Synchronous Pancreatic and Rectal Carcinoma in the Same Patient: Case Report and Review of the Literature
Article Information
John K. Triantafillidis1*, Malgarinos George1, Vagianos Costas2, Papalois E. Apostolos3,4
1GI Medicine Department, Metropolitan General Hospital, Holargos, Athens, Greece
22nd Propedeutic Surgical Department, Laikon Hospital, University of Athens, Greece
3Experimental, Educational and Research Center ELPEN, Pikermi Athens, Greece
4European University Cyprus, School of Medicine, Cyprus, Greece
*Corresponding Author: Dr. John K. Triantafillidis, GI Medicine Department, Metropolitan General Hospital, Holargos, Athens, Greece
Received: 16 October 2019; Accepted: 26 November 2019; Published: 13 January 2020
Citation: John K. Triantafillidis, Malgarinos George, Vagianos Costas, Papalois E. Apostolos. Synchronous Pancreatic and Rectal Carcinoma in the Same Patient: Case Report and Review of the Literature. Archives of Clinical and Medical Case Reports 4 (2020): 059-064.
View / Download Pdf Share at FacebookAbstract
We report a patient with an exceptionally rare combination of synchronous pancreatic and rectal cancer diagnosed within a 4 months’ interval. A 69-year-old female was evaluated for continuous epigastric pain and anorexia over the last three months. The tumor was classified as non-operable, as infiltration of large vessels was detected. The patient responded well to chemo/radiotherapy. However, infiltration of the large vessels was persisted. Four months later the patient started complaining of tenesmus and bloody stools. A digital rectal examination revealed the presence of a mass starting 7-8cm from the anal margin. Sigmoidoscopy showed a mass protruding into the bowel lumen. The patient died 16 months after the diagnosis of pancreatic cancer and 12 months after the diagnosis of rectal cancer. We suggest that clinicians should be aware about the possibility of the existence of multiple primary tumors in the same patient and subsequently to optimize their investigational plans.
Keywords
Pancreatic cancer; Rectal cancer; Synchronous neoplasms; Digestive cancer
Pancreatic cancer articles, Rectal cancer articles, Synchronous neoplasms articles, Digestive cancer articles
Article Details
1. Introduction
During the last years an increasing number of publications describing multiple primary cancers in the same patient appeared in the medical literature. The appearance of synchronous tumors in the same patient represents a significant therapeutic challenge as multi-disciplinary therapeutic approaches should be applied [1]. Multiple primary malignancies are divided into two categories. Synchronous cancers are two different malignant neoplasms occurring simultaneously or within 6 months after diagnosis of the first malignancy. Metachronous malignancies are cancers that developed more than 6 months after diagnosis of the first malignancy [2]. However, others suggest that the definition of synchronous cancers means the existence of two or more histologically distinct simultaneously detected malignancies, two or more malignancies diagnosed during the same hospital admission, or two or more malignancies following each other in sequence by less than two months [3].
Hereby, we present a case of a female patient with pancreatic cancer who, soon after, was diagnosed with a malignant rectal neoplasm. The diagnostic and therapeutic manipulations applied in this patient along with a literature review are presented.
2. Patient and Methods
A 69-year-old non-smoker female patient was referred to us because of epigastric pain, loss of weight, fatigue and anorexia. Her illness started three months earlier with continuous moderate back pain. Peroral NSAIDs were prescribed. Two weeks later she started complaining of moderate pain in the epigastrium. NSAIDs were interrupted and treatment with esomeprazole was introduced without improvement. During the next 4 weeks anorexia, fatigue and loss of 3 Kg BW were added to her previous symptoms. The patient was referred to us for further evaluation. Previous medical history included thyroidectomy and hyperlipidemia. Physical examination apart from mild sensitivity on the palpation of epigastrium was negative.On admission, an abdominal CT scan revealed the presence of a mass in the body of the pancreas of 5 × 4.3 cm. Multiple large local lymph nodes and infiltration of the larger vessels were also seen. No distal metastases were detected. An endoscopic ultrasound confirmed the presence of the tumor and fine needle aspiration cytology confirmed the diagnosis of the primary pancreatic adenocarcinoma. Immunochemical study showed that the tumor was positive for MUC5ac, CK7, Ca 19-9 and CEAm. Blood investigation was unremarkable except of a small increase in the value of CA 19-9 (112, NV: <37U/ml).
