LIBS Technology and its Application: Overview of the Different Research Areas
Article Information
Mputu Kanyinda Jean-Noël1,3,*, Kaniki Tshamala Arthur2, Baele Jean-Marc3
1,3Department of chemistry and industry, faculty of science University of Kinshasa. B. P 190 Kin XI Kinshasa, D.R. Congo
2Department of Chemical Engineering, Faculty of polytechnic, University of Lubumbashi, B. P 1825 Lubumbashi, D.R. Congo
3Department of Geology, Faculty of polytechnic, University of Mons, 7000 Mons Belgium
*Corresponding Author: Dr. Mputu Kanyinda Jean-Noël, Department of chemistry and industry, faculty of science University of Kinshasa, B. P 190 Kin XI Kinshasa, D.R. Congo
Received: 15 June 2020; Accepted: 22 June 2020; Published: 01 July 2020
Citation: Mputu Kanyinda Jean-Noëla, Kaniki Tshamala Arthurb, Baele Jean-Marcc. LIBS technology and its application: overview of the different research areas. Journal of Environmental Science and Public Health 4 (2020): 134-149.
View / Download Pdf Share at FacebookAbstract
Laser-induced plasma atomic emission spectroscopy (LIBS) which is a less costly and simple to implement, technique is starting to prevail in qualitative and quantitative analyses of the various elements of the periodic system. She is based on the interaction of a laser pulse that only lasts a few nanoseconds with the material to be analyzed. LIBS technology has several advantages such as its situ use, it speeds in carrying out analyses in real time, the use of samples without prior treatment, analysis of minute quantitative of samples. She may be portable for use in any space without contact with the sample reason she was on board the Discovery robot for ground exploration Martian. She entered several fields of research such as medicine, agronomy, nuclear research, biology, the food industry, the research archeological, space research, in the pharmaceutical field, in mineralogy for determination of minerals and environment for monitoring pollution of metallic trace elements (MTE) in soil, water and air. LIBS work on all kinds of materials in solid, liquid, or gaseous state.
Keywords
Ablation; Environment; ETM; Laser; LIBS; Spectroscopy; Target sample
Ablation articles, Environment articles, ETM articles, Laser articles, LIBS articles, Spectroscopy articles, Target sample articles
Ablation articles Ablation Research articles Ablation review articles Ablation PubMed articles Ablation PubMed Central articles Ablation 2023 articles Ablation 2024 articles Ablation Scopus articles Ablation impact factor journals Ablation Scopus journals Ablation PubMed journals Ablation medical journals Ablation free journals Ablation best journals Ablation top journals Ablation free medical journals Ablation famous journals Ablation Google Scholar indexed journals Environment articles Environment Research articles Environment review articles Environment PubMed articles Environment PubMed Central articles Environment 2023 articles Environment 2024 articles Environment Scopus articles Environment impact factor journals Environment Scopus journals Environment PubMed journals Environment medical journals Environment free journals Environment best journals Environment top journals Environment free medical journals Environment famous journals Environment Google Scholar indexed journals ETM articles ETM Research articles ETM review articles ETM PubMed articles ETM PubMed Central articles ETM 2023 articles ETM 2024 articles ETM Scopus articles ETM impact factor journals ETM Scopus journals ETM PubMed journals ETM medical journals ETM free journals ETM best journals ETM top journals ETM free medical journals ETM famous journals ETM Google Scholar indexed journals Laser articles Laser Research articles Laser review articles Laser PubMed articles Laser PubMed Central articles Laser 2023 articles Laser 2024 articles Laser Scopus articles Laser impact factor journals Laser Scopus journals Laser PubMed journals Laser medical journals Laser free journals Laser best journals Laser top journals Laser free medical journals Laser famous journals Laser Google Scholar indexed journals LIBS articles LIBS Research articles LIBS review articles LIBS PubMed articles LIBS PubMed Central articles LIBS 2023 articles LIBS 2024 articles LIBS Scopus articles LIBS impact factor journals LIBS Scopus journals LIBS PubMed journals LIBS medical journals LIBS free journals LIBS best journals LIBS top journals LIBS free medical journals LIBS famous journals LIBS Google Scholar indexed journals Spectroscopy articles Spectroscopy Research articles Spectroscopy review articles Spectroscopy PubMed articles Spectroscopy PubMed Central articles Spectroscopy 2023 articles Spectroscopy 2024 articles Spectroscopy Scopus articles Spectroscopy impact factor journals Spectroscopy Scopus journals Spectroscopy PubMed journals Spectroscopy medical journals Spectroscopy free journals Spectroscopy best journals Spectroscopy top journals Spectroscopy free medical journals Spectroscopy famous journals Spectroscopy Google Scholar indexed journals industrial environment articles industrial environment Research articles industrial environment review articles industrial environment PubMed articles industrial environment PubMed Central articles industrial environment 2023 articles industrial environment 2024 articles industrial environment Scopus articles industrial environment impact factor journals industrial environment Scopus journals industrial environment PubMed journals industrial environment medical journals industrial environment free journals industrial environment best journals industrial environment top journals industrial environment free medical journals industrial environment famous journals industrial environment Google Scholar indexed journals biology articles biology Research articles biology review articles biology PubMed articles biology PubMed Central articles biology 2023 articles biology 2024 articles biology Scopus articles biology impact factor journals biology Scopus journals biology PubMed journals biology medical journals biology free journals biology best journals biology top journals biology free medical journals biology famous journals biology Google Scholar indexed journals food industry articles food industry Research articles food industry review articles food industry PubMed articles food industry PubMed Central articles food industry 2023 articles food industry 2024 articles food industry Scopus articles food industry impact factor journals food industry Scopus journals food industry PubMed journals food industry medical journals food industry free journals food industry best journals food industry top journals food industry free medical journals food industry famous journals food industry Google Scholar indexed journals
Article Details
1. Introduction
The LIBS (laser-introduced Breakdown Spectroscopy) technique belongs to the family of atomic emission spectroscopy technique [1]. LIBS allow obtaining a quantitative and qualitative analysis of the elementary chemical composition of a sample in a solid, liquid, gaseous or aerosol form in situ and in real time [2, 3]. She also allows the mass concentration of a pollutant to be determined as well at the dissolved state than in the particulate state with particles of size limit of 2 nm for concentration rangers from ng/L to mg/L [4-6]. This LIBS technology has the advantage of taking different form adaptation to any type of situation, be it online measurement in an industrial environment, direct analyzes in the field or analyzes in laboratory [1].
