Atypical Progeroid Syndrome with Familial Partial Lipodystrophy due to A Missense c.1045 C > T LMNA Mutation: A Case Report and an Innovative Therapeutic Approach
Article Information
Benedetta Russo1,2, Marika Menduni1,2, Andrea Mari3, Caterina Pelosini4, Francesco Brancati5,6, Maria Rosaria D’Apice7, Fabiana Picconi1*, Simona Frontoni1,2, Ilaria Malandrucco8
1Unit of Endocrinology, Diabetes and Metabolism, Fatebenefratelli Isola Tiberina-Gemelli Isola Hospital, 00186 Rome, Italy
2Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
3CNR Institute of Neurosciences, Padua, Italy
4Chemistry and Endocrinology Laboratory, University Hospital of Pisa, 56124 Pisa, Italy
5Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy
6IRCCS San Raffaele Rome, 00163 Rome, Italy
7Laboratory of Medical Genetics, Tor Vergata University Hospital, Rome, Italy
8Endocrinology and Metabolic diseases Frosinone Hospital, Frosinone, Italy
*Corresponding Author: Fabiana Picconi, Unit of Endocrinology, Diabetes and Metabolism, Fatebenefratelli Isola Tiberina-Gemelli Isola Hospital, 00186 Rome, Italy.
Received: 30 July 2022; Accepted: 16 August 2022; Published: 24 September 2022
Citation: Benedetta Russo, Marika Menduni, Andrea Mari, Caterina Pelosini, Francesco Brancati, Maria Rosaria D’Apice, Fabiana Picconi, Simona Frontoni, Atypical Progeroid Syndrome with Familial Partial Lipodystrophy due to a Missense c.1045 C > T LMNA Mutation: A Case Report and an Innovative Therapeutic Approach. Archives of Clinical and Medical Case Reports 6 (2022): 647-652.
View / Download Pdf Share at FacebookAbstract
Familial partial lipodystrophy (FPLD) associated with LMNA gene mutations is a rare form of lipodystrophy disorder characterized by partial absence of subcutaneous adipose tissue and metabolic complications. Recently, this peculiar phenotype has been associated to cardiac disease or to atypical progeroid syndrome (APS). We present a case of 31-yearold woman with progeria features, partial lipodystrophy, type 2 diabetes mellitus (T2D), hypertriglyceridemia and hepatic steatosis. Genetic analysis revealed a missense heterozygous LMNA mutation c.1045 C > T, p. (Arg349Trp) that established APS diagnosis with FPLD, so far studied and described in only 10 patients worldwide. The patient was initially treated with metformin, fenofibrate, omega-3 and low carb and low fat diet; later, liraglutide (a Glucagon-Like Peptide-1 analog, GLP-1) therapy was added and follow-up was arranged. During the initial follow-up we observed a significant improvement in metabolic and anthropometric conditions, body composition, liver volume and distribution of fat mass with a reduction of visceral fat. However, with disease progression, the patient’s lipid profile worsened, and focal segmental glomerulosclerosis (FSGS) and peripheral neuropathy developed. This case highlights the clinical signs and the progression of this rare lipodystrophy disorder and propose an innovative therapeutic approach to manage the disease.
Keywords
Atypical Progeroid Syndrome; Case Report; Familial Partial Lipodystrophy; Focal Segmental Glomerulosclerosis; Hypertriglyceridemia; Liraglutide; Type 2 Diabetes Mellitus
Article Details
1. Introduction
The lipodystrophies are rare heterogeneous group of disorders characterized by the general or partial absence of subcutaneous adipose tissue and predisposition to metabolic complications related to insulin-resistance [1,2]. Familial partial lipodystrophy (FPLD) is a rare form of lipodystrophy characterized by selective but variable loss of subcutaneous adipose tissue typically localized in the limbs, causing prominent musculature, while adipose tissue loss is variable in the trunk. However, subcutaneous adipose tissue can accumulate in other regions of the body such as face, neck, perineal and intra-abdominal areas, especially in women [1-3]. Most of FPLDs are associated with mutations of the LMNA which is located at chromosome 1q21-22 and contains 12 exons. The LMNA gene encodes A and C-type lamins - proteins, which contribute to the maintenance of nuclear structure and function. The majority of LMNA mutations are heterozygous and lead to defective differentiation and premature death of adipocytes [4]. The most frequent form of laminopathies associated with partial lipodystrophy is type 2 FPLD or Dunnigan type (FPLD2). However, there are LMNA gene pathogenetic variants associated with non-Dunnigan FPLD variety recently described that can cause diseases that share cardiac phenotype or features with accelerated aging, known as progeria [5,6]. Progerias associated with LMNA mutations are distinguished in typical (Hutchinson-Gilford progeria syndrome [HGPS], type a mandibulo-acral dysplasia [MADA]) and atypical progeroid syndrome (APS), which includes atypical Werner syndrome [7] sometimes used as a synonym of APS. HGPS and MADA are all well-characterized syndromes