Radiochemistry of Bangladeshi Phosphogypsum and Leachate Study
Article Information
Satyajit Ghose1,*, Brian Heaton2
1Division of Nuclear Safety and Security, Bangladesh Atomic Energy Regulatory Authority, Dhaka, Bangladesh
2Bio-Medical Physics, University of Aberdeen, Aberdeen AB25 2ZD, U.K.
*Corresponding Author: Dr. Satyajit Ghose, Division of Nuclear Safety & Security,Bangladesh Atomic Energy Regulatory Authority, Dhaka, Bangladesh
Received: 08 April 2020; Accepted: 15 April 2020; Published: 30 July 2020
Citation: Satyajit Ghose , Brian Heaton. Radiochemistry of Bangladeshi Phosphogypsum and Leachate Study. Journal of Environmental Science and Public Health 4 (2020): 184-196.
View / Download Pdf Share at FacebookAbstract
Phosphogypsum is a high volume by-product from Phosphate fertilizer factory, named the Triple Superphosphate complex of Bangladesh containing naturally occurring radionuclide. Distribution of 226Ra, 210Pb and 210Po in the fertilizer factory byproduct, phosphogypsum, has been determined using alpha and gamma-spectrometry. Both nuclides (226Ra and 210Po) were found to be concentrated in the finest-grained materials of phosphogypsum. The investigated radionuclide concentration values in PG obtained were comparable with data reported in the phosphogypsum literature, while lower values were found for these radionuclides. Moreover, laboratory radium and polonium leaching experiments of phosphogypsum by deionized water and selective extraction solution have been performed. Radium leaching results showed that fresh phosphogypsum produced the highest concentration of Ra in solution and old phosphogypsum showed a very low transfer to the water. Polonium leaching experiments have shown that only a small amount of polonium is dissolved in filtrated fresh water, this amount is increased when the pH is decreased. It was observed that some extraction solution is more effective at releasing polonium to water from the gypsum matrix. Approximately 32% of 210Po is present on the gypsum surface and not inside the lattice; this position is easily exchangeable with contact of external media (i.e., MgCl2 extraction solution) and 95% of PG was soluble with concentrated cocktail acid solution and a value of 14% 210Po was released with contact of 0.02M sulfuric acid solution. The results obtained in this study can be utilized to verify the environmentally safe use of phosphogypsum as an amendment to agricultural soils.
Keywords
Phosphogypsum ; Radiochemistry ; Leachate
Phosphogypsum articles, Radiochemistry articles, Leachate articles
Phosphogypsum articles Phosphogypsum Research articles Phosphogypsum review articles Phosphogypsum PubMed articles Phosphogypsum PubMed Central articles Phosphogypsum 2023 articles Phosphogypsum 2024 articles Phosphogypsum Scopus articles Phosphogypsum impact factor journals Phosphogypsum Scopus journals Phosphogypsum PubMed journals Phosphogypsum medical journals Phosphogypsum free journals Phosphogypsum best journals Phosphogypsum top journals Phosphogypsum free medical journals Phosphogypsum famous journals Phosphogypsum Google Scholar indexed journals Radiochemistry articles Radiochemistry Research articles Radiochemistry review articles Radiochemistry PubMed articles Radiochemistry PubMed Central articles Radiochemistry 2023 articles Radiochemistry 2024 articles Radiochemistry Scopus articles Radiochemistry impact factor journals Radiochemistry Scopus journals Radiochemistry PubMed journals Radiochemistry medical journals Radiochemistry free journals Radiochemistry best journals Radiochemistry top journals Radiochemistry free medical journals Radiochemistry famous journals Radiochemistry Google Scholar indexed journals Leachate articles Leachate Research articles Leachate review articles Leachate PubMed articles Leachate PubMed Central articles Leachate 2023 articles Leachate 2024 articles Leachate Scopus articles Leachate impact factor journals Leachate Scopus journals Leachate PubMed journals Leachate medical journals Leachate free journals Leachate best journals Leachate top journals Leachate free medical journals Leachate famous journals Leachate Google Scholar indexed journals gypsum articles gypsum Research articles gypsum review articles gypsum PubMed articles gypsum PubMed Central articles gypsum 2023 articles gypsum 2024 articles gypsum Scopus articles gypsum impact factor journals gypsum Scopus journals gypsum PubMed journals gypsum medical journals gypsum free journals gypsum best journals gypsum top journals gypsum free medical journals gypsum famous journals gypsum Google Scholar indexed journals fresh water articles fresh water Research articles fresh water review articles fresh water PubMed articles fresh water PubMed Central articles fresh water 2023 articles fresh water 2024 articles fresh water Scopus articles fresh water impact factor journals fresh water Scopus journals fresh water PubMed journals fresh water medical journals fresh water free journals fresh water best journals fresh water top journals fresh water free medical journals fresh water famous journals fresh water Google Scholar indexed journals pH articles pH Research articles pH review articles pH PubMed articles