In Vitro Evaluation of The Antioxidant and Anti-Skin Aging Properties of Green Algal Sulfated Polysaccharides
Article Information
Berness Falcao, Jyoti Vishwakarma, Helly Jadav, Sirisha L. Vavilala*
School of Biological Sciences, UM-DAE-Centre for Excellence in Basic Sciences, Nalanda Building, Opp. National Centre for Nanoscience and Nanotechnology, University of Mumbai, Kalina campus, Santacruz (East), Mumbai 400098, India.
*Corresponding Author: Sirisha L. Vavilala, Assistant Professor, School of Biological Sciences, UM-DAE-Centre for Excellence in Basic Sciences, Nalanda Building, Opp. National Centre for Nanoscience and Nanotechnology, University of Mumbai, Kalina campus, Santacruz (East), Mumbai, 400098, India
Received: 03 June2020; Accepted: 14 June 2020; Published: 17 June 2020
Citation:
Berness Falcao et al. In Vitro Evaluation of The Antioxidant and Anti-Skin Aging Properties of Green Algal Sulfated Polysaccharides. Archives of Microbiology & Immunology 2020; 4 (2): 75-90.
View / Download Pdf Share at FacebookAbstract
Abstract
Skin aging is a natural phenomenon witnessed by humans. However various intrinsic and extrinsic factors lead to early skin aging. There have been a variety of approaches to combat skin aging one approach uses antioxidants that are known to fight oxidative stress as well as combat problems of aging. In this study, the antioxidant and anti-skin aging properties of sulfated polysaccharides (SPs) from fresh water microalgae Chlamydomonas reinhardtii (Cr) are evaluated. Sulfated polysaccharides were isolated by hot water extraction method and purified by anion-exchange chromatography. The biochemical composition of the extract showed carbohydrate content of 785.07 mg/g, 324.26 mg/g of sulphate and 393.32 mg/g of uronic acid. These extracts which are enriched with SPs were further used for checking antioxidant and anti-skin aging properties. Cr-SPs showed Superoxide anion scavenging activity of 38-92% at 0.1-2 mg/mL, 51-89% of nitric oxide scavenging ability at 0.2-2 mg/mL, 10-58% of hydrogen peroxide scavenging ability at 1- 10 mg/mL, and 28-68% of ferric ion reducing potential at 0.5-5 mg/mL respectively. Furthermore, Cr-SPs showed 90% anti-elastase enzyme activity at 1 mg/mL, 83% and 89% anti-collagenase and anti-hyaluronidase activities at 1 mg/mL and 2 mg/mL respectively. These promising antioxidant and anti- skin aging properties of Cr-SPs pave way to explore the potential of Cr-SPs in cosmeceutic and pharmaceutical formulations as anti-skin aging agents in a cost-effective manner.
Keywords
Skin aging; Oxidative stress; Chlamydomonas reinhardtii; Antioxidant; Sulfated polysaccharides; Anti-collagenase; Anti-elastase; Anti-hylauronidase
Skin aging articles, Oxidative stress articles, Chlamydomonas reinhardtii articles, Antioxidant articles, Sulfated polysaccharides articles, Anti-collagenase articles, Anti-elastase articles, Anti-hylauronidase articles
Article Details
Introduction
Aging is the atrophy that occurs with time. Skin aging is a slow and complex process, it causes structural changes in skin such as thinning, dryness, laxity, fragility, enlarged pores, fine lines, and wrinkles. Aging is induced by extrinsic and intrinsic factors resulting in structural disorganization in extracellular matrix components. Intrinsic factors involve metabolic activities, hormonal responses, stress, lifestyle etc. Extrinsic factors involve UV exposure, pollution, reactions with toxins etc. [18, 47]. Even though there are many more factors known that lead to skin damage, the exact mechanism of skin aging is not known. However, formation of reactive oxygen species in excess can be a major contributor to skin aging [24].
Dermis is the innermost layer of the skin which is composed of three crucial enzymes collagenase, elastase and hyaluronidase that are responsible for skin aging. Collagen is the most abundant protein present in the dermal layer. About 70% of the dermis dry weight consist of the collagen protein [67]. Skin layers show presence of collagen type I, III, IV and V [43]. Cutis Laxa is a collagen defect observed in skin when collagen fibres are unable to crosslink with the help of Lysyl oxidase. In a similar manner lack of Collagen III fibres makes the skin and blood vessels more fragile. This phenomenon is termed as Ehlers–Danlos syndrome. Elastin are less abundant as compared to collagen in the skin. 1-2% of the skin layer is composed of cross-linked elastin fibres [67]. Elastin fibres play a key role in providing elasticity to the skin. In case of skin aging, elastosis is observed that shows an altered morphology and functioning of the elastin fibres. Glycosaminoglycans (GAG) are the most abundant type of polysaccharides and are the major organic extracellular matrix component. Glycosaminoglycans (GAG) include Hyaluronic acid (HA), heparin sulfate (HS), chondroitin sulfate, and dermatan sulphate. HA is present both in the dermal and epidermal layer and shows water holding activity [43]. The visible signs of skin aging are spots and wrinkles, reduction in elasticity and firmness, thinning of the dermal matrix and inflammation. These skin proteins are degraded by the action of elastase, collagenase and hyaluronidase enzymes [5]. Therefore, the decline in the activity of these enzymes is an important way of monitoring the aging phenomenon. It is observed that higher amount of reactive oxygen species in the body can lead to increased activity of these enzymes [48].
Reactive oxygen species (ROS) are a result of imbalance in the free radicals generation and free radical scavenging ability of the body. There are broadly 2 types of ROS generated: oxygen molecule with unpaired electrons and oxygen molecules in their excited state [41]. Even though free radicals are generated as by-products of metabolic activities, exposure to UV radiation, smoking etc. can lead to higher production of these radicals [8]. ROS can have several detrimental effects like aging of skin, cancer, cardiac diseases, brain disorders etc. [9]. The human body has enzyme-based antioxidants like catalase, superoxide dismutase etc. and non-enzymatic scavenging substances like carotenoids, flavonoids etc. [9, 57]. But these inherent antioxidants may not be able to scavenge the excessive radicals produced. Therefore various plant sources are used as external supplementation of antioxidants. Antioxidants can also be obtained from macro and micro algae. Various secondary metabolites produced by macro-algae are reported to have antioxidant and anti-aging properties [14].
