
32.6 |
No |
|
5 |
90 |
36.31 |
32.96 |
800mL |
|
6 |
75 |
35.91 |
32.41 |
1L |
|
7 |
72 |
35.09 |
32.59 |
1L |
|
8 |
73 |
35.97 |
33.91 |
1L |
|
9 |
75 |
36.31 |
33.08 |
No |
|
10 |
84 |
36.3 |
33.8 |
No |
|
Mean |
77.4 (±10.7) |
36.1 (± 0.46) |
33.1 (± 0.65) |
Table 2: Description of baseline and 18 minutes’ core temperatures.
|
Temperature |
Saline - CCS group |
No Saline - NCCS group |
p value |
|
Mean baseline temperature |
36 (± 0.6) |
36.3 (± 0.5) |
0.27 |
|
Mean 18 min temperature |
33.0 (± 0.6) |
33.3 (± 0.8) |
0.59 |
|
Mean delta-Temperature |
3.0 (± 0.6) |
3.0 (± 0.5) |
0.63 |
|
Mean 30 min temperature |
32.0 (± 0.4) |
32.04 (± 0.5) |
0.12 |
|
Temperature < 35ºC at 18 min |
5 (100%) |
5 (100%) |
1 |
|
Temperature 33ºC ±1.0C at 18 min |
5 (100%) |
4 (80%) |
0.8 |
Table 3: Comparison of temperature variations between CCS and NCCS groups.
|
Saline - CCS group |
No Saline - NCCS group |
Total (p= ns) |
|
|
Death |
0 |
1 (20%) |
1 (10%) |
|
Infarct |
0 |
2 (40%) |
2 (20%) |
|
Revascularization |
0 |
2 (40%) |
2 (20%) |
|
MACE |
0 |
2 (40%) |
2 (20%) |
|
Ventricular fibrillation |
0 |
2 (40%) |
2 (20%) |
|
Paroxistical atrial fibrillation |
2 (40%) |
1 (20%) |
3 (30%) |
|
Cardiogenic shock |
1 (20%) |
2 (40%) |
3 (30%) |
|
Stent Thrombosis |
0 |
2 (40%) |
1 (10%) |
|
Renal impairment |
1 (20%) |
2 (40%) |
3 (30%) |
|
Infection |
2 (40%) |
2 (40%) |
4 (40%) |
|
Major bleeding |
1 (20%) |
1 (20%) |
2 (20%) |
MACE: major adverse cardiac events
Table 4: Adverse events.
4. Discussion
As far as we know, this is the first trial demonstrating the role played by cold saline in the endovascular cooling procedure. The current ETH protocols advocate that 1 liter of saline solution frosted to 1 – 4ºC should be infused in the patient adjunctive to the ETH catheter, but no study had ever been done before evidencing the impact of this additional saline infusion. This is supposed to make the cooling procedure faster and easier, so that temperatures below 35ºC, ideally under 33ºC, can be reached in less than 18 minutes [15].Our data demonstrated that ETH is feasible and that low target temperatures of 33.0 ± 1.0ºC could be reached in 18 minutes with the new Proteus™ Cooling System, much more powerful than the current commercial cooling devices [15]. At 18 minutes of ETH, all 10 patients (100%) reached core temperature < 35ºC, and 90% of them within the pre-specified 33.0 ± 1.0ºC target temperature. In the CCS group, all five patients (100%) reached the target temperature, as compared with four patients (80%) in the NCCS group. The additional 30% decrease delivered by the cold saline solution may have had some influence in this result, but the total number of patients was low to stablish definitive conclusions. The current ETH algorithms are based upon COOL-MI [18] data, which was performed using less powerful cooling equipment, which take over an hour to reduce the core temperature.
Although an incremental decrease in 0.3ºC by the time of reperfusion, as seen in Table 3 and Graphic 1, does not look quite a lot, especially with powerful ETH devices such as Proteus, it represents an additional 30% reduction as compared to the previous trials’ reduction of 1ºC [18]. Therefore, in the real-world scenario in tertiary centers, in which the current commercial available cooling devices are less powerful [15], this additional 30% decrease is really important and significant in STEMIs. Even though randomized clinical trials including COOL MI [18], ICE-IT [19], CHILL MI [20] and VELOCITY [21] failed to show a significant reduction in infarct size, endovascular cooling appears to be safe and well tolerated. Despite neutral overall results, subsequent unpublished post hoc subgroup analysis of COOL MI [21] and ICE-IT [22], and combined analysis of RAPID MI-ICE [22] and CHILL MI [20] showed significant reduction in infarct size in a subgroup of early presenters with anterior STEMI who were cooled below 35°C prior to reperfusion [23]. Thereby, benefits of therapeutic hypothermia might be achieved by using a rapid cooling to decrease core temperature below 35ºC prior to the opening of acute coronary occlusion [23]. In our trial, only one (10%) patient underwent mechanical ventilation due to cardiogenic shock, and all other patients (90%) had a well-tolerated ETH procedure, conscious, calm and awake during the whole PCI.