pH PubMed Central articles pH 2023 articles pH 2024 articles pH Scopus articles pH impact factor journals pH Scopus journals pH PubMed journals pH medical journals pH free journals pH best journals pH top journals pH free medical journals pH famous journals pH Google Scholar indexed journals agricultural soils articles agricultural soils Research articles agricultural soils review articles agricultural soils PubMed articles agricultural soils PubMed Central articles agricultural soils 2023 articles agricultural soils 2024 articles agricultural soils Scopus articles agricultural soils impact factor journals agricultural soils Scopus journals agricultural soils PubMed journals agricultural soils medical journals agricultural soils free journals agricultural soils best journals agricultural soils top journals agricultural soils free medical journals agricultural soils famous journals agricultural soils Google Scholar indexed journals Phosphate rock articles Phosphate rock Research articles Phosphate rock review articles Phosphate rock PubMed articles Phosphate rock PubMed Central articles Phosphate rock 2023 articles Phosphate rock 2024 articles Phosphate rock Scopus articles Phosphate rock impact factor journals Phosphate rock Scopus journals Phosphate rock PubMed journals Phosphate rock medical journals Phosphate rock free journals Phosphate rock best journals Phosphate rock top journals Phosphate rock free medical journals Phosphate rock famous journals Phosphate rock Google Scholar indexed journals phosphoric acid articles phosphoric acid Research articles phosphoric acid review articles phosphoric acid PubMed articles phosphoric acid PubMed Central articles phosphoric acid 2023 articles phosphoric acid 2024 articles phosphoric acid Scopus articles phosphoric acid impact factor journals phosphoric acid Scopus journals phosphoric acid PubMed journals phosphoric acid medical journals phosphoric acid free journals phosphoric acid best journals phosphoric acid top journals phosphoric acid free medical journals phosphoric acid famous journals phosphoric acid Google Scholar indexed journals
Article Details
1. Introduction
1.1 Production and characteristics of phosphogypsum
In Bangladesh, there is a big Phosphate fertilizer factory, named the Triple Superphosphate (TSP) complex at Potanga area of Chittagong. It consists of two units known as TSP-1 and TSP-II having an installed capacity of 32000 MT and 120000 MT of TSP fertilizers respectively. Initially the factory started with the production of TSP but from 1990 manufacturing of Single Superphosphate (SSP) has been undertaken. The total production of fertilizers is 2247613 MT (1705172 MT of TSP and 542441 MT of SSP) and, as by-products, 2157473 MT of phosphogypsum has been produced (TSPC profile). Phosphate rock is the starting materials for the production of phosphate products. The complex is fully dependent on imported phosphate rock as raw materials. All the phosphate rocks are imported from China, Algeria, Morocco, and Russia.
Phosphogypsum is an acidic by-product produced by the phosphate fertilizer industry during the production of phosphoric acid from phosphate rock. The by-product is composed mainly of gypsum and the phosphorus content is usually below 1% (Rutherford et al, 1994) [1]. Although phosphoric acid can be made in a variety of ways, the most common method is the so-called “wet–process” production in which phosphate rock is reacted with sulfuric acid. The process also results in the production of substantial quantities of by–product gypsum known as “phosphogypsum”. Industrial processing of phosphate rock to manufacture phosphate fertilizers involves the production of phosphoric acid according to the following chemical reaction:
Ca10(PO4)6F2 + 10H2SO4 + 20H2O ------> 10CaSO4.2H2O + 6H3PO4 +2HF
(Phosphate rock)(Phosphogypsum)
While the mole ratio between phosphogypsum and phosphoric acid is 5:3, the mass ratio is closer to 3:1, i.e.; almost 3 tons of gypsum is produced for every ton of phosphoric acid (Hull and Burnett, 1996) [2]. The mass ratio of phosphogypsum produced to phosphate ore rock reacted is about 1.7:1 based on this equation, but is probably less and varies with the proportion of sand and unreacted solids in the ore rock (Mazzilli et al., 2000) [3]. As a consequence of this approach, the phosphate industry, as it operates in most parts of the world, could more accurately be termed a gypsum industry. Since the phosphate industry measures its phosphoric acid production in terms of many millions of metric tons per year, the amount of by-product gypsum produced is clearly very substantial. According to Ferguson (1988), the worldwide production of phosphogypsum, estimated for the year 2000, was 280 million tonnes. Approximately 2.2 million tonnes of this phosphogypsum (1.37 g of PG per gm of TSP production) have already been produced by the phosphate fertilizer industry in Bangladesh, with a production capacity of 6 tonnes per day. Although phosphogypsum is mainly calcium sulphate drihydrate, it contents elevated levels of impurities which originate preliminary from the source phosphate rock used as a raw material in phosphate fertilizer production.