Algae are a group of aquatic organisms which are a habitat of marine water, fresh water or even soil dwelling. Based on their cellular nature, they are classified into macro-algae and microalgae. Extracts of seaweeds that come under the category of macro-algae are used in cosmetics, food supplements etc. Microalgae are unicellular in nature but undergo photosynthesis like most macro-algae. Production of pigments like beta-carotene and astaxanthin, and polymeric units like alginate, carrageenan and agar by these microalgae have also contributed in the food industry [59]. Microalgae are known to produce various compounds like fatty acids, peptides, terpenes, sulfated polysaccharides, alcohols, ketones, aldehydes etc. which showed wide range of bioactivities [5].
Sulfated polysaccharides are anionic polymeric units studied mostly in macro-algae that show antibacterial, antioxidant, immunomodulatory activities. Their activity is correlated to the sulphate content in the polysaccharide and their position, molecular weight, sugar content etc. [41]. Earlier studies on SPs from Porphyridium cruentum showed increased antioxidant activities and also inhibition of aging enzymes [5, 9]. Chlamydomonas reinhardtii is a green-microalgae and is known to produce bioactive SPs upon exposure to stress. These SPs were found to have a lot of bioactive properties [27, 13, 66, 51]. However, the anti-skin aging properties of Cr-SPs have not been explored till now. In the current study the antioxidant and anti-skin aging properties of Cr-SPs have been explored which will help pave way to develop these natural compounds as skin care formulations.
Materials and Methods
Sub-Culturing and Maintenance of Culture
Chlamydomonas reinhardtii (CC124 strain) was procured from Chlamydomonas Genetic Centre, Duke University, USA (http://www.chlamy.org). The cells were grown on TAP agar plates. The cells were sub cultured every 7-15 days in agar plates. For experimental purposes, colonies from these plates were inoculated in liquid TAP media and kept in shaker conditions at 120 rpm, 25°C for 72 h.
Extraction and Purification of Sulfated Polysaccharides
The extraction and purification of SPs from Chlamydomonas reinhardtii was carried out according to a modified hot water extraction method as mentioned in Kamble et al. 2018 [27]. The biochemical composition of the extract was carried out using standard protocols and the purified extract having highest carbohydrate and sulfate content was used to check its antioxidant and anti-skin aging properties.
Determination of Antioxidant Properties of the Cr-Sps
Ferric Ion Reducing Anti-Oxidant Potential (FRAP) Assay.
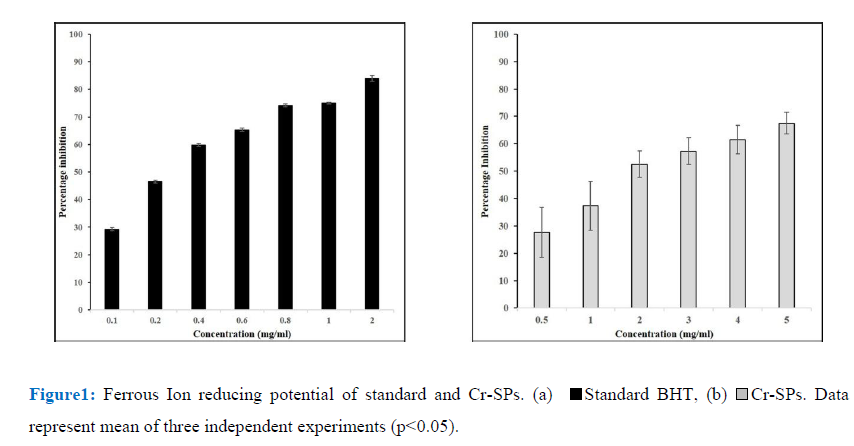
The FRAP assay was used to determine the ferric ion chelating property of Cr-SPs by a modified method described by Oyaizu et al. (1986) [50]. Briefly, 100µL of different concentrations of Cr-SPs (0.1-2 mg/ml), 900 µL of 96% ethanol, 5ml of distilled water, 1.5ml of 1% potassium ferricyanide and 0.5 mL of 1% SDS were added and mixed well. Then 0.5mL of 0.2% ferric chloride was added and incubated in a hot water bath at 50°C for 20 min. The increase in absorbance values at 750 nm which measures the reducing potential of Cr-SPs were taken using Tecan infinite M200 spectrophotometer [15]. Butylated Hydroxytoulene (BHT) is used as a standard for this experiment. The ferric ion reducing ability of Cr-SPs was determined by plotting the concentration of Cr-SPs on X-axis Vs the O.D of the sample.
Super-Oxide Anion Radical Scavenging Assay
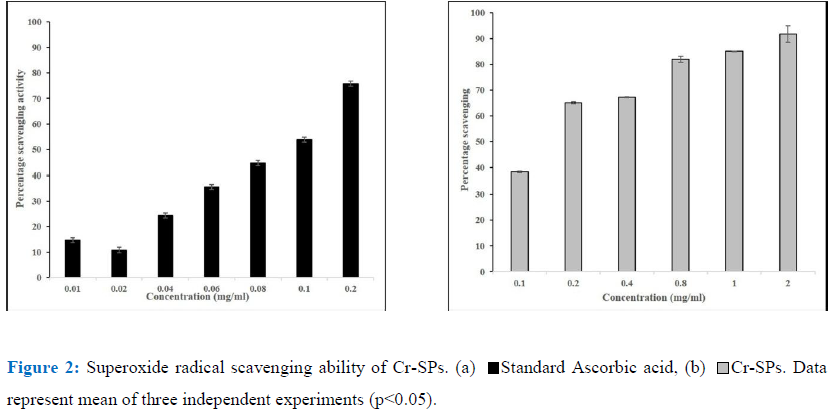
The Superoxide radicals are generated in PMS-NADH systems by oxidation of NADH and assayed by the reduction of NBT [49]. The reaction mixture contains 3 ml of 20 mM phosphate buffer containing 72 µM NBT, 338 µM NADH, 30 µMPMS and different concentrations of Cr-SPs ranging from 0.1 to 2 mg/mL. The reaction mixture was incubated at 25°C for 5 min and the absorbance was read at 562 nm by spectrophotometer. Ascorbic acid is used as a standard for this experiment.