To date, LIBS technology is brought to meet the needs detection and analysis in very varied fields such as for the analysis of metals more particularly the metallic trace elements [2, 7, 8], metallic alloys [8, 9], in sample environment [4, 10-14], archeological materials [15, 16], in products, food products such as milk [17, 18], in the biomedical field [19], in biological and pharmaceutical products [5, 20-22]. In parallel, portable instruments LIBS have been developed and have made it possible to explode the use of it [23-26] ; which enabled its use in space research with the curiosity robot rover sent by NASA on Mars which is currently traveling on this planet with an instrument LIBS potable in search of condition favorable to the appearance of life. Spectra LIBS from Martian soil are thus regularly transmitted to Earth for analysis [27, 28]. LIBS can be used for online analysis in the determination sulfur in ones or a multi-elemental analysis of radioactive glasses [5, 29, 30].
1.1 Historic
Historically speaking, this technique has seen the light of day since the invention of the laser in the 1960 [6]. In 1962, two years after the invention of the laser, two American physicists Frederick Brech and LIyod Cross, discovered laser-introduced plasma when they focused a momentum on the surface of a large [6]. In 1963, the work of Debras-Guédon and Lindec two French physicists reveal a rich spectrum of emission of elements (ions and atoms) but also molecules (and radicals [31]. During the same decade, the LIBS technology was developed simultaneous in Russia by Korunchikov and Yankovaskii in 1966 and in the USA Wiggins at al. in 1966 [32, 33]. It was abandoned for a long time until the 1980s when an American research team renovated the technique to analysis of chlorine in the air and provokes a renewed interest in other operation, thus, the number of works related to the spectrochemical analysis by laser addition has increased on the one hand thanks to the improvement of the quality of solid teams of the Nd: YAG type [34] and on the other hand by improving the performance of spectrometers and detectors. It was not until 1988 to see the appearance of a prototype devoted to analysis in situ of Beryllium particles in the air [6]. It was not until the 2000s that the industries and various defense ministries find in LIBS a new way to identification of gases, explosives but also biological agents (viruses and bacteria) in if you and immediate [6].
2. Principles of LIBS
2.1 General principles of the LIBS method;
The analysis by LIBS is based on the interaction of a laser pulse (duration of a few nanoseconds) with the material to be analyzed (analysis is also possible with team picosecond and femtosecond, however more expensive and more restrictive in use) the principle of which is presented in figure1. The laser beam is focused on the surface of the sample which induced a significant energy deposit in a short time on a surface reduced: the irradiance (power per unit area) achieved in LIBS is of the order of GW.cm-2 [35, 36]. Sudden heading of the materials then leads to ablation and vaporization of the material. The vapor absorbs part of the laser radiation, it heats up and ionized. A plasma containing electrons, atoms and ions in an excited state, the forms. All these mechanisms occur during and after the laser pulse (a few microseconds) [35, 37].
The interaction of laser matter is governed by the laws of quantum mechanics describing how photons are absorbed or emitted by atoms. If an electron absorbs a photo, the electron reaches a quantum mechanical state of higher energy. The electron tends towards the lowest possible energy levels, and in the process of disintegration, the electron emits a photon (de-excitation of the atom). The different levels of energy from each type of atom, with narrow band emissions due to the quantification, with an uncertainty defined by Heisenberg’s uncertainty principle [35, 38, 39]. These emissions are the spectral emission line found in the LIBS spectra, its characteristics and associated energy levels are well known for each atom [40]. During its propagation in the surrounding atmosphere, atoms and ions emit photons at wavelengths characteristic of the emitting atomic elements. Thus by collecting the radiation from the plasma and analyzing its spectrum, it is possible identify the elements present in the plasma and therefore in the analyzed sample, from databases of produced emission line [5, 13].
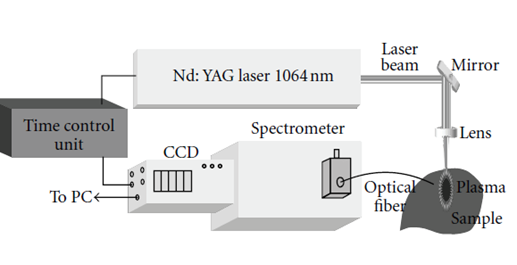
Figure 1: Experimental schema of laser-induced plasma spectroscopy [35].
The radiation is collected through an optical fiber connected to a spectrometer coupled to a detector. These make it possible to record time spectra in the length range wave from near ultraviolet (UV) to near infrared (IR) through visible (200-800 nm). If the position of the line gives information to the element in the sample (spectrum optical emission identical to that obtained to the atomic emission spectrometry by inductively coupled plasma ICP-AES [13, 41].
There are several variants of the different LIBS assemblies, however the assembly below above is the common basis for all existing LIBS instrumentation versions right now. An infrared laser delivering a right pulse with the duration of nanoseconds order (commonly Nd: YAG at 1064-532-355 or 266 nm) is focused on a target by a fiscal length silica lens (f = 10 cm) and created a plasma. Light is collected by an achromatic lens and injected into a fiber wide spectral band optic (280-760 nm) connected to an ARYELLE 400 spectrometer (laser Teknik Berlin) equipped with a CCD detector and a disk with multiply perforated blades (called a chopper) rotating at a frequency of 25.000 revolution per minute [35, 41-44].