As the tumor was classified as a non-operable one, the patient was submitted to chemotherapy and later-on to upper abdomen radiotherapy with a linear accelerator Precise in three levels. The total number of sessions was 25. The daily dose of radiation was 2 Gy and the total dose administered was 50Gy. Chemotherapy consisted of 9 continuous cycles given during four months. Six chemotherapy cycles were repeated 8 months later, as the patient refused to continue treatment according to initial treatment schedule. Radiotherapy was administered during the first chemotherapy cycles. The results were quite favorable as both, the intensity of the pain and the size of the tumor was reduced. Both, chemotherapy and radiotherapy were well tolerated.
3. Results
Four months after diagnosis of pancreatic cancer the patient started complaining of tenesmus and bloody stools. A digital rectal examination revealed the presence of a mass starting 7-8 cm from the anal margin. Sigmoidoscopy revealed a mass protruding into the rectal lumen and histology confirmed the diagnosis of primary rectal adenocarcinoma. Magnetic Resonance Imaging showed a large tumor protruding into the bowel lumen and infiltrating the bowel wall and surrounding tissues including a number of lymph nodes. The size of the tumor was 3 × 6 cm. Because of the possibility the rectal tumor to represent metastases in the bowel wall from the pancreatic adenocarcinoma, special immunostaining studies confirmed the origin of this malignancy from the rectal wall.
On the basis of these data the patient was considered as suffering from two different synchronous malignant neoplasms namely pancreatic and rectal adenocarcinoma. The oncology team decided the rectal tumor to be treated with topical radiotherapy only without adjuvant chemotherapy. The neoplasmatic tissue of the patient was tested for microsatellite instability with immunohyperoxidase linked with chromogene DAB. For the study of various antibodies the following clones were tested.: for index MLH1 the clone M1 (VENTANA), for index MSH2 the clone G219-1129 (CELL MARQUE), for the index MSH6 the clone 44 (VENTANA) and for the index PMS2 the clone EPR3947 (CELL MARQUE). For all indices a control index was used. The results based on the expression of the nuclear proteins were as follows: MLH1: 100%, MSH2: 100%, MSH6: 90% and PMS2: 100% of the malignant cells, thus classifying the neoplasm as MSI-stable carcinoma.
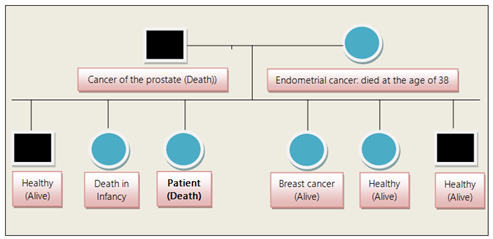
The patient died sixteen months after the diagnosis of pancreatic cancer and twelve months after the diagnosis of rectal cancer. The pedigree of the patient’s family revealed that the patient’s parents died from malignancy and one of her sisters died from breast cancer (Figure 1).
4. Discussion
In this report an exceptionally rare case of synchronous appearance of pancreatic and rectal cancer is presented. The different origin of the two neoplasms was confirmed histologically, as always there is a possibility the second tumor to be a metastatic from the first one [4]. Multiple primary tumors are classified as synchronous and metachronous. Most of the authors suggest that synchronous tumors are tumors that are diagnosed either at the same time or within six months from the diagnosis of the first tumor, while metachronous are tumors that are diagnosed at least six months after diagnosis of the first tumor [5]. In our case, since the two tumors were diagnosed within four months, we considered them as synchronous cancers [6].
The development of multiple synchronous or metachronous tumors may be related to an individual predisposition or the influence of some carcinogenic factor(s) acting on different organs at different times. So far, the risk factors implicated in the pathogenesis of the synchronous malignancies are related to an increasing discovery rate due to the application of modern diagnostic techniques; an increased number of patients with malignant disorder due to population aging; a preexisting immunologic and genetic defect including familial susceptibility; and the previously applied radiation/chemotherapy for the primary cancer [7, 8].
Among the previously mentioned factors, the combination of environment and genetic factors seems to be the most important. In our patient her mother and father died from prostate and endometrial cancer respectively, while one of her sisters developed breast cancer. Gastrointestinal tract cancers having a hereditary predisposition include Cowden syndrome, Peutz-Jeghers syndrome, Lynch syndrome, Serrated Polyposis syndrome, Familial Adenomatous Polyposis syndrome, attenuated Familial Adenomatous Polyposis syndrome, MUTYH-associated polyposis syndrome, Hereditary Pancreatic Cancer, and Hereditary Gastric Cancer. Concerning genetic abnormalities in these syndromes the presence of microsatellite instability is a characteristic feature of Lynch syndrome. The existence of microsatellite instability support the assumption that mismatch repair deficiency may be responsible for a proportion of colorectal cancers. Germ-line mutations of P53 tumor suppressor gene could also be found in some patients with multiple primary cancers [9]. Somatic genomic analysis of the synchronous adenocarcinomas with the next-generation DNA sequencing rise the possibility that genomic alterations might be responsible for modulating the phenotypic or clinical expression of the tumors [10].