Nitric-Oxide Scavenging Ability
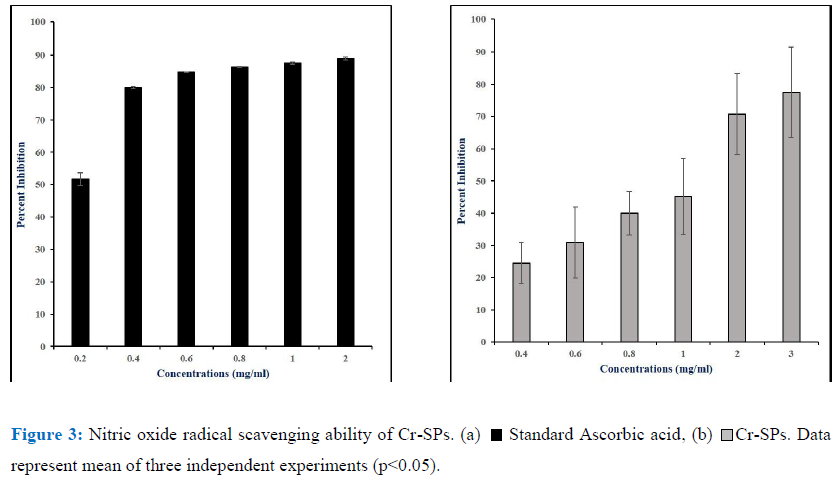
The Nitric-oxide scavenging ability of Cr-SPs was determined by a modified protocol of Rao MNA (1997) [61]. The reaction mixture contains 1mL of different concentrations of Cr-SPs (0.2- 2 mg/mL) with 5mM of Sodium nitroprusside prepared in Phosphate buffer saline (pH 7). The tubes were incubated at 25°C for 15 min. Post-incubation, 0.05mL of Griess reagent was added and the absorbance values were measured at 540 nm. The Griess reagent was made using 1% sulfanilamide, 2% Phosphoric acid and 0.1% N-(1-Naphthyl) ethylenediamine Ascorbic acid was used as a standard. The percentage of nitric oxide scavenging ability of Cr-SPs was measured using the below formula.
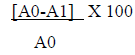
A0= Absorbance of control; A1- Absorbance of the sample
Hydrogen Peroxide Scavenging Assay
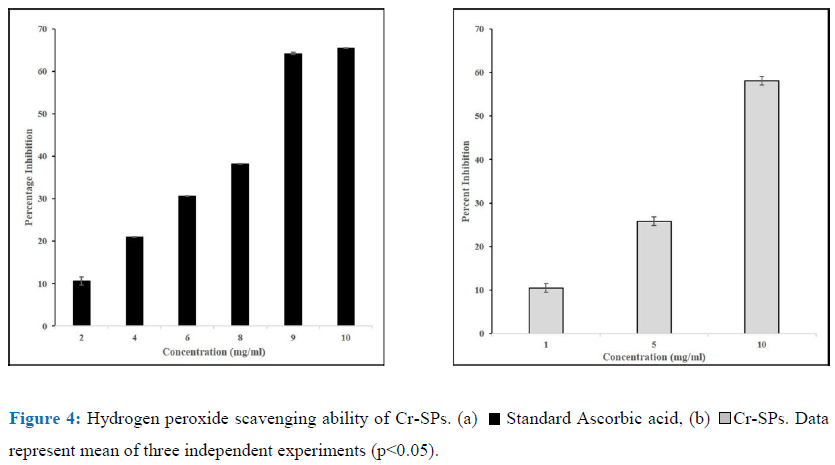
The ability of Cr-SPs to scavenge hydrogen peroxide was determined by FOX (ferrous ion oxidation-xylenol orange) assay using a modified protocol of Long et al (2000) [38]. The reaction mixture contains, 0.1 mL of hydrogen peroxide (50 mM) and 0.1 mL of different concentrations of Cr-SPs (0.1-2 mg/mL). The tubes were mixed well and incubated at room temperature for 30 min. Post incubation, 0.09 mL of the mixture was taken out and added to 0.01 mL methanol and 0.9 mL of FOX reagent. The FOX reagent was prepared using 9 volumes of BHT (4.4 mM) and 1 volume of Xylenol orange (1mM) and 2.56 mM of ammonium ferrous sulphate in sulfuric acid (0.25 M). The tubes were incubated for 30 min. After incubation the absorbance values were measured at 560 nm using Tecan infinite M200 spectrophotometer. Ascorbic acid was used as a standard for the experiment. The ability of Cr-SPs to scavenge H2O2 is measured by using the below equation:
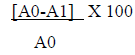
A0= Absorbance of control; A1- Absorbance of the sample
Determination of Anti-Skin Aging Properties of the Cr-Sps.
Anti-Elastase Assay
The elastin protein in the skin layers provides elasticity to the skin. In contrary, the elastase enzyme leads to the inactivation of this protein function by binding to the active site of the protein. The inhibition of the elastase enzyme by Cr-SPs can be determined by measuring the activity of elastase enzyme as described by a modified protocol by Thring et al. (2009) [65]. Briefly, 0.5 and 1.5 mg/mL concentrations of Cr-SPs were subjected to Tris HCl (pH 8.0) and elastase enzyme (1 μg/mL). After incubation for 15 min at room temperature, activation of the enzyme takes place. Further 100 µL of N Succinyl-L-alanyl-L-alanyl-L-alanine reagent (1 mM) was added. This reagent is used a substrate in in vitro enzyme activity experiments. After incubation, readings were taken at 410 nm using Tecan infinite M200 spectrophotometer. EDTA was used a positive control and solvent control was used to calculate the inhibition activity [31].