2.2 Physical interactions
The physical mechanisms involved in the different phases governing an analysis LIBS are presented here in a simplify way. The many physical phenomena and the plasma expansion dynamic make the process very complex. The understanding of these physical phenomena allows better control of many parameters which govern the laser-matter interaction and better understand the signal that it is possible to get into LIBS. Three main phases are identified:
- A laser-matter interaction phase during which the sample material is vaporized under the effect of the transfer of energy from laser to the sample;
- The creation of the plasma by interaction between the atomic vapor in the formation and the laser, then its spatial and temporal evolution;
- The emission of a large spectrum, its detection and its transformation into a signal exploitable [36, 38].
2.3 Parameters influencing the signal
Understanding the physics of LIBS plasma is essential to provide a framework optimized for LIBS. There are many environment factors affecting the lifetime and characteristics of the plasma, modifying the spectral emission and performance of this technique for chemical analysis at the atomic level. The LIBS spectroscopy is characterized by its simplicity of implementation. Indeed, a system basic LIBS require only a few elements to function: a pulsed laser, a focusing lens, signal collection system, optical fiber, spectrometer, and a computer. There are of course more sophisticated LIBS devices that we can meet in research laboratories working with this technique everywhere in the world [35, 45].
2.3.1 The laser: The main equipment of the LIBS is the laser. It generates the energy necessary to induce plasma and mainly determines the characteristics of the plasma. The main laser related parameters are pulse time, pulse energy, length, and the number of pulses. Obviously, each app works better with a combination of these parameters. Nanoseconds pulses lasers are the more common for LIBS [35].
The energy parameters linked to the interaction of the laser material are fluence (energy per unite area, J/cm2) and irradiance (energy per unit area and time, W/cm2). The ablation process (erosion, fusion, sublimation, etc.) have fluence thresholds different. The effect of changes in laser energy is related to the laser wavelength and at pulse time. It therefore seen obvious that all the parameters linked to the laser will have affect the LIBS signal analyzed. The laser used must reach a fluence sufficient on the target to cause ablation of the material. Pulses lasers allow to reach powers suitable for this application. The laser will therefore be characterized by its wavelength, its energy and its pulse duration as well as by its rate of repetition [35, 42, 45].
The nature and characterized of laser-induced plasma are strongly affected by laser operating condition, i.e. laser wavelength (λ) pulse duration (τ) and energy (E). At the same time it should be remembered that, if the specific mechanisms governing the absorption of the laser energy in the target depend on the type of material, the surrounding atmosphere, both in composition and in pressure, plays an important role because it is a surrounding environment where the plasma evolves. The mechanisms physical factors involved in the creation of plasma by laser are:
2.3.1.1 Laser-matter interaction: The processes involved in laser ablation are photo-physical in nature. All first the photons of the laser radiation are absorbed by the target material. This absorption depends on the wavelength of the laser and the optical properties of the sample, the energy communicated will change the state of the material, mainly by thermal effect and possible by photochemical effect. The level of illumination will determine how whose material will behave [35, 42]. From a certain threshold, variable depending on the material and the laser conditions there is the injection of the heated material which can be in the form of vapor [39]. The laser wavelength and the illumination are the parameters control of the excitation of matter. They influence the process fundamentals of ablation. The lighting is fixed for the pulse duration imposed by the laser used. For a given energy and laser / matter interaction surface, the illumination is defined as [40] :
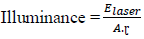
Elaser : Energy of a laser pulse, A: laser / matter interaction surface, τ: laser pulse duration
2.3.1.2 The wavelength: The influence of wavelength on LIBS can be explained from two points of view; laser-material interaction (energy absorption) and the development and properties of plasma (plasma-material interaction). Wavelength plays an important role in laser-matter interaction, it influences ablation mainly by the absorbency of the material at the laser excitation wavelength. Light absorption mechanisms often depending on the nature of the material. Two main classes can be distinguished conductors and dielectrics (consisting of insulators and semiconductors). The drivers are optically very absorbent by their capacity to absorb electrons from the conduction. Dielectrics are less absorbent, or even completely transparent when UV-VIS laser radiation. The interaction mechanisms are different from those of metals and the influence of the wavelength differ a lot depending on their different materials [35, 42].
2.3.1.3 The laser pulsion duration: The pulse duration in the time characteristics of the laser pulse. According to its nature (nanosecond, picosecond, femtosecond), the lifetime of the plasma can vary for example, for nanosecond pulse, the plasma is generated if the irradiation persists. Following the laser-matter interaction, there may be an additional interaction between the laser beam and the plasma. Indeed, the plasma can be heated by the laser However for femtosecond pulse, energy is used only through laser-matter interaction for a short time and before plasma formation. The laser-plasma interaction does not exist in this case. In these pulse regimes sub-nanoseconds i.e. picosecond and femtosecond, the rapid transition from the state of vaporization at the formation of the plasma, is dominate and the emission of the plasma is short and low. The laser pulse duration also influences the laser-matter interaction with the nature of sample. Indeed, in the cause of solids, the diameter of the crater obtained, are reduced for short pulse duration[14, 35, 42].
2.3.1.4 The influence of energy per purse: For constant beam diameters, increasing the energy per pulse results in an increase in the quality of vaporized material and therefore in the LIBS signal. So, Sirven at al. [14] observed a linear increase in the intensity of a Manganese line in the energy function per laser pulse. Pulse energy is also related to diameter of the beam on the target and the pulse duration by influence and irradiation. The signal behavior as a function of the energy per pulse is therefore dependent on focusing condition [35, 42].
2.3.2 Target: In the broad sense, the term “target” here designates the sample and its ambient environment, the psycho-chemical characteristics influence the interaction with the laser and the properties plasma [13]. The environment of the sample has a great influence on the ablation and plasma emission. Let us cite in particular the effect of the pressure and the nature of the gas ambient. The effect of atmospheric conditions on the LIBS signal is widely studied, on the one hand to understand the interaction of ambient gas molecules with plasma and secondly for certain application of the LIBS spectroscopy in atmosphere different from the Earth’s atmosphere [35, 42].