The main environmental concern associated with phosphogypsum in connection to radioactive contamination is the presence of the naturally occurring radionuclides, the 238U decay series daughter products 226Ra, 210Pb and 210Po. 226Ra is considered to be the major source of radioactivity in phosphogypsum and it is chemically analogous to calcium (Haridasan et al., 2001; Rutherford et al.,1994) [1, 4]. It has been shown from the literature data reported by Bolivar et al. (1995) [5] that about 80% of the U, 3% of Po and <1% of Ra stay in the filter phosphoric acid fraction while 15% of U and 90% of Ra and Po remain with the phosphogypsum.
Phosphogypsum can be used for construction or similar applications if it does not produce a radiological risk for the public and the environment. On the other hand, large amounts of phosphogypsum are dumped often into water supplies through at the world. The Adjorf plant in Morocco (which is contributing two-third of phosphates commercialized worldwide) discharges per day 25 thousand tonnes of phosphogypsum directly into the Atlantic Ocean when operating at normal capacity (Becker, 1989; Becker, 1989). The French also discharge into the mouth of the Seine river. The UK has discharged phosphogypsum into rivers or the sea (McDonald et al., 1992, Germain et al., 1994) up to recently. McDonald et al (1992) carried out a radiological assessment in UK marine environment from the discharge effect of the dose received by the public in the UK showing that there was actually higher potential exposure from eating seafood near the Whitenhaven phosphogypsum out fall than from the nearby Sellafield operations which have had significant discharge from a plutonium reprocessing facility.
In Bangladesh, a significant amount of phosphogypsum has been dumped into the Karnaphuli river from the TSP complex and most of the phosphogypsum is considered waste and is usually stored near a processing factory in piles where the surrounding environment may be affected by leaching of the radioactive materials. These discharges may results in serve radiological problem for aquatic creatures and humans due to consumption of aquatic food and river water. It is reported that a significant amount of phosphogypsum is used in agriculture to improve water movement in saline-alkaline soil and as a substitute for lime or limestone in alkaline soil (Alam et al.,1997) [6]. Phosphogypsym, is also used as a substitute for natural gypsum in the manufacture of cements, wall board, plaster and as building materials in dwellings and significant radiation exposure may occur in those materials.
1.2 Aim of the work
The primary aim of this research was to characterise the Bangladeshi phosphogypsum by radiochemical analysis. Although there are many studies on the phosphogypsum worldwide, little information is available about phosphogypsum produced in Brazil. Therefore, the primary objective of this study was to characterize the naturally occurring radionuclides in Brazilian phosphogypsum, aimed at providing a database for the assessment of environmental radiological impacts due to the uses of this material. It was also intended to investigate the radiochemical associations to the phosphate rocks from which the phosphogypsum was derived. In addition, some sampling of ‘old phosphogypsum’ of various ages has been completed in order to assess if during weathering some radioelements migrate preferentially to others during long-tern storage on gypsum piles. According to TSP profile data, the major part of by-product phosphogypsum (~80%) is usually stored near a processing factory in piles where the surrounding environment may be affected by leaching of the radioactive materials and the rest of the phosphogypsum (20%) are discharged to the adjacent tubular of the Karnafulli river. The aim of the reported work is to study the 226Ra and 210Po leaching behaviour from Phosphogypsum for a better understanding of the characteristics of the radioactive impact caused in the contaminated environment and aquatic systems.
2. Materials and Method
2.1 Sample collection and preparation
Two category of (fresh and old) phosphogypsum samples were collected from some piles placed near the TSP complex. Some samples of the raw material (phosphate rock) from which the phosphogypsum was derived were also collected. All the phosphogypsum samples were prepared by air drying at a room temperature, homogenised, sieved and stored in a sealed container for radionuclide analyses. Some phosphogypsum samples were stored for individual analysis of radionuclides in each particle size of the phosphogypsum samples; in these samples, the phosphogypsum cake was ground and sieved to separate it into various particle sizes. The phosphate rock was treated in the same way as the phosphogypsum samples.
2.2. Radioanalytical methods
The radionuclides analyses of 238U decay series particularly, 226Ra, 210Pb and 210Po in the phosphogypsum and phosphate rock samples can be measured using various nuclear measuring techniques gives access to a significant number of nuclides in the natural radioactive series. In this work, the gamma spectrometry technique was used for radionuclide analyses of 226Ra and 210Pb and an alpha spectrometry technique was used for 210Po analyses in phosphogypsum (PG) and phosphate rock (PR) samples. Both techniques were used for 210Pb analyses to compared the results with measured these techniques.