Anti-Collagenase Assay
The collagenase enzyme inhibits the activity of the collagen protein which leads to disturbing the firmness in the skin. The inhibition of the enzyme is tested by a modified method described by Mandl et al. (1953) [40]. In this method, the reaction mixture contains 25 µL of collagenase (1 mg/mL), 25 µL of TES buffer (50 mM) with 0.36 mM calcium chloride (pH 7.4) and 25µl of Cr-SPs (1 mg/ml and 2 mg/ml). Blank contains 75 µL of TES buffer. Positive control 25 µL collagenase, and 25 µL each of TES buffer and 1mg/mL EDTA solution. The reaction mixtures are then incubated at 37°C for 20 min. Then the 100 μl of substrate which is FALGPA (N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala) was added to the tubes and incubated at 37°C for 60 min. room temperature and then 200 µL of isopropanol was added. Collagenase enzyme activity was measure at 540 nm and percent inhibition of collagenase Post-incubation, 200 µL of solution containing Ninhydrin and 200 mM citric acid were added, incubated at 100°C for 5 min, allowed to cool down to activity was calculated using the below equation:
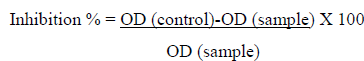
Anti-Hyaluronidase Assay
The Hyaluronidase assay was performed by using the modified protocol as mentioned by Reissig et al. (1955) and Ndlovu et al. (2013) [54, 48]. In this assay the activity of the enzyme hyaluronidase on the substrate sodium hyaluronate was tested in the presence of Cr-SPs. The reaction mixture contained 50 µl of different concentrations of Cr-SPs (1 mg/mL and 2 mg/mL) and 50 µL of hyalurinodase. Blanks contained acetate buffer instead of extract. The tubes were incubated at 37°C for 20 min. then 50 µL of calcium chloride and 250 µL of substrate sodium hyaluronate were added, incubated at 37°C for another 40 min to carry out enzyme-substrate reaction. Later, 100 µl of 0.2 M sodium borate, 50 µl of 0.4 M NaOH were added, incubated at 100°C for 5 min, and then allowed to cool down to room temperature. Then for color development, 1.5 mL of p-Dimethylaminobenzaldehyde solution was added and the samples were incubated at 37°C for 20 min. The color intensity was then measured at 585 nm. Gallic acid was used as a standard. The inhibitory effect of Cr-SPS was measured by using the below equation:
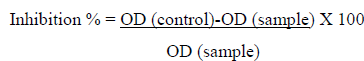
Statistical Analysis
The experiments were performed thrice, and the data was compared with controls using origin pro 8.5 software. Furthermore, the data was analyzed by one-way analysis of variance (ANOVA) and Turkey’s t-test (p<0.05).
Results
Extraction of Sulfated Polysaccharides from Chlamydomonas Reinhardtii.
The method used to extract SPs from Chlamydomonas reindardtii (CC-124) cells was based on the separation of cell wall components from the intact whole cell after giving it treatments like mechanical breakdown of cells by maceration and boiling water bath treatment for 4 h. These steps help us to accomplish separation of cell wall bound Cr-SPs successfully. The cells after centrifugation were discarded and the crude SPs were present in the solvent. The crude extract was then purified by passing through anion exchange column chromatography. Determination of the biochemical composition of the extract found to have 785.07 mg/g of total carbohydrate content, 324.26 mg/g of sulphate, 393.32 mg/g of uronic acid and very low protein. Cr-SPs was characterized by FTIR and NMR and showed structural features of SPs [66, 51] These results clearly indicate that the extract is enriched with polysaccharides which are sulfated. These Cr-SPs were then used to check their antioxidant and anti-skin aging properties.
Antioxidant Properties of the Cr-Sps
Ferric Ion Reducing Antioxidant Power Assay (FRAP)
Transition metals play a key role in generation of radicals. Iron in combination with superoxide or hydrogen peroxide lead to formation of reactive oxygen species. As ferric ions act as precursors for generation of free radicals, it becomes easier to target free radical generation. The ferric ion reducing potential of Cr-SPs was found to be dose dependent. Cr-SPs at 0.5-2 mg/mL concentrations showed FRAP of 27-68%. The maximum reducing potential of 68% was found at 5 mg/mL of Cr-SPs. The IC50 value of Cr-SPs was found to be ~1.9 mg/mL (Figure1). BHT was used as standard at the concentrations of 0.1-2 mg/mL and the IC50 was found to be ~0.3 mg/mL. Both Cr-SPs and BHT showed a positive correlation between the ferric ion reducing potential and concentration of the compound in a dose dependent manner.
Super-Oxide Anion Radical Scavenging Assay
Superoxide radical scavenging assay helps in determining the antioxidant properties of Cr-SPs that leads to the reduction of NBT. In the current study Cr-SPs at concentrations of 0.1-2 mg/mL showed 38-92% of superoxide radical scavenging potential with an IC50 of around ~0.14 mg/mL (Figure 2). A maximum scavenging of 92% was showed at 2 mg/mL concentration of Cr-SPs. In case of standard ascorbic acid at concentrations of 0.01-0.2 mg/mL showed and efficient superoxide scavenging of 14-78% with an IC50 of around 0.1 mg/mL. Both Cr-SPs and standard an increasing scavenging ability with increased concentration of the extract/standard.
Nitric Oxide Radical Scavenging Assay
Reactive oxygen species and reactive nitrogen species have a lot of physiological importance in the body. However, an imbalance in either of them can lead to formation of excessive radicals that can have detrimental effects on the body. Peroxynitrite (ONOO), nitric oxide (NO), nitrogen dioxide (NO2) are nitrogen containing oxidants that act as free radical moieties. The ability to chelate the nitric oxide radicals by Cr-SPs was evaluated by Nitric oxide radical scavenging assay using Griess reagent. It was observed that Cr-SPs at 0.4-3 mg/mL showed NO scavenging ability of 25-77%, with maximum scavenging potential at 3 mg/mL (Figure 2). The IC50 was found to be ~1 mg/mL (Figure 3). Ascorbic acid at concentrations of 0.2-2 mg/mL showed scavenging ability of 51-89%, with an IC50 of ~0.2 mg/mL.
Hydrogen Peroxide Inhibition Assay
Hydrogen Peroxide is not a very reactive radical. As mentioned above ferric ions along with hydrogen peroxide can lead to formation of reactive oxygen species. Toxic radicals such as OH by Fenton reaction or hypochlorous acid from conversion of hydrogen peroxide. Cr-SPs at 1-10 mg/mL showed H2O2 scavenging activity of 10%-58% with an IC50 of ~8 mg/mL. At 10 mg/mL Cr-SPs showed a maximum of 58% H2O2 scavenging activity (Figure 4). Ascorbic acid showed a 65% activity at 10 mg/mL concentration.