The plasma created by laser absorption develops in the ambient gas and interacts necessarily with him. With regard to the intensity of the plasma emission, this confinement is optimum at low pressure and increase the signal by 1 to 2 orders of magnitudes compared to atmospherics pressure. Beyond this optimal pressure, the plasma cools quickly by free expansion in the gas, it is not maintained by collision. Conversely, at higher pressure, the energy of the plasma is dissipated at the contact of the ambient gas by thermal condition and by collision. On the other hand, the author shows that beyond the raw signal, the signal to background ratio also benefits from a considerable improvement at optimal pressure over atmospherics pressure it is up to 100 time higher. Finally, the decrease in pressure increase the volume of the plasma, therefore the decreases in its density [35, 43].
2.3.3 Signal collection and analysis: The plasma created by laser ablation is a three-dimensional luminous object, strongly heterogeneous and whose life is limited in time. Know how to collect the radiation it emits is therefore not an obvious question. It is then a question of analyzing this light using a spectrometer and a suitable detector [35].
2.3.3.1 The collection system: The focusing of the laser on the studied surface is carried out via a set of lenses. In most configuration, the plasma signal is collected by a lens, which transmits an image of the plasma at the entrance spectrometer with a certain magnification radio or reduction. These lens mounts are very easy to use but cause chromatic aberrations (different focus depending on the wavelength). These chromatic aberrations are all the more important as the spectral range analyzed by the spectrometer is wide. Another solution is to use optical fibers or a set of mirrors that direct light through multiply reflections up to spectrometer, which solves the problem chromatic aberration. The montages of mirrors are however complicated to adjust and generate geometric aberration after the crossing of the optimal system the rays far from the axis of the system do not converge at the same place at the paraxial rays and the image formed by the optical system is blurred [35, 42].
2.3.3.2 The optical fiber: Optical fibers are integrated in LIBS system to bring light from the plasma to the spectrometer and occasionally to transport the laser beam, even though the most common optical fiber in LIBS are fused silica (with diameters between 50 µm and 1 mm), different types fibers such us crystalline fibers, photonic are also used. Fiber optic technologies can be used to detect other important plasma signals such as shock waves [35].
2.3.3.3 Detection: Detection is increasingly carried out using a “ladder spectrometer” developed from the 1950s with its first application for LIBS analysis dating from the late 90s [45, 46]. Once the plasma light has been collected, it must go through a suitable detection to be able to analyze it. This system includes two parts: a spectrometer to select one or more wavelengths of interest and a detector. The spectrometer is a device which diffracts the light emitted by the plasma. There are different models, but the most used in the LIBS is the Czemy-Turner. This spectrometer is composed of an entrance slit, two mirrors and a diffraction grating. The light passes through slit and reach the first mirror which collimates the light, directly it on the network. Light is reflected from different angles depending on it wavelength. The second mirror concentrate the light on the focal plane where the detector is placed. The different detectors used in LIBS depends on the application or analysis to perform [35]. It allows to select in the plasma radiation the length (s) wave of interest. It is characterized in the case of a slit spectrometer by: its range spectral which determines the wavelength range over which the emission lines can be detected, it brightness, characterized by the maximum light flux transmissible which influence the detection limit of the instrument and finally its resolution which is a function of its ability to separate lines for a given wavelength [35, 42].
The detector is chosen according to the spectrometer and its ability one or more several wavelengths. For the detection of a single wavelength it is possible to use photodiodes or even photomultiplier tubes. In the case of the simultaneous detection of several lines. CCDs light sensors (Charge Coupled Device) or CCD camera will be used instead. The start of signal acquisition with one of these detections is always offset by one or two microseconds relative to the formation of plasma to avoid the predominance of the background continued. Elements called Micro-Channels Plates (MCP) can boost the signal by applying the voltage between the terminal of MCP after a certain delay and during defined direction. This amplification makes it possible to define a specific window acquiring a signal [35].
3. Advantages and Applications
3.1 Advantages and limitation of the LIBS
The advantages of this material analysis technique are numerous: detection possible of all elements, high selectivity and sensitivity (ppm) the realization of non-contact measurement, a rapid technique usually form a few second to a few minutes, it allows a simultaneous multi-elemental analysis, all of the chemical elements of the periodic table can be detected simultaneously, with a detection limit specific to each element, which can reach ppm in the most favorable [1, 47]. It is a technique capable of analyzing solids, liquids, gases and aerosols, and whose instrumentation goes from the fully portable system to device transportable in a vehicle, or even fully robotized, depending on the application and needs. The detection limit compared to exiting analytical technique, the LIBS reach detection limit even higher, in the order of ppm up to the hundred ppm according to the element considered [27, 28, 47]. LIBS does not only have advantages, it also suffers from some limitations. Reproducibility poses a problem because plasma generation is a stochastic process which is highly depend on conditions but also the surface condition of the material. The use of high lasers power can damage the optics or cause ionization of the air in contact with lenses if the fluence is too strong. Technological integration in environment can be difficult.
3.2 Applications
For nearly 30 years, analysis technique based on laser ablation have been considerably developed since these allow a direct analysis of materials without going through an ample preparation [48]. It should also be noted that laser ablation reduces the risk of contamination and require a good quantity of material less compared to the required for a solution. A few years ago, a LIBS has taken on an industrial dimension for analysis in several area because of the easy of analysis on the one hand and the portability of the device on the other. The analyzes to distance are also of particular importance in the use of LIBS [1]. This technique is distinguished by its ability to analyze all type of materials, under any physical form: solid, liquid or gas. But also, this multi-elementary method could meet the analytical needs expressed in the environment field ETM-related mining because it can be used in situ, remotely, without preparation of the sample, and allow to know the elementary composition of the desired material [5, 19, 49].