2.2.1 Gamma-spectrometric analysis: The activity concentration of 226Ra was measured in twenty samples of phosphogypsum and two samples of phosphate rocks using gamma spectrometry. 226Ra was determined by measuring its single characteristics g-energy peak 186 keV directly. However, its (226Ra) daughter products 214Bi (609 keV) and 214Pb (352 keV) were also measured (Ghose 2005). Two sizes of Perspex holder geometry were used for radionuclide analysis of phosphogypsum and phosphate rock samples (Ghose, 2005). The system was calibrated using the prepared calibration source in this study as well as the same sample geometry. The referred geometry CSPH1 and CSPH2 (Ghose, 2005) were used for solid sample analyses. For the analyses of 226Ra in liquid samples (for leaching solution) 200 ml Marinelli geometry was used and the same geometry was used for the calibration of the system (Ghose, 2005). The concentration of 210Pb was determined by direct measuring of the activity of its low-energy peak (46.5 keV) in homogenized samples of phosphate rock and phosphogypsum by gamma spectrometry (Ghose, 2005).
2.2.2 Alpha spectrometric analysis: Radiochemical separation and alpha spectrometry were used to determine the activity of 210Po in the phosphogypsum and phosphate rock samples. A method for the determination of 210Po in different sample types was set up involving acid digestion of the samples followed by adjustment of pH, buffering and then spontaneous deposition from solution of both 210Po and 208Po (tracer) onto silver discs. An alpha spectrometer was then employed to count the discs and the resulting ratio of counts between the two polonium isotopes was analysed in order to determine the activity of 210Po in the sample. The determination of 210Pb involved further deposition of all polonium remaining in the solution onto silver discs and then leaving the samples for at least six months for 210Po to ingrown from the decay of any 210Pb present. This was then plated out and counted in the same fashion as before, and, with the assumption that the 210Po and 210Pb had reached a state of equilibrium, the activity of the 210Pb at the time of sampling was calculated (Ghose, 2005).
2.3 Leaching experiments
The laboratory leaching experiments have been carried out in two different ways with regard to the contact of filtered fresh water: (i) Batch-wise leaching and (ii) Continuous leaching. These two leaching processes have also been applied to determine if radium and polonium is mobilized during leaching and to provide additional information on how radium and polonium is bound within the phosphogypsum matrix. Two different samples of PG have been considered for the extended leaching experiment: “fresh PG” collected for one of the factories before it is transported to the piles and “old PG”, after two years from the first collection. The radioactivity concentrations of these samples are shown in Table 2. The PG and water ratio was chosen according to the usual mixtures used by the factories to transport PG to the piles, because it has been found that liquid to solid ratio plays an important role in the leachability of radium from sulphate scale and phpsphogypsum (Ghose and Heaton, 2002; Haridasan et al., 2002 [7]. An effective method of agitating the phosphogypsum and fresh water mixtures was devised applying a proposed technique in this study (Ghose, 2005). After the desired leaching time had elapsed each sample was removed from the agitation equipment and then the solution filtered. The leaching solution was extracted via a vacuum filtration system. Then the leachates were subjected to 226Ra analysis using a Marinelli container.
3 Results and Discussion
3.1 Characteristics of phosphogypsum
3.1.1 Physical characteristics: Phophogypsum has physical properties similar to natural gypsum. Rutherford et al. (1994) [1] reported on the basis of literature data that the particle density of phosphogypsum ranged between 2.27 and 2.40 g.cm-3 and the bulk density within a range between 0.9 and 1.7 g.cm-3. Phosphogypsum generally has a large proportion of medium to fine-organized particles. May and Swened (1984a) reported medium sized particles (0.250 –0.045 mm diameter) to account for 36-60% of the mass of seven phosphogypsum samples. Fine particles (0.045-mm diameter) accounted for 2-49% of the mass and 50% of the phosphogypsum was of fine size or smaller in samples from Alberta. In the investigated phosphogypsum samples, it was observed that approximately 50% (an average value) of the phosphogypsum weight was finer than 0.045 mm of particle size and 40% was medium size larger than 0.060 mm of the mass. The apparent densities of the set of investigated samples ranged between 2.5 and 2.8 g cm-3. The pH of a newly produced phosphogypsum ranged from 2.3 and 4.2 while the pH in an old one reached a value of 7 probably because of leaching processes.