ETH was feasible without any further delays within the 90 minutes’ door-to-balloon (DTB) guidelines recommendation, once it was done at the Cath Lab concomitant to the angiogram [15]. This was possible because of this new powerful ETH technology, where the cooling catheter is inserted through a simple femoral vein puncture, which takes less than 5 minutes to be done by a non-specialist physician. Other recent similar trials failed to show no delays in DTB when EHT was performed. In the COOL AMI EU Pilot trial [15], the mean DTB time was 105 min, and there was a 17 minutes cooling-related delay in the DTB. Maybe we got lower DTB because of intensive simulation and training before starting the Cooling Protocol. By that, focusing on optimization of the logistics from the ER to the Cath Lab, and them to the Coronary Unit, there were no unnecessary delays and ideal DTB was reached. In our sub-study, the mean DTB time was 77.2 (± 28.9), and 90% (9) of the patients got primary PCI with DTB < 90 minutes, which is a benchmark of a center of excellence, once the guidelines suggest that DTB should be < 90 minutes in more than 90% of patients in top-performing institutions [8, 24, 25, 26, 27, 28]. According to previous clinical trials, there was significant reduction in infarct size in a subgroup of early presenters with anterior STEMI who were cooled below 35°C prior to reperfusion [18, 19, 20, 22, 23, 25]. Thereby, our findings that it is possible to reach a mean temperature of 33.3°C by the time of coronary reperfusion, i.e., way below 35°C, may signalize that all benefits from ETH might be achieved. Even though our sub-study demonstrated adverse events including death, paroxysmal atrial fibrillation, ventricular fibrillation, renal impairment, stent thrombosis, infection and major bleeding, previous trials already demonstrated the safety of ETH for STEMI patients [15]. The patients presented arrhythmias during cooling, often disappearing during the rewarming phase, especially paroxysmal atrial fibrillation, so the concern of major adverse events is never forgotten. Nevertheless, further studies are needed to incisively demonstrate the safety of ETH in the STEMI scenario.
Limitations
Our results, however, should be interpreted in the light of several limitations. Firstly, the study was single-center, with a small number of patients included in this sub-analysis, which markedly contributed to the non-significant statistical results. Secondly, these were the first 10 patients included from a total of 50 patients by the end of the main trial, so it was performed during the learning curve for the physicians, causing eventual delaying of the procedure time. Last, there was no physician exclusively responsible for the cooling procedure, it was performed concomitant to the interventional procedure, which might imply in delays in the DTB.
5. Conclusions
As far as we know, this is the first trial showing the role played by cold saline in the endovascular cooling procedure. We conclude that when using more powerful endovascular cooling systems such as Proteus, concomitant infusion of cold saline does not significantly improve the cooling rate, but cold saline may still play an important role in less powerful systems (such as the current available commercial devices) and thus should continue to be one of the steps in the ETH protocol.
References
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357 (2007): 1121-1135.
- Piegas LS, Timerman A, Feitosa GS, Nicolau JC, Mattos LAP, Andrade MD, et al. V Diretriz da Sociedade Brasileira de Cardiologia sobre Tratamento do Infarto Agudo do Miocardio com Supradesniivel do Segmento ST. Arq Bras Cardiol 105 (2015): 1-105.
- Maria Margarita Gonzalez, Sergio Timerman, Renan Gianotto de Oliveira, Thatiane Facholi Polastri, Luis Augusto Palma Dallan, Sebastião Araújo, et al. Sociedade Brasileira de Cardiologia. I Diretriz de Ressuscitacao Cardiopulmonar e Cuidados Cardiovasculares de Emergeencia da Sociedade Brasileira de Cardiologia. Arq Bras Cardiol 101 (2013): 1-221.