3.2.1 The use of LIBS in archeological search: Archeological sample or even sample form cultural sites are difficult to analyze. Some cannot be moved on risk of damage it. Certain chemical techniques necessary for their studies require preparation for preliminary sample for laboratory analysis which is small task. LIBS has considerable advantages over conventional methods for this purpose of analyzes [50]. First, portable LIBS devices can be used, solving this problem of moving samples and secondly it doesn’t require a contact with a sample for analysis, preventing damage to samples precious. LIBS is used to determine the element content of material in a variety of object, including painted art, icons, polychromes, weapons, sculptures, pottery, sculpture, and object made from metal, glass, and stones. The LIBS can also be used for the restoration of works of art, thereby removing small amounts of material, typically a few micrograms per pulse laser. In the field of painting, it makes it possible to determine the element that make up the pigments, this analyze of pigments can help date and authenticate frescoes or paintings. In additional, LIBS can be used, combined with other technique, to add the potential, like Raman or X-ray fluorescence (XRF), ICP-MS [15, 50].
3.2.2 LIBS technology in biomedical research: LIBS technology entered the biomedical field a few years ago only. It has the capacity to analyze the chemical composition of samples biological fluids such as human fluids, bones, and tissues. It can also help detect toxic elements as well as an excess or a deficit of mineral in the tissues, teeth, nails, or bones. Likewise, cancer detection will be possible with LIBS and it can provide a surgical device that would detect and destroy tumor cell at the same time [51]. In addition, the classification of bacteria or pathogenic virus is also possible. Exogenous and endogenous metal which constitute biological tissue can also be highlighted by technology LIBS (P, Fe, Na, Ca, and Mg). it can also be easily used in laboratories of research for routine element analyzes in the field of nanotechnology [19, 35, 47].
3.2.3 LIBS technology in the food industry and agronomy: LIBS technology has seen its use spread in the agricultural field and food industry [12, 52, 53]. It made it possible, for example, to detect exogenous sugars in honey [54]. Augusto et al. [55] were able to dose calcium (Ca), potassium (K) and magnesium (Mg) in commercial samples of milk powder and food supplements. Alfarraj et al. [56] were able to dose problem, liquid, calcium magnesium and potassium with LIBS. Xavier et al. [18] compared LIBS to SAA to measure calcium in infant formula and have shown that LIBS have the advantage over PAC thanks to its simplicity. Speed of an analysis and minimal preparation of the sample. Ahmed et al. [57] used LIBS to determine soil fertility from southern Iraqi. Marangoni et al. [58] were able to quantify phosphorus in the sample fertilized thanks to LIBS. Erler et al. [59] detected a series of nutrients Ca, K, Mg, N, P, Mn and Fe in the soil for agriculture.
3.2.4 LIBS technology in industry: Industrial process monitoring is of the preferred application of LIBS [60, 61]. It is generally associated with rapid and remote analysis, systems must be adapted to the constraint of the industrial process in order to allow analysis in environment that are not easily accessible to human, sometime with hot, corrosive or radioactive. They can be combined with small device to identify the position of the sample and adjust the focusing distance. Correctly, LIBS find its place in industrial development such as the metallurgical industry for analysis [62]. In geology, two main issues are addressed: identifying minerals in the field, and measuring weakly concentrated elements in rocks [24, 41]. The works of Noll. R et al. [63] have highlighted the different LIBS application between 2014 and 2018, LIBS is used to the space sector to the analysis of rocks on the planet Mars [5, 19, 64], in defense it is used in the detection explosives or biological agents [65] and in the nuclear field it is used in the detection and quantification uranium and other metals [66-68]. The works of Rifaia et al [8] using a commercial LIBS device, were able to detect six majors elements namely nickel, iron, cobalt, copper, magnesium, and sulfur (Ni, Fe, Co, Cu, Mg and S) on pressed nickel-copper ore powder. The works of Li et al. [20] ont permis de doser le cuivre dans un échantillon de concentré de cuivre. Lu et al. [9] were able to measure manganese, cadmium, and copper in an aqueous solution.
3.2.5 LIBS technology in the environment: The possibility of making fields measurements with compact instruments very early motivated the development of LIBS for environment monitoring, be it air, water or soil. For these applications, it is generally metal pollution heavy that are wanted. In the air, the objective is to detect in real time and continuously suspended particles, to monitor air quality (fine particular by example), or the discharges from industrial installations such as glass foundries or metal, or incinerators. New application is emerging for the control of exposure to (nano) particles in the workplace [41]. Soil and rocks are also an important field for application of LIBS thus, one can quickly and locally maps the pollution of a site, using a portable, fiber-optic even embedded LIBS instrument in a vehicle, and which may include a device for rapid preparation of the sample taken (sieving, tableting, drying etc.) [13].
Zhao et al [69] were able to measure lead in a soil sample while Sugito et al [4] used LIBS with a nd: YAG pulsed laser (1064 mm, 8 ns, 200 mJ) on a sample polluted soil from the paper industry and highlighted Fe, Cd and Mg. Wang et al. [10] analyzed pollution in a high voltage insulators by LIBS and succeeded to dose solution , magnesium, silicon, iron, oxygen and Carbon (Na, Mg, Si, Fe, O and C). The qualitative and quantitative characterization of metals in materials particles from a diesel engine service by LIBS made it possible to dose C, Fe, Mg, Ai, Cr, Zn, Na and Ca [70]. Rifaia et al. [8] were able to measure P, Si, Mg, C, Fe and Ai using LIBS in phosphate waste. Vinicius C.C et al [71] used LIBS to the identification and classification of electron polymer waste.
The LIBS CURIOSITY analyzer used for principal component analysis (PCA) of the almost half a million spectra captured on the scanned area of 30 mm 40 mm allowed to identify seven classes of mineral on the surface of the sample [60]. They identify the braggite, pentlandite, chalcopyrite, pyntholite, bytownite, olivine and actinolite. The analysis elementary has shown in particular that, unlikely the light metals Al, Na and K, the heavy metals Fe, Cu, Ni, Pt and Pd are mainly contained in minerals based sulfides which are incorporated in rich silicon bytownite [8].