3.1.2 Chemical composition: The chemical composition of phosphogypsum depends on the nature of the phosphate ore, the type of wet process used, the efficiency of plant operation, the age of the stockpile and any contaminants which may be introduced into phosphogypsum at the production plant. The major chemical composition of the investigated phosphogypsum samples are: BaSO4 (0.1%), SrSO4 (0.1%), CaSO4 (33.7%) and the elemental composition of phosphogypsum varies according to the type of wet phosphoric acid process used are in Table 8.1. It can be seen that from the Table, the element composition of PG are barium, strontium, calcium, magnesium, silicon, alunium and iron, where the percentage of these element 0.1%, however for calcium it was ~10%.
|
Elemental (%) |
Phosphogypsum |
|
Acid soluble % wt |
98.6 |
|
Barium |
0.1 |
|
Strontium |
0.1 |
|
Calcium |
9.9 |
|
Magnesium |
<0.1 |
|
Silicon |
0.1 |
|
Aluminum |
<0.4 |
|
Potassium |
<0.1 |
|
Sodium |
<0.1 |
|
Iron |
|
|
Possible composition |
|
|
Acid soluble Barium sulphate |
0.1 |
|
Acid soluble strontium sulphate |
0.1 |
|
Acid soluble calcium sulphate |
33.7 |
|
Acid soluble silica |
0.1 |
|
Total % wt allocated |
34.0 |
Table 1: Chemical composition in Phosphogypsum.
3.1.3 Radioactivity in phosphogypsum: The activity concentrations for 226Ra, 210Po and 210Pb in two types of phosphogypsum samples collected from TPS complex, obtained by alpha and gamma spectrometry are listed in Table 2. The results obtained for 226Ra in PG varied from 295 to 337 Bqkg-1, and for 210Po within a range between 42.5 and 50.7 Bqkg-1.
The second measurement of 226Ra and 210Pb concentrations in different depth profile phosphogypsum samples and phosphate rock, obtained by gamma spectrometry, are listed in Table 3. The results for the activity concentrations of 210Po and 210Pb in phosphogypsum and phosphate rock, obtained by alpha spectrometry, are presented in the same Table 3 (in column 3 and 4 respectively). Table 4 shows the 226Ra, 210Pb and 210Po distribution in different particle size of phosphogypsum sample. From these results (Table 3) it can be seen that the 226Ra and 210Po activity increases when the depth increases until a depth of 3 meter. These differences may be due to two reasons. Firstly, radium or other nuclides may be transferred by rainwater from the upper layer of the phosphogypsum pile to lower layer phosphogypsum pile, after that there is no movement where there is no water after 3-meter depth piles. Secondly, low 226Ra activity in surface samples may be due to the particle size distribution where smaller particles contain more 226Ra and 210Po than larger particles as shown in Table 4. Therefore, radium and polonium activities in the upper layers of phosphogypsum piles, which are attached to larger particles, can be released very easily by rainwater and hence the surface activity is reduced.
210Pb concentration in phosphogypsum has not been reported in the literature as extensively as 226Ra concentration. Horton et al. (1988) found 210Pb content to be slightly higher than 226Ra content in phosphogypsum derived from the Florida phosphate rock. Beaker (1989) reported that most of the Pb contained within phosphate rock is transferred into PG. However, in the present study 210Pb and 210Po are showing the similar activity levels in the phosphogypsum samples, but these nuclides concentrations are much lower than 226Ra in the same samples.
|
Nuclides |
Fresh PG |
Old PG |
|
226Ra |
337 ± 52 |
295 ± 49 |
|
210Po |
50.7 ± 4.8 |
42.5 ± 4.5 |
|
210Pb |
45.2 ± 5.2 |
39.1 ± 5.8 |
Table 2: Specific activities (Bqkg-1 ± S.D.) of 226Ra, 210Pb and 210Po in phosphogypsum (PG) samples.