It is of interest that patients with pancreatic cancer often exhibit a multiple primary cancer history and that their relatives have an increased risk for the development of malignant solid tumors, a predisposition that has been attributed to an inherited component [11]. Mu-Ni Hu et al recently described a patient with syncronous multiple primary gastrointestinal cancers, in whom E-cadherin expression was downregulated in the malignant cells, and β-catenin was translocated to the cytoplasm and nucleus. DNA sequencing indicated C.57T>G and C.1418A>T in CDH1, thus emphasizing the role of CDH1 mutations in the pathogenesis of synchronous primary GI malignancies [12].
Although few cases of synchronous gastrointestinal cancers have underlying genetic predisposition, most of the authors emphasize that molecular genetic studies could identify dependencies which are at the moment not certain. Even if we consider the appearance of the synchronous pancreatic and colorectal cancer as sporadic (of course excluding both, the intraductal pancreatic mucinous neoplasm, whose association with colorectal cancer is greater than an invasive ductal carcinoma [13] and the Lunch syndrome), genetic studies could finally influence our therapeutic decisions. Unfortunately genetic studies in our patient were not performed. Regarding the treatment strategy most of the authors agree that the more advanced cancer should be treated first. In our case, the pancreatic adenocarcinoma was treated first as it was diagnosed earlier.
In conclusion, the described case underlines the importance of performing an adequate preoperative staging and follow-up in every patient in order to exclude the possibility of a second synchronous tumor. Although it seems excessive to suggest a complete investigation in all patients with one primary cancer, clinicians should be aware about the possibility of the existence of multiple primary tumors in the same patient and subsequently to optimize their investigational plans.
Informed Consent
Written informed consent was obtained from the relatives of the patient after her death.
Financial Disclosure
The authors declare that received no financial support for this study.
Author Contributions
JKT was responsible for the patient; he designed and wrote the paper
PA, VK, and MG interpreted the data and made suggestions
References
- Jena A, Patnayak R, Yadagiri A, et al. Multiple primary cancers: An enigma. S Asian J Cancer 5 (2016): 29-32.
- Irimie A, Achimas-Cadariu P, Burz C, et al. Multiple primary malignancies – Epidemiological analysis at a single tertiary institution. J Gastrointestin Liver Dis 19 (2010): 69-73.
- Howe HL. A review of the definition for multiple primary cancers in the United States. Workshop Proceedings, December 4–6, 2002, in Princeton, New Jersey. Springfield (IL): North American Association of Central Cancer Registries (2003).
- Boussios S, Zerdes I, Batsi O, et al. Pancreatic resection for renal cell carcinoma metastasis: An exceptionally rare coexistence. Int J Surg Case Rep 27 (2016): 198-201.
- Sakellakis M, Peroukides S, Iconomou G, et al. Multiple primary malignancies: report of two cases. Chin J Cancer Res 26 (2014): 215-218.
- Nanashima Α, Tominaga Τ, Nonaka Τ, et al. A case of multiple synchronous quadruple cancers of the stomach, sigmoid colon, rectum, and pancreas. J Surg Case Rep 35 (2017): 4-7.
- Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am 14 (2000): 17-23.
- Crocetti E, Buiatti E, Falini P. The Italian Multiple Primary Cancer Working Group Multiple primary cancer incidence in Italy. Eur. J. Cancer 37 (2001): 2449-2456.
- Kimura K, Shinmura K, Hasegawa T, et al. Germline p53 mutation in a patient with multiple primary cancers. Jpn J Clin Oncol 31 (2001): 349-351.
- Castro M, Vierkoetter K, Prager D. Montgomery S. Sedgwick K. Synchronous onset of breast and pancreatic cancers: results of germline and somatic genetic analysis. Case Rep Oncol 9 (2016): 387-394.
- Gerdes B, Ziegler Z, Ramaswamy A, et al. Multiple primaries in pancreatic cancer patients: indicator of a genetic predisposition? International J Epidemiol 29 (2000): 999-1003.
- Mu-Ni Hu, Wei Lv, Rui-Yue Hu, et al. Synchronous multiple primary gastrointestinal cancers with CDH1 mutations: A case report. World J Clin Cases 7 (2019): 1703-1710.
- Eguchi H, Ishikawa O, Ohigashi H, et al. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery 139 (2006): 749-754.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