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118 (2008): 2452-2483.
- Robert W Neumar, Michael Shuster, Clifton W Callaway, Lana M Gent, Dianne L Atkins, Farhan Bhanji, et al. Part 1: executive summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132 (2015): S315-S367.
- Nolan JP, Hazinski MF, Aicken R, Bhanji F, Billi JE, Callaway CW, et al. Part 1: executive summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation 95 (2015): e1-e32.
- Patrick T O'Gara, Frederick G Kushner, Deborah D Ascheim, Donald E Casey Jr, Mina K Chung, James A de Lemos, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127 (2013): e362-e425.
- Glenn N Levine, Eric R Bates, James C Blankenship, Steven R Bailey, John A Bittl, Bojan Cercek, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: an Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol (2015).
- Koenraad G Monsieurs, Jerry P Nolan, Leo L Bossaert, Robert Greif, Ian K Maconochie, Nikolaos I Nikolaou, et al. European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 95 (2015): 1-80.
- Mary Fran Hazinski, Jerry P Nolan, Richard Aickin, Farhan Bhanji, John E Billi, Clifton W Callaway, et al. Part 1: executive summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 132 (2015).
- Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol 270 (1996): H1189-H1199.
- Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol Heart Circ Physiol 282 (2002): H1584-H1591.
- Erlinge D. A Review of Mild Hypothermia as an Adjunctive Treatment for ST-Elevation Myocardial Infarction. Ther Hypothermia Temp Manag 1 (2011): 129-141.
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123 (2013): 92-101.
- Noc M, Erlinge D, Neskovic A, Kafedzic S, Merkely B, Zima E, et al. COOL AMI EU Pilot Trial: a multicentre, prospective, randomized controlled trial to assess cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction. Eurointervention 13 (2017): 1-9.
- Willms JF, Boss O, Keller E. Safety, Feasibility, and Efficiency of a New Cooling Device Using Intravenous Cold Infusions for Fever Control. Neurocrit Care 30 (2019): 149-156.
- Electronic randomization website Sealed Envelope.
- Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction – COOL MI Trial. J Am Coll Cardiol 40 (2002): 1928-1934.
- O’Neill WW, Dixon SR, Grines CL. The year in interventional cardiology. J Am Coll Cardiol 45 (2005): 1117-1134.
- Erlinge D, Götberg M, Lang I, Holzer M, Noc M, Clemmensen P, et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol 63 (2014): 1857-1865.
- Nichol G, Strickland W, Shavelle D, Maehara A, Ben-Yehuda O, Genereux P, et al. VELOCITY Investigators. Prospective, multicenter, randomized controlled pilot trial of peritoneal hypothermia in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 8 (2015): e001965.
- Götberg M, Olivecrona GK, Koul S, Carlsson M, Engblom H, Ugander M, et al. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction - RAPID-MI ICE Trial. Circ Cardiovasc Interv 3 (2010): 400-407.
- Erlinge D, Götberg M, Noc M, Lang I, Holzer M, Clemmensen P, et al. Therapeutic hypothermia for the treatment of acute myocardial infarction-combined analysis of the RAPID MI-ICE and the CHILL-MI trials. Ther Hypothermia Temp Manag 5 (2015): 77-84.
- Götberg M, Olivecrona GK, Engblom H, Ugander M, van der Pals J, Heinberg E, et al. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc Disord 8 (2008): 7.
- Villablanca PA, Rao G, Briceno DF, Lombardo M, Ramakrishna H, Bortnick A, et al. Therapeutic hypothermia in ST elevation myocardial infarction: a systematic review and meta-analysis of randomised control trials. Heart (2016): 1-8.
- Daniel S Menees, Eric D Peterson, Yongfei Wang, Jeptha P Curtis, John C Messenger, John S Rumsfeld, et al. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med 369 (2013): 901-909.
- Harlan M Krumholz, Jeph Herrin, Lauren E Miller, Elizabeth E Drye, Shari M Ling, Lein F Han, et al. Improvements in door-to-balloon time in the United States, 2005 to 2010. Circulation 124 (2011): 1038-1045.
- Stone GW, Selker HP, Thiele H, Patel MR,
- Udelson JE, Ohman EM, et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol 67 (2016): 1674-1683.


 Impact Factor: * 3.5
Impact Factor: * 3.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 14.80%
Acceptance Rate: 14.80%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 