LIBS technology has been used to identify impunities and dangerous elements in the materials during the manufacturing process, several chemical elements have been detected such as AI, Si, P, Ca, Mg, N, Br, AI and C [53]. The works of Rai et al [72] on the Dergaon meteorite in India were able to highlight metallic elements such as Na, K, Mg, Ni and Cr using LIBS technology. Air quality can also be monitored by using LIBS technology. LIBS can also be used for detecting products toxic like heave metal in industrial waste [13]. This waste must be recycled or stored, and knowledge of the elements they contain can provide key data to reduce the environment impact of the process.
4. Conclusion
Laser induced plasma atomic emission spectroscopy (LIBS) is a technique analysis of the chemical composition of materials, which presents a wide range of attractive features: simultaneous multi-element analysis, application to all type of material (solid, liquid, gas, aerosol) in real time from a distance. She was born a day ago half a century and to days it stands out as an essential technique for both quantitative as well as qualitative analyzes. It has several advantages including its ease of use in the field, in industry in real time, the non-preparation of sample, cost and portability. It was the latter that earned him to be taken on board. In the curiosity robot to explore the soils of planet Mars. Its ability to provide in situ chemical analyzes and without prior preparation of samples have done a simple technique for monitoring pollution in trace metal elements (ETM) of soil, water, and air.
Acknowledgements
We would like to thank the entire department of Fundamental and Applied Geology of the Polytechnic Faculty of Mons for their moral and material support.
Conflicts of interest
The authors declare no conflict of interest with respect to the research, authorship and/or publication of this article.
References
- Noll R, Fricke-Begemann C, Conneman S, et al. LIBS analyses for industrial applications – an overview of developments from 2014 to 2018. Journal of Analytical Atomic Spectrometry 33 (2018): 945-956.
- Kondo H, Hamada N, Wagatsuma K. Determination of phosphorus in steel by the combined technique of laser induced breakdown spectrometry with laser induced fluorescence spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy 64 (2009): 884-890.
- Sarah C Jantzi VMR, Florian Trichard, Yuri Markushin, et al. Sample treatment and preparation for laser-induced breakdown spectroscopy. spectrochimica Acta Part B 115 (2016): 52-63.
- Sugito H, Khumaeni A, Binu QM. Detection of heavy metal containment of soil pollution due to waste of paper industry using Nd:YAG laser induced breakdown spectroscopy. ournal of Physics: Conference Series (2020): 1428.
- Jean-Marc B. Faire la lumière sur les mystères du sous-sol avec… de la lumière. Lumière dans les Sciences de l’Ingénieur. Polytech-News 55 (2017): 21.
- Yu, J.a.M.-R V. Spectroscopie du plasma induit par laser pour l'analyse de matière organique. Photoniques 63 (2013): 38-43.
- Lau S.K.a.C NH. Minimally Destructive and Multi-Element Analysis of Steel Alloys by Argon Fluoride Laser-Induced Plume Emissions. Applied Spectroscopy 63 (2009): 835-838.
- Rifai K, Özcan L, Doucet F, et al. Rapid analysis of phosphate slurries and pressed pellets using laser-induced breakdown spectroscopy. Spectrochimica Acta Part B: Atomic Spectroscopy 163 (2020): 163.
- Jiazhe Lu JL, Mingliang Li, Xun Gao. Quantitative Analysis of Mn, Cd and Cu Elements in Aqueous Solution Based on LIBS Technology. Applied Physics 10 (2020): 103-109.
- Wang X, Lu S, Wang T, et al. Analysis of Pollution in High Voltage Insulators via Laser-Induced Breakdown Spectroscopy. Molecules 25 (2020).
- Pandhija S, Rai NK, Rai AK, et al. Contaminant concentration in environmental samples using LIBS and CF-LIBS. Applied Physics B 98 (2010): 231-241.
- Santos D, Nunes LC, Trevizan LC, et al. Evaluation of laser induced breakdown spectroscopy for cadmium determination in soils. Spectrochimica Acta Part B: Atomic Spectroscopy 64 (2009): 1073-1078.
- Sirven J, Bousquet B, Canioni L, et al. Laser-induced plasma spectroscopy: an emerging technique for on-site analysis of polluted soils. Environment, Spectra analysis (2005).
- Sirven JB, Sallé B, Mauchien P, et al. Feasibility study of rock identification at the surface of Mars by remote laser-induced breakdown spectroscopy and three chemometric methods. Journal of Analytical Atomic Spectrometry 22 (2007): 1471.
- Olga Syta BW, Ewa Bulska, Dobrochna Zielinska, et al. Elemental imaging of heterogeneous inorganic archaeological samples by means of simultaneous laser induced breakdown spectroscopy and laser ablation inductively coupled plasma mass spectrometry measurements. Talanta 179 (2018): 784-791.
- Guirado S, Fortes F, Lazic V, et al. Chemical analysis of archeological materials in submarine environments using laser-induced breakdown spectroscopy. On-site trials in the Mediterranean Sea. Spectrochimica Acta Part B: Atomic Spectroscopy 74 (2012): 137-143.
- Bader A Alfarraj HKS, Chet R Bhatt, Fang Y Yueh, et al. Qualitative Analysis of Dairy and Powder Milk Using Laser-Induced Breakdown Spectroscopy (LIBS) Applied Spectroscopy 72 (2018): 89-101.
- Cama-Moncunill X, Markiewicz-Keszycka M, Dixit Y, et al. Feasibility of laser-induced breakdown spectroscopy (LIBS) as an at-line validation tool for calcium determination in infant formula. Food Control 78 (2017): 304-310.
- Leprince M, Sancey L, Coll JL, et al. L’imagerie élémentaire par spectroscopie LIBS. Médecine/sciences 35 (2019): 682-688.
- Li H, Huang M, Xu H. High accuracy determination of copper in copper concentrate with double genetic algorithm and partial least square in laser-induced breakdown spectroscopy. Optics Express 28 (2020): 2142.
- Carvalho GGAD, Nunes LC, Souza PFD, et al. Evaluation of laser induced breakdown spectrometry for the determination of macro and micronutrients in pharmaceutical tablets. Journal of Analytical Atomic Spectrometry 25 (2010): 803-809.