Anti-Skin Aging Properties of the Cr-Sps.
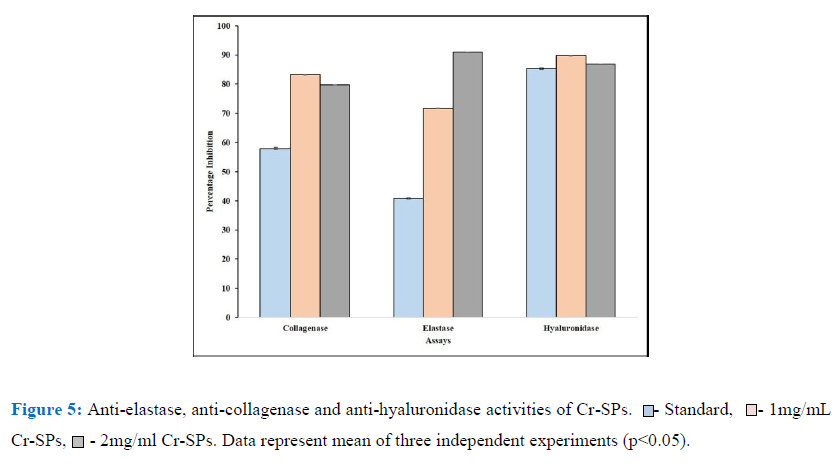
In order to determine the anti-skin aging properties of Cr-SPs, its ability to inhibit crucial enzymes for skin aging such as elastase, collagenase and hyaluronidase was carried out. These enzymes, when in their active state will bind to the proteins and lead to degradation of the protein which are observed as signs of aging. The anti- elastase activity of Cr-SPs were evaluated using N-Methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide as the substrate. It was observed that Cr-SPs at 1 mg/ml and 2 mg/mL showed 71% and 90% inhibition of elastase enzyme. However, the standard EDTA showed around 41% inhibition in enzyme activity at 1 mg/mL. Similarly, the ability of Cr-SPs to inhibit collagenase enzyme was tested using FALGPA as the substrate. The results showed that Cr-SPs at 1 and 2 mg/mL showed around ~80% collagenase enzyme inhibition (Figure 5). It is known that hyaluronidase enzyme leads to degradation of glycoasminoglycans (GAG’s) which are essential proteins that help in skin hydration. Inhibiting or reducing the activity of hyaluronidase is very essential for maintaining skin hydration. It was observed that Cr-SPs at 1 and 2 mg/mL concentration 80% hyaluronidase enzyme inhibition was observed (Figure 5). Gallic acid which is used as a standard for these experiments showed around showed 60% collagenase and 85% hylauronidase enzyme inhibitions at 1 mg/mL. These results clearly showed that Cr-SPs has promising antioxidant and anti-skin aging properties.
Discussion
Skin aging is a complex process, induced by extrinsic and intrinsic factors resulting in structural disorganisation in extracellular matrix components. The exact mechanism of aging is not yet known [35]. However, among the various causative factors known, oxidative stress is known to be an important contributor in aging. Mitochondria in the cells helps in production of cellular energy. However, in the process, mitochondria produce reactive oxygen species (ROS) during metabolism of oxygen. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system also lead to intercellular ROS production like mitochondria. NOX enzymes generate superoxide and other forms of ROS, by transferring electrons from NADPH to oxygen [47]. Excess accumulation of ROS by external environment makes it difficult for the body to abolish the harmful effects of ROS, which ultimately degrade the crucial enzymes of skin and leads to premature skin aging. BHT and Butylated Hydroxyanisole (BHA) are commercially available antioxidants. However, it is observed that these antioxidants on consumption are unstable and are known to be carcinogenic [41]. Therefore, consumers have shifted their use from synthetic antioxidants to antioxidants produced from natural sources like plants, algae etc. In the current study, SPs from C. reinhardtii were extracted and evaluated for their antioxidant and anti-skin aging properties.
The biochemical composition of the extract was found to have total carbohydrate content of 785.07 mg/g, 324.26 mg/g of sulphate and 393.32 mg/g of uronic acid. Similarly, earlier reports in Spirulina platensis showed that extraction of sulfated polysaccharides was carried out using ethanol and it gave significantly more carbohydrate content than water extraction [1]. The total carbohydrate content was estimated to be 12% with sulphate content of 4%. However, the percentage of carbohydrate content and sulphate content produced by C. reinhardtii is much more than Spirulina Platensis with ethanol extraction method. In earlier reports, the SPs from green algae Ulva lactuca, red algae Gracilaria lemaneiformis and Sarcodia ceylonensis, and brown alga Durvillaea antarctica by using microwave-assisted method were found to have total polysaccharide content of about 9% [22], which is much lower than the total carbohydrate content extracted in the current study.
Superoxide anion radical is one of the strongest reactive oxygen species among the free radicals that are generated after the oxygen is taken into living cells. Superoxide anion changes to other harmful ROS and free radicals such as hydrogen peroxide and hydroxyl radical, which induce oxidative damage [30]. It was reported that compounds that allow addition of electron-withdrawing groups to pyrrole showed enhanced antioxidant activity [68, 69]. Therefore, sulphate groups in SPs can effectively scavenge the free radicals generated during oxidative stress. In the present study, with increased concentrations of Cr-SPs ranging from 0.1 to 2 mg/mL, there was 53-92% of super oxide anion radical scavenging activity was observed (Figure 2). Earlier reports showed the extract of C. adhaerens at 1000 μg /mL, maximum scavenging activity of 59%, was observed [62, 26]. Mahendran and Saravanan (2013) [39] reported that the superoxide anion radical scavenging activity of polysaccharide from C. racemosa was found to be 66%. And in our present study, at concentration of 1mg/mL, Cr-SPs exhibited activity of 89% which is much higher compared to these green algae Codium sp.
Hydrogen peroxide is a reactive oxygen species (ROS) which is produced as a byproduct of metabolic processes of the cells which in turn can produce highly active ROS like hydroxyl radicals that lead oxidative damage to the cells [52]. In the current study, Cr-SPs showed to have efficient H2O2 scavenging potential in a dose-dependent manner. The ability of an antioxidant to scavenge H2O2 is mainly attributed to its ability to donate an electron to H2O2, thereby neutralizing it to water (Figure 4). Similarly, SPs such as Fucoidan and heterofucan from algae Fucus vesiculosus and Padina gymnospora showed efficient hydrogen peroxide scavenging ability [55, 25]. Moreover, several seaweeds have been reported to possess H2O2 scavenging potential [60, 62].