|
Sample code |
Activity in Bq.kg-1 ± S.D |
|||
|
226Ra (g-spectrometry) |
210Po (a-spectrometry) |
210Pb (a-spectrometry) |
210Pb (g-spectrometry) |
|
|
PGDP-1/0 |
302 ± 44 |
30.1 ± 4.2 |
38.2 ± 3.8 |
43.2 ± 4.6 |
|
PGDP-1/1 |
320 ± 47 |
50.7 ± 4.7 |
37.5 ± 3.7 |
39.1 ± 5.8 |
|
PGDP-1/3 |
328 ± 50 |
35.7 ± 2.9 |
45.3 ± 4.2 |
39.0 ± 4.2 |
|
PGDP-2/0 |
274 ± 39 |
22.9 ± 2.0 |
15.4 ± 4.0 |
31.2 ± 3.8 |
|
PGDP-2/1 |
285 ± 51 |
25.3 ± 3.0 |
23.8 ± 2.8 |
30.3 ± 5.4 |
|
PGDP-2/3 |
285 ± 42 |
26.4 ± 2.5 |
28.9 ± 3.9 |
35.6 ± 4.0 |
|
PGDP-3/0 |
271 ± 46 |
32.9 ± 2.4 |
34.7 ± 2.7 |
31.6 ± 7.2 |
|
PGDP-3/1 |
286 ± 55 |
28.1 ± 3.2 |
30.3 ± 3.0 |
35.6 ± 3.8 |
|
PGDP-3/3 |
284 ± 39 |
22.5 ± 1.9 |
24.3 ± 2.1 |
29.4 ± 3.2 |
|
PR-1 |
2378 ± 259 |
117.4 ± 12.0 |
130.6 ± 13.0 |
101.2 ± 11.0 |
|
PR-2 |
158 ± 27 |
67.1 ± 7.0 |
69.7 ± 5.0 |
67.3 ± 8.2 |
* 0, 1, and 3 denotes the depth in the piles ( 0 m, 1 m and 3 m) of the phosphogypsum
Table 3: Radionuclide concentration in depth profile of Phosphogypsum and Phosphate rock samples taken from TSP fertilizer factory.
|
Particle size |
Activity in Bq.kg-1 ± S.D |
||
|
226Ra (g-spectrometry) |
210Po (a-spectrometry) |
210Pb (a-spectrometry) |
|
|
>45 mm |
373 ± 43 |
81.7 ± 9.2 |
84.5 ± 7.9 |
|
90 mm |
329 ± 50 |
79.9 ± 10 |
67.3 ± 6.7 |
|
120 mm |
297 ± 52 |
52.6 ± 7.2 |
52.0 ± 8.1 |
|
200 mm |
279 ± 48 |
25.5 ± 5.1 |
29.5 ± 5.3 |
|
500 mm |
260 ± 49 |
35.7 ± 4.7 |
28.4 ± 4.7 |
Table 4: 226Ra, 210Po and 210Pb distribution in different size particles of phosphogypsum sample.
3.2 Radium Leaching Experiments
3.2.1 Batch-wise leaching: Laboratory leaching experiments were carried out to examine the leachability of the 226Ra from “fresh PG“ with respect to change in contacts time. On the other hand another experiment, with “old PG” was repeatedly conducted with respect to change in contact times.
Figure 1 shows the 226Ra transferred to water ratio obtained from the batch-wise leaching experiments with contact of fresh de-ionized water and fresh phosphogypsum sample and for different stirring times. The transfer coefficient was quite variable at short times of agitation but showing a steady decline for longer contact times (0 - 240 hrs). The percentage leached out varied in a range of 3.3 to 0.7% and the maximum value was observed during the short contact times. The solution concentration of 226Ra activity showed decreasing trend with increasing contacts the times. This is possible due to re-adsorption of the released 226Ra activity for long time stirring.
The high level of radium leachates 1.12 Bq.l-1 (short time), and the low level of radium leachates 0.36 Bq.l-1 (long times). So, the leachability of 226Ra to water could be related to the solubility of the PG itself. However, the 226Ra concentration found in the leachates was in agreement with the results described in previous works (Rutherford et al.1995; Al-Marisi et al.,1999; Haridason et al., 2002) [8, 9 7 ].
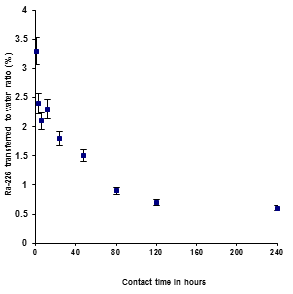
Figure 1: Plot of 226Ra transferred from “Fresh phosphogypsum” to filtered fresh water versus contact time.
When similar leaching experiment was carried out with “Old PG sample”, the radium transferred ratio into water was found for the same time of stirring was very uniform (0.22 to 1.4%). The concentration levels in the leachate also varied in a quite narrow rang 0.65 to 0.09 Bql-1. The result was in agreement with a previous reported results about the fractionation of 226Ra in PG, and shows that a major proportion of 226Ra could be extracted by dissolution with water from fresh PG, the corresponding 226Ra concentration in the acidic liquid fraction obtained pH of the leachates were close to 3.0. These experimental results were clearly related to the acidic content of the liquid fraction. If phosphoric acid is present in the phosphogypsum samples, the pH of the leachate is less than 3 and radium tends to higher values.