- Green RL, Mowery MD, Good JA, et al. Comparison of Near-Infrared and Laser-Induced Breakdown Spectroscopy for Determination of Magnesium Stearate in Pharmaceutical Powders and Solid Dosage Forms. Applied Spectroscopy 59 (2005): 340-347.
- Vítková G, Novotný K, Prokeš L, et al. Fast identification of biominerals by means of stand-off laser-induced breakdown spectroscopy using linear discriminant analysis and artificial neural networks. Spectrochimica Acta Part B: Atomic Spectroscopy 73 (2012): 1-6.
- Anderson RB, Morris RV, Clegg SM, et al. The influence of multivariate analysis methods and target grain size on the accuracy of remote quantitative chemical analysis of rocks using laser induced breakdown spectroscopy. Icarus 215 (2011): 608-627.
- Palanco S, Alises A, Cuñat J, et al. Development of a portable laser-induced plasma spectrometer with fully-automated operation and quantitative analysis capabilities. J. Anal. At. Spectrom 18 (2003): 933-938.
- Palanco SaL J. Remote sensing instrument for solid samples based on open-path atomic emission spectrometry. Review of Scientific Instruments 75 (2004): 2068-2074.
- Lanza NL, Clegg SM, Wiens RC, et al. Examining natural rock varnish and weathering rinds with laser-induced breakdown spectroscopy for application to ChemCam on Mars Applied Optics 51 (2012): B74-B82.
- Cousin A, Forni O, Maurice S, et al. Laser induced breakdown spectroscopy library for the Martian environment. Spectrochimica Acta Part B: Atomic Spectroscopy 66 (2011): 805-814.
- Gaft M, Nagli L, Fasaki I, et al. Laser-induced breakdown spectroscopy for on-line sulfur analyses of minerals in ambient conditions. Spectrochimica Acta Part B: Atomic Spectroscopy 64 (2009): 1098-1104.
- Yun JI, Klenze R, Kim, JI. Laser-Induced Breakdown Spectroscopy for the On-Line Multielement Analysis of Highly Radioactive Glass Melt Simulants. Part II: Analyses of Molten Glass Samples. Applied Spectroscopy 56 (2002): 852-858.
- Debras-Guédon JaL N. The use of a ruby laser as an excitation source in emission spectroscopy. Comptes Rendus de l’Académie des Sciences 257 (1963): 3336-3339.
- Korunchikov AIaY AA. Some aspects of the plasma uptake and spectrum excitation produced by laser radiation. Journal of Applied Spectroscopy 5 (1966): 429-434.
- Wiggins TH, Wick RV, Rank DH, et al. Laser-Induced Breakdown in Oxygen Gas at High Pressure. Applied Optics 5 (1966): 166.
- Cremer LJRaDA. Spectrochemical Analysis using laser plasma excitation, Laser Induced Plasmas and Apllications International Atomic Energy Agency (IAEA) 21 (1989): 21.
- Anabitarte F, Cobo A, Lopez-Higuera JM. Laser-Induced Breakdown Spectroscopy: Fundamentals, Applications, and Challenges. ISRN Spectroscopy 2012 (2012): 1-12.
- Hahn DWaO N. Laser-Induced Breakdown Spectroscopy (LIBS), Part I: Review of Basic Diagnostics and Plasma-Particle Interactions: Still-Challenging Issues within the Analytical Plasma Community. Applied Spectroscopy 64 (2010): 12.
- Seyyed Ali Davari PAT, Robert W Standley, Dibyendu Mukherjee. Detection of interstitial oxygen contents in Czochralski grown silicon crystals using internal calibration in laser-induced breakdown spectroscopy (LIBS). Talanta 193 (2018): 192-190.
- Clair GaLH D. 1D modelling of nanosecond laser ablation of copper samples in argon at P = 1 atm with a wavelength of 532 nm. Journal of Applied Physics 110 (2011): 8.
- Fornarini L, Fantoni R, Colao F, et al. Theoretical Modeling of Laser Ablation of Quaternary Bronze Alloys: Case Studies Comparing Femtosecond and Nanosecond LIBS Experimental Data. The Journal of Physical Chemistry A 113 (2009): 14364-14374.
- Aguilera JaA C. Characterization of laser-induced plasmas by emission spectroscopy with curve-of-growth measurements. Part II: Effect of the focusing distance and the pulse energy. Spectrochimica Acta Part B: Atomic Spectroscopy 63 (2008): 793-799.
- (CEA) C.à.l.é.a.e.a.é.a. La LIBS : les applications d’un laser d’analyse, des systèmes nucléaires à l’exploration spatiale. CEA SACLAY (2014).
- Cristoforetti G, Giacomo AD, Dell'aglio M, et al. Local Thermodynamic Equilibrium in Laser-Induced Breakdown Spectroscopy: Beyond the McWhirter criterion. Spectrochimica Acta Part B: Atomic Spectroscopy 65 (2010): 85-95.
- Miziolek AW, Palleschi V, Schechter I. Laser Induced Breakdown Spectroscopy. Cambridge University Press (2006).
- Philippe Aubourg FF, Patrick Mauchien, François Salin. Deux exemples d’applications industrielles des lasers : La fabrication des cellules photovoltaïques et la spectroscopie d'émission. Reflets de la Physique 927 (2010): 83-87.
- Bauer H, Leis F, Niemax K. Laser induced breakdown spectrometry with an échelle spectrometer and intensified charge coupled device detection. Spectrochimica Acta Part B: Atomic Spectroscopy 53 (1998): 1815-1825.
- Hiddemann L, Uebbing J, Ciocan A, et al. Simultaneous multi-element analysis of solid samples by laser ablation-microwave-induced plasma optical emission spectrometry. Analytica Chimica Acta 283 (1993): 152-159.
- Kaiser J, Novotný K, Martin MZ, et al. Trace elemental analysis by laser-induced breakdown spectroscopy-Biological applications. Surface Science Reports 67 (2012): 233-243.