Nitric oxide (NO) and its derivatives were the main source of reactive nitrogen species in vivo. NO is enzymatically synthesized by oxidation of L-arginine by nitric oxide synthase (NOS). NOS are either inducible (iNOS) or constitutive (cNOS). iNOS is known to be expressed in various cells in the body such as endothelial cells, hepatocytes, neurons, macrophages, astrocytes etc. after exposure to cytokines. NO plays a crucial role in the regulation of various physiological activities of an organism and excess accumulation of NO is known to cause various pathological conditions such as diabetes, arthritis, cancer, inflammation, [44, 58, 46]. In the current study the scavenging ability of Cr-SPS on NO was evaluated. NO is known to react with oxygen and form stable nitrate and nitrite, which can be detected by Griess reagent [16]. As shown in the Figure 3 there was a positive correlation between the concentration of Cr-SPs and its NO scavenging ability. Similarly, earlier reports showed that natural compounds such as tocopherol, curcumin, catechin showed NO scavenging ability [3, 10, 28, 7].
The reducing potential of Fe3+ to Fe2+ by ferric reducing antioxidant potential assay (FRAP) is an indicator of antioxidant capacity of natural compounds [21]. The chelating ability of a compound is defined as the ability to form two or more binding sites within the same molecule, and a single central atom and polysaccharides are known to show this specificity [42]. In the present study it is being shown that there is a significant increase in chelating ability of iron atoms by Cr-SPs with increased dose (Figure 1). It has been reported earlier that, the ability of SPs to chelate free radicals is dependent on the molecular weight of the SPs. A low molecular weight SPs has greater potential to exhibit antioxidant properties. A lower molecular weight SPs from Laminaria japonica showed around 75% superoxide radical chelating activity at 0.4 mg/mL [12]. In another study it has also been stated that SPs are a mixture of polymer chains of different molecular weights. According to Sun et al. (2014) [63], when non-fractionated high molecular weight SPs are evaluated for anti-oxidant activities, the activity is moderately lower than lower molecular weight SPs. The antioxidant activity of SPs is also dependent on other factors like sulfate content and their position in the polysaccharide structure. The high percentage of polysaccharide content in the extract is responsible for the high iron chelating ability. From the results obtained, it was clear Cr-SPs has the promising ability to scavenge free radicals and also can terminate chain reaction.
Anti- SkinAging potential of Sulfated Polysaccharides from Chlamydomonas reinhardtii
Cr-SPs were further evaluated for their anti-aging properties with regards anti-elastase, anti-collagenase and anti-hylauronidase activities. Statistical analysis of the results showed that Cr-SPs has efficient inhibiting potential of all these three enzymes (Figure 5). Elastin is an enzyme that is present in the connective tissue of the skin and is responsible for elasticity of the skin and lungs [64, 19, 33]. In the relaxed state, the elastin protein is linked to lysine at various positions in a staggered manner. Therefore, in ideal conditions, when the skin is stretched these lysine residues arrange the elastin protein in a linear manner. With age these linkages break and lead to reduction in elasticity. Earlier studies reported that there is an increase in the activity of elastase enzyme over age and also after exposure to UV-B radiation. It was observed that after exposure to UV-A rays, the activity of the enzyme was enhanced due to which there was degradation of elastin protein, attacking and damaging several connective tissue proteins leading to inflammation, weakening of dermal matrix thereby leading to skin aging [36, 34, 47]. In the current study the elastase activity of Cr-SPs showed efficient inhibitory potential (Figure 5). Redini et al. (1988) [53] hypothesized that the probable mechanism of inhibitory action of SPs could be the interaction between the negative charges of the sulfate groups in SPs with the guanidium groups of the arginine residue present on the surface of the human leukocyte elastase and inactivating it [6, 4, 53, 5]. Earlier studies showed that SPs from Porphyridium cruentum exhibited anti-elastase activity at 0.25-2.5 mg/mL concentration.
Collagen is another important protein present in the connective tissue and gives firmness to the skin. During skin aging, there is an imbalance between production and degradation of collagen [45]. Collagen gets degraded by collagenase and the levels of collagenase increases drastically. It was reported that oxidative stress enhances ROS production that activates collagenase which in turn causes the breakdown of collagen [23]. In the present study, Cr-SPs found to have efficient inhibitory activity against collagenase enzyme activity. At 1 mg/mL concentration, Cr-SPs showed 83% collagenase enzyme inhibition (Figure 5). It is known for long that collagenase are a group of metalloproteins which have zinc ions at the catalytic site [37]. Therefore Cr-SPs probably binding to these zinc ions and inactivating the enzyme. Similarly, catechins of green tea showed reduced collagenase activity mainly because of their interaction with Zn2+ ion of the enzyme and inhibits its binding to the substrate [29, 65]. Also, extracts from C. glabrum, P. africanum and S. brachypetala showed efficient anti-collagenase activities [11; 48].
Hyaluronic acid and chondroitin sulphate are important constituents of amorphous substances of connective tissues. Hylauronidase hydrolyzes hyaluronic acid and leads to inflammatory diseases, and destruction of extracellular matrix promoting aging [17]. Earlier literature reported that various polyphenols such as tannic acid, apigenin, and quercetin significantly inhibited hylauronidase activity [20, 56]. In the present study a significant inhibition of hyaluronidase enzyme activity was observed at 1 and 2 mg/mL concentration of Cr-SPs (Figure 5). This inhibitory activity of Cr-SPs could be the electrostatic interaction of negatively charged sulfate and uronic acid groups in the SPs with positively charged hyaluronidase amino acids, that make the enzyme unavailable to bind to hyaluronic acid and therefore protect it from degradation. Similarly, SPs from Porphyridium cruentum exhibited anti hyaluronidase activity [5].