3.2.2 Continuous leaching: The continuous leaching experiment was carried out to examine the effect of radium leaching from a reused PG sample with respect to change in time and agitating with additional fresh de-ionized water. This leaching was conducted using 1 day tumbling periods for 7 cycles with contact of fresh water in each cycle. Several sample solutions were filtered through the 0.45 micron pore size membrane filter, the solid quantity again transferred back for each step into the agitating tube, additional fresh water added and the leaching continue until the end of the preset time. The results obtained from this experiment are presented in Figure 2.
It can be seen that the 226Ra leached for the first additional periods was increased, but after that the leach-out of activity remains nearly constant. The initial leachate is responsible for the low leach-out of radium in the first batch that mentioned the leachate radium from phosphogypsum reduce to about 0.2 Bq.l-1. These results assumed that chemical equilibrium is expected between the liquid and solid phase in this leaching procedure. Once equilibrium has been achieved, there will be no net transfer of contaminant from the solid to the liquid as the rate of diffusion is very slow. The total 226Ra activity leached out was found to be around 6% in filtered fresh water after a leaching out 7-cycle period of agitation from the same phosphogypsum sample. Haridasan et al. (2002) [7] reported the concentration of 226Ra in the leachate varied from 0.08-0.38 Bql-1 in the case of distilled water and Rutherford et al. (1995) [8] reported 0.19-0.65 Bql-1 of 226Ra activity in distilled water leachates of phosphogypsum, which is comparable to the values observed in the present study. It can be seen that there was a loosely bound fraction, which come out immediately from the matrix, as the activity levels in the initial extractions were higher in comparison to the subsequent leaching, as observed in earlier experiments.
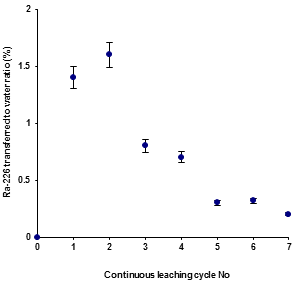
Figure 2: Plot of 226Ra transferred from “Reused phosphogypsum” to filtered fresh water versus continuous leaching cycle.
3.3 Polonium leaching study
In this study, the investigated polonium values in phosphogypsum from Bangladesh samples are lower when compared with those reported for Florida, Spanish, Syrian and Morocco phosphogypsum . Upchurch et al. (1991) reported that 210Po is quite mobile and has been found to account for most of the gross alpha radioactivity within groundwater below phosphate mined lands in Florida. However, no literature has been published on leachability of 210Po from phosphogypsum. In the present work, a preliminary investigation was undertaken to evaluate the leaching mechanisms of polonium from phosphogypsum in order to understand the transfer mechanisms of polonium to the environment. Some leaching solutions were used for this investigation, these are as follows: i) de-ionized water to determine the amount of 210Po soluble in water, ii) MgCl2 solution to determine the amount of exchangeable 210Po, which is adsorbed on phosphogypsum particle surface, iii) 0.02M H2SO4 was used to determine the polonium sulphate solubility with this sulphate solution, iv) concentrated acid solution (HNO3 + HCl + HClO3 with 30% of H2O2) was used to determine the amount of 210Po leachate in acids. All the experimental results are given in Table 5-7. The leaching results with distilled water are given in Table 5 from these results it can be seen that the amount of 210Po transferred from phosphogypsum to water does not exceed 4.4% after 6 hrs stirring. These results indicate that it is difficult to dissolve 210Po from phosphogypsum with deionized water. The amount of 210Po transferred to the water may be due to the acidic nature of fresh phosphogypsum (pH 2.3). The experimental results are in good agreement with other reported results (Hurst and Arnold, 1982; Al-Masri and Al-Bich, 2002 [10]). The leaching results in Table 6 were completely difference when some reused PG sample was used for a second test. In this experiment, the initial leaching percentage was decreased from 6% to 1.5% when the pH value was increased after each batch-leaching step from pH vales 2 to 7, respectively. The leaching results of fresh PG with contact of some extraction solution is given in Table 7. It was observed that some extraction solution is more effective at releasing polonium to water from the gypsum matrix. Approximately 32% of 210Po is present on the gypsum surface and not inside the lattice; this position is easily exchangeable with contact of external media (i.e., MgCl2 extraction solution) and 95% of PG was soluble with concentrated cocktail acid solution and a value of 14% 210Po was released with contact of 0.02M sulfuric acid solution.
|
Agitation time (min) |
210Po activity in solution (mBq ± S.D.) |
% of leaching |
|
30 |
22 ± 4 |
4.3 |
|
60 |
18 ± 3 |
3.5 |
|
120 |
20 ± 6 |
2.9 |
|
240 |
16 ± 4 |
3.2 |
|
360 |
24 ± 7 |
4.4 |
Table 5: The results of 210Po leaching (by continuous leaching method) of phosphogypsum with contact of distilled water for different agitation times (pH value 2.5 and 210Po = 50.7 Bqkg-1).