- David W, Hahn NO. Laser-Induced Breakdown Spectroscopy (LIBS), Part II: Review of Instrumental and Methodological Approaches to Material Analysis and Applications to Different Fields. Applied Spectroscopy 6 (2012): 347-419.
- Stehrer TBP, Viskup RJ, Jasik H, et al. Laser-induced breakdown spectroscopy of iron oxide powder. Journal of Analytical Atomic Spectrometry 24 (2009): 973-978.
- Giakoumaki A, Melessanaki K, Anglos D. Laser-induced breakdown spectroscopy (LIBS) in archaeological science-applications and prospects. Analytical and Bioanalytical Chemistry 387 (2006): 749-760.
- A K Pathak NKR, Ankita Singh AK Rai, Pradeep K Rai, et al. Medical Applications of Laser Induced Breakdown Spectroscopy. Journal of Physics: Conference Series 548 (2014): 1-8.
- Senesi GSaS N. Laser-induced breakdown spectroscopy (LIBS) to measure quantitatively soil carbon with emphasis on soil organic carbon. A review. Analytica Chimica Acta 938 (2016): 7-17.
- Farooq W, Al-Johani AS, Alsalhi M, et al. Analysis of polystyrene and polycarbonate used in manufacturing of water and food containers using laser induced breakdown spectroscopy. Journal of Molecular Structure (2020): 1201.
- Jiyu Peng WX, Jiandong Jiang, Zhangfeng Zhao, et al. Fast Quantification of Honey Adulteration with Laser-Induced Breakdown Spectroscopy and Chemometric Methods. Foods 9 (2020): 1-10.
- Augusto ADS, Barsanelli PL, Pereira FMV, et al. Calibration strategies for the direct determination of Ca, K, and Mg in commercial samples of powdered milk and solid dietary supplements using laser-induced breakdown spectroscopy (LIBS). Food Research International 94 (2017): 72-78.
- Alfarraj BA, Sanghapi HK, Bhatt CR, et al. Qualitative Analysis of Dairy and Powder Milk Using Laser-Induced Breakdown Spectroscopy (LIBS). Applied Spectroscopy 72 (2017): 89-101.
- Hameed MA, Al-Ali ASA, Ali OA, et al. Determination of the Fertility of Southern Iraqi Soil Using Laser - Induced Breakdown Spectroscopy System. Journal of Physics: Conference Series 1279 (2019).
- Marangoni BS, Silva KSG, Nicolodelli G, et al. Phosphorus quantification in fertilizers using laser induced breakdown spectroscopy (LIBS): a methodology of analysis to correct physical matrix effects. Analytical Methods 8 (2016): 78-82.
- Erler A, Riebe D, Beitz T, et al. Soil Nutrient Detection for Precision Agriculture Using Handheld Laser-Induced Breakdown Spectroscopy (LIBS) and Multivariate Regression Methods (PLSR, Lasso and GPR). Sensors 20 (2020): 418.
- Unnikrishnan VK, Choudhari KS, Kulkarni SD, et al. Analytical predictive capabilities of Laser Induced Breakdown Spectroscopy (LIBS) with Principal Component Analysis (PCA) for plastic classification. RSC Advances 3 (2003): 25872.
- Yanwei Yang XH, Lili Zhang, Long Ren. Application of Scikit and Keras Libraries for the Classification of Iron Ore Data Acquired by Laser-Induced Breakdown Spectroscopy (LIBS). Sensors 20 (2020): 1-11.
- Shunsuke KASHIWAKURA* KW, Selection of Atomic Emission Lines on the Mutual Identification of Austenitic Stainless Steels with a Combination of Laser-induced Breakdown Spectroscopy (LIBS) and Partial-least-square Regression (PLSR). ISIJ International, Advance Publication 549 (2020): 1-9.
- Noll R. Terms and notations for laser-induced breakdown spectroscopy. Analytical and Bioanalytical Chemistry 385 (2006): 214-218.
- Maurice S, Wiens RC, Saccoccio M, et al. The ChemCam Instrument Suite on the Mars Science Laboratory (MSL) Rover: Science Objectives and Mast Unit Description. Space Science Reviews 170 (2012): 1-4.
- Lucia FCDaG JL. Classification of explosive residues on organic substrates using laser induced breakdown spectroscopy. Applied Optics 51 (2012): 7.
- Judge EJ, Barefield JE, Berg JM, et al. Laser-induced breakdown spectroscopy measurements of uranium and thorium powders and uranium ore. Spectrochimica Acta Part B: Atomic Spectroscopy 83-84 (2013): 28-36.
- Chinni RC, Cremers DA, Radziemski LJ, et al. Detection of Uranium Using Laser-Induced Breakdown Spectroscopy. Applied Spectroscopy 63 (2009): 1238-1250.
- Carlos E. Ararat-Ibarguen AL, Carolina Corvalan, Nicolas Di Lalla, et al. Laser Induced Breakdown Spectroscopy Application to Reaction-Diffusion Studies in Nuclear Materials. Spectrochimica Acta Part B: Atomic Spectroscopy 19 (2020): 30551-30559.
- Zhao S, Gao X, Chen A, et al. Effect of spatial confinement on Pb measurements in soil by femtosecond laser-induced breakdown spectroscopy. Applied Physics B 126 (2019): 1.
- Viskup R, Wolf C, Baumgartner W. Qualitative and Quantitative Characterisation of Major Elements in Particulate Matter from In-use Diesel Engine Passenger Vehicles by LIBS. Energies 13 (2020): 368.
- Vinicius Camara Costa FWBA, Caio Marcio Paranhos, Edenir Rodrigues Pereira-Filho. Identification and classification of polymer e-waste using laserinduced breakdown spectroscopy (LIBS) and chemometric tools. Polymer Testing 59 (2017): 390-395.
- Abhishek K Rai JKP, Christian G Parigger, Sonali Dubey, et al. The Plasma Spectroscopic Study of Dergaon Meteorite, India. Molecules 25 (2020): 1-10.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 