Conclusion
In conclusion, the sulfated polysaccharides were extracted from Chlamydomonas reinhardtii by hot water method and using ethanol as solvent. Biochemical analysis showed that the extract is highly enriched with carbohydrates which have sulphate groups and uronic acid. Antioxidant assays showed that Cr-SPs have efficient free radical scavenging ability and profound chain terminating potential in a dose-dependent manner. There is close correlation between skin aging and oxidative stress, the property of antioxidant potential of the sulfated polysaccharides could be used to treat aging. Furthermore, in vitro anti-skin aging properties of Cr-SPs were explored which showed efficient inhibition of elastase, collagenase and hyaluronidase enzyme activities. These promising antioxidant and anti-aging properties of Cr-SPs provides opportunities to develop them in skin aging formulations and help in fighting skin aging problems in a cost-effective manner.
Funding: This work is sponsored by Department of Atomic Energy, Government of India.
Conflict of Interest: The authors do not have any conflict of interest in publishing this manuscript.
Author Contribution Statement: VLS- concept, design, data analysis and editing the manuscript, BF-conducted antioxidant experiments and wrote the manuscript, JV- extraction of Cr-SPs, HJ- performed Anti-skin aging experiments.
References
- El-Baky AH, El Baz HK, El-Latife SA. Induction of sulfated polysaccharides in Spirulina platensis as response to nitrogen concentration and its biological evaluation. Journal of Aquaculture Research & Development 5 (2014): 1.
- Akagawa M, Shigemitsu T, Suyama K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Bioscience, Biotechnology, and Biochemistry 67 (2003): 2632-2640.
- Arroyo PL, Hatch-Pigott V, Mower HF, et al. Mutagenicity of nitric oxide and its inhibition by antioxidants. Mutation Research Letters 281 (1992): 193-202.
- Baici A, Bradamante P. Interaction between human leukocyte elastase and chondroitin sulfate. Chemico-Biological Interactions 51 (1984): 1-1.
- Bayona KD, Navarro SM, Lara AD, et al. Activity of sulfated polysaccharides from microalgae Porphyridium cruentum over degenerative mechanisms of the skin. International Journal of Science and Advanced Technology 2 (2012): 85-92.
- Bode W, Wei AZ, Huber R, et al. X-ray crystal structure of the complex of human leukocyte elastase (PMN elastase) and the third domain of the turkey ovomucoid inhibitor. The EMBO Journal 5 (1986): 2453-2458.
- Bor JY, Chen HY, Yen GC. Evaluation of antioxidant activity and inhibitory effect on nitric oxide production of some common vegetables. Journal of Agricultural and Food Chemistry 54 (2006): 1680-1686.
- Brieger K, Schiavone S, Miller Jr FJ, et al. Reactive oxygen species: from health to disease. Swiss Medical Weekly 142 (2012): w13659.
- Çakmak ZE, Ölmez TT, Çakmak T, et al. Antioxidant response of C hlamydomonas reinhardtii grown under different element regimes. Phycological Research 63 (2015): 202-211.
- Chan MM, Fong D, Ho CT, et al. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochemical Pharmacology 54 (1997): 1281-1286.
- Chiang HM, Lin TJ, Chiu CY, et al. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food and Chemical Toxicology 49 (2011): 309-318.
- Choi DS, Athukorala Y, Jeon YJ, et al. Antioxidant activity of sulfated polysaccharides isolated from Sargassum fulvellum. Preventive Nutrition and Food Science 12 (2007):65-73.
- Choudhary S, Save SN, Vavilala SL. Unravelling the inhibitory activity of Chlamydomonas reinhardtii sulfated polysaccharides against α-Synuclein fibrillation. Scientific Reports 8 (2018): 1-2.
- Connan S, Delisle F, Deslandes E, Gall EA. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Botanica Marina 49 (2006): 39-46.
- Dasgupta S, Pandya M, Patel N. Antioxidant activities of some less utilized edible fruits. Int J Curr Pharm Res 9 (2017): 28-31.
- Dirsch VM, Stuppner H, Vollmar AM. The Griess assay: suitable for a bio-guided fractionation of anti-inflammatory plant extracts?. Planta Medica 64 (1998): 423-426.
- Facino RM, Carini M, Aldini G, et al. Direct characterization of caffeoyl esters with antihyaluronidase activity in crude extracts from Echinacea angustifolia roots by fast atom bombardment tandem mass spectrometry. Farmaco (Societa Chimica Italiana: 1989) 48 (1993): 1447-1461.
- Farage MA, Miller KW, Elsner P, et al. Intrinsic and extrinsic factors in skin ageing: a review. International Journal of Cosmetic Science 30 (2008): 87-95.
- Fulop T, Khalil A, Larbi A. The role of elastin peptides in modulating the immune response in aging and age-related diseases. Pathologie Biologie 60 (2012): 28-33.
- Girish KS, Kemparaju K. Inhibition of Naja naja venom hyaluronidase by plant-derived bioactive components and polysaccharides. Biochemistry (Moscow) 70 (2005): 948-952.
- Gullón B, Eibes G, Moreira MT, et al. Antioxidant and antimicrobial activities of extracts obtained from the refining of autohydrolysis liquors of vine shoots. Industrial Crops and Products 107 (2017): 105-113.
- He J, Xu Y, Chen H, et al. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. International Journal of Molecular Sciences 17 (2016): 1988.
- Hooda R. Antiwrinkle herbal drugs-an update. Journal of Pharmacognosy and Phytochemistry 4 (2015): 277.
- Jiao G, Yu G, Zhang J, et al. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Marine Drugs 9 (2011): 196-223.
- Jose GM, Radhakrishnan A, Kurup GM. Antioxidant and antimitotic activities of sulfated polysaccharide from marine brown algae Padina tetrastromatica. Journal of Phytology (2015): 39-51.
- Kallswari G, Mahendran S, Subalakshmi P, et al. Purification, characterization and antioxidant activity of green seaweed Codium sp. Adv. Pharm. Pharm 4 (2016): 16-21.
- Kamble P, Cheriyamundath S, Lopus M, et al. Chemical characteristics, antioxidant and anticancer potential of sulfated polysaccharides from Chlamydomonas reinhardtii. Journal of Applied Phycology 30 (2018): 1641-1653.
- Kawada N, Seki S, Kuroki T, et al. Regulation of stellate cell proliferation by lipopolysaccharide: role of endogenous nitric oxide. Journal of Gastroenterology and Hepatology 13 (1998): S6-S13.