|
Batch number |
pH |
210Po activity in solution (mBq ± S.D.) |
% of leaching |
|
1 |
2.5 |
14.2 ± 4 |
3.6 |
|
2 |
4.7 |
25.3 ± 6 |
6.5 |
|
3 |
5.5 |
7.4 ± 4 |
1.9 |
|
4 |
6.1 |
6.4 ± 4 |
1.6 |
|
5 |
7.2 |
6.2 ± 3 |
1.6 |
Table 6: Results of batch-wise leaching of Phosphogypsum with distilled water (leaching time for each batch 30 min and 210Po = 39.0 Bqkg-1).
|
Extraction solutions |
210Po activity in solution (mBq ± S.D) |
(%) of leaching |
|
De-ionized water |
16 ± 4 |
4.4 |
|
1.0 M MgCl2 solution |
57 ± 11 |
32.0 |
|
0.02M H2SO4 |
26 ± 4 |
14.5 |
|
Concentrated acid solutions (HNO3+HCl+HClO3 with 30% of H2O2 |
65 ± 11 |
92.8 |
Table 7: Results of 210Po leaching from phosphogypsum with contact of different extraction solutions (210Po = 35.7 Bqkg-1).
4. Conclusions
Based on the observations made in this paper, the following conclusions can be drawn. The activity concentration of 210Pb and 210Po measured in phosphogypsum was relatively lower than 226Ra. In general, the investigated polonium values in PG samples are also relatively lower in comparison with other reported value in the world. Both nuclides (226Ra and 210Po) were found to be concentrated in the finest-grained materials of phosphogypsum. The fractionation between 226Ra and 210Pb implies that these radionuclides either exist in phosphogypsum as in a different phase or display different adsorption characteristics. Leachates obtained from these leaching experiments, showed that fresh phosphogypsum produced the highest concentration of 226Ra in solution and old phosphogypsum showed a very low transfer to the water. It was found that Ra mobilization from this by-product waste is dependent on the presence of phosphoric acid in the phosphygypsum and is also dependent on the amount present. Polonium leaching experiments have shown that only a small amount of polonium is dissolved in filtrated fresh water, this amount is increased when the pH is decreased. Finally it can be concluded that the dissolution characteristics of 226Ra and 210Po from PG depend largely on the leaching condition such as solution, contact time, acidic of the solution (pH), etc. The high insolubility of 226Ra in PG does not mean that 226Ra in PG leachate are so low as to be a non-issue. The highest concentration of soluble 226Ra measured in this study was 1.12 Bql-1; however this levels of 226Ra still exceeds the WHO (1993) prescribed limit 1 Bql-1 for 226Ra.
References
- Rutherford PM, Dudas MJ, Samek RA. Environmental impacts of phosphogypsum. Science of the Total Environment 149 (1994): 1-38.
- Hull CD, Burnett WC. Radiochemistry of Florida phosphogypsum. J.Envirn. Radioactivity 32 (1996): 213-238.
- Mazzilli B, Palmiro V, Saueia C, et al. Radiochemical characterization of Brazilian phosphogypsum. J.Environ. Radioactivity 49 (2000): 113-122.
- Haridasan PP, Paul AC, Desai MVM. Natural radionuclides in the aquatic environment of a phosphogypsum disposal area. J.Environ. Radioactivity 53 (2001): 155-165.
- Bolivar JP, Garcia-Tenorio R, Garcia-Leon. Fluxes and distribution of natural radionuclides in the production and use of fertilizer. Applied Radiation Isotopes 46 (1995): 717-718.
- Alam MN, Chowdhury MI, Kamal M, et al. Radioactivity in chemical fertilizer used in Bangladesh. Applied Radiation Isotopes 48 (1997): 1165-1168.
- Haridasan PP, Maniyan CG, Pillai PMB, Khan AH. Dissolution characteristics of 226Ra from phosphogypsum. J.Environ. Radioactivity 62 (2002): 287-294.
- Rutherford PM, Dudas MJ, Arocena JM. Radium in phosphogypsum leachates. J. of Environmental Quality 24 (1995): 307-314.
- Al-Marsi MS, Ali AI, Khietou M, et al Leaching of Ra-226 from Syrian phosphogypsum. in: Environmental Radiochemical Analysis G.W.A. NEWTON (Ed.), Royal Society of Chemistry, UK (1999): 21.
- Al-Marsi MS, Al-Bich F. Polonium-210 distribution in Syrian phosphogypsum. J. of Radioanalytical and Nuclear Chemistry 251 (2002): 431-435.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 