- Kim YJ, Uyama H, Kobayashi S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochemical and Biophysical Research Communications 320 (2004): 256-261.
- Korycka-Dahl MB, Richardson T, Foote CS. Activated oxygen species and oxidation of food constituents. Critical Reviews in Food Science & Nutrition 10 (1978): 209-241.
- Kraunsoe JA, Claridge TD, Lowe G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 35 (1996): 9090-9096.
- Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clinical and Experimental Immunology 113 (1998): 147.
- Kurtz A, Oh SJ. Age related changes of the extracellular matrix and stem cell maintenance. Preventive Medicine 54 (2012): S50-S56.
- Labat-Robert J, Fourtanier A, Boyer-Lafargue B, et al. Age dependent increase of elastase type protease activity in mouse skin: Effect of UV-irradiation. Journal of Photochemistry and Photobiology B: Biology 57 (2000): 113-118.
- Lapiere CM. The ageing dermis: the main cause for the appearance of ‘old’skin. British Journal of Dermatology 122 (1990): 5-11.
- Lee KK, Kim JH, Cho JJ, et al. Inhibitory effects of 150 plant extracts on elastase activity, and their anti-inflammatory effects. International Journal of Cosmetic Science 21 (1999): 71-82.
- Leite SR. Inhibitors of human collagenase, MMP1. Eclética Química 34 (2009): 87-102.
- Long LH, Clement MV, Halliwell B. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin,(−)-epigallocatechin gallate,(+)-catechin, and quercetin to commonly used cell culture media. Biochemical and Biophysical Research Communications 273 (2000): 50-53.
- Mahendran S, Saravanan S. Purification and in vitro antioxidant activity of polysaccharide isolated from green seaweed Caulerpa racemosa. Int J Pharm Bio Sci 4 (2013): 1214-1227.
- Mandl I, MacLennan JD, Howes EL, et al. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. The Journal of Clinical Investigation 32 (1953):1323-1329.
- Masaki H. Role of antioxidants in the skin: anti-aging effects. Journal of Dermatological Science 58 (2010): 85-90.
- Melo-Silveira RF, Fidelis GP, Viana RL, et al. Antioxidant and antiproliferative activities of methanolic extract from a neglected agricultural product: corn cobs. Molecules 19 (2014): 5360-5378.
- Menon GK. Skin basics; structure and function. InLipids and Skin Health (2015): 9-23.
- Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. European Journal of Clinical Investigation 21 (1991): 361-374.
- Mukherjee PK, Maity N, Nema NK, et al. Bioactive compounds from natural resources against skin aging. Phytomedicine 19 (2011): 64-73.
- Murphy MP. Nitric oxide and cell death. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1411 (1999): 401-414.
- Naidoo K, Birch-Machin MA. Oxidative stress and ageing: the influence of environmental pollution, sunlight and diet on skin. Cosmetics 4 (2017): 4.
- Ndlovu G, Fouche G, Tselanyane M, Cordier W, Steenkamp V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complementary and Alternative Medicine 13 (2013): 304.
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications 46 (1972): 849-854.
- Oyaizu M. Studies on products of browning reaction. The Japanese Journal of Nutrition and Dietetics 44 (1986): 307-315.
- Panigrahi GP, Rane AR, Vavilala SL, Choudhary S. Deciphering the anti-Parkinson’s activity of sulphated polysaccharides from Chlamydomonas reinhardtii on the α-Synuclein mutants A30P, A53T, E46K, E57K and E35K. The Journal of Biochemistry 166 (2019): 463-474.
- Ramani R, Sudini S, Boddupalli BM, et al. Antioxidant, free radical scavenging and invitro cytotoxic studies of ethanolic extract of Leucas indica var lavandulifolia and Leucas indica var nagalapuramiana. Asian Pacific Journal of Tropical Biomedicine 2 (2012): S1637-S1642.
- Redini F, Tixier JM, Petitou M, et al. Inhibition of leucocyte elastase by heparin and its derivatives. Biochemical Journal 252 (1988): 515-519.
- Reissig JL, Strominger JL, Leloir LF. A modified colorimetric method for the estimation of N-acetylamino sugars. Journal of Biological Chemistry 217 (1955): 959-966.
- de Souza MC, Marques CT, Dore CM, et al. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. Journal of Applied Phycology 19 (2007): 153-160.
- Sahasrabudhe A, Deodhar M. Anti-hyaluroiüdase, Anti-elastase Activity of Garcinia indica. Int J Bot 6 (2010): 1-0.
- Scaife MA, Nguyen GT, Rico J, et al. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. The Plant Journal 82 (2015): 532-546.
- Schmidt HH, Pollock JS, Nakane M, et al. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proceedings of the National Academy of Sciences 88 (1991): 365-369.
- Shannon E, Abu-Ghannam N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Marine Drugs 14 (2016): 81.
- Siriwardhana N, Lee KW, Jeon YJ, et al. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Science and Technology International 9 (2003): 339-346.
- Rao MN. Nitric oxide scavenging by curcuminoids. Journal of Pharmacy and Pharmacology 49 (1997): 105-107.
- Sudha KM, Kumarin V, Palanichamy V. Screening of antioxidant potential of green alga Codium adhaerens. Int J Drug Dev Res 6 (2014): 103-111.
- Sun L, Wang L, Li J, et al. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food chemistry 160 (2014): 1-7.
- Debelle L, Tamburro AM. Elastin: molecular description and function. The International Journal of Biochemistry & Cell Biology 31 (1999): 261-272.
- Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complementary and Alternative Medicine 9 (2009): 27.
- Vishwakarma J, Vavilala SL. Evaluating the antibacterial and antibiofilm potential of sulphated polysaccharides extracted from green algae Chlamydomonas reinhardtii. Journal of Applied Microbiology 127 (2019): 1004-1017.
- Yagi M, Yonei Y. Glycative stress and anti-aging: glycative stress and skin aging. Glycative Stress Res 5 (2018): 50-54.
- Yanagimoto K, Ochi H, Lee KG, et al. Antioxidative activities of volatile extracts from green tea, oolong tea, and black tea. Journal of Agricultural and Food Chemistry 51 (2003): 7396-7401.
- Zhang Z, Wang F, Wang X, et al. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydrate Polymers 82 (2010): 118-121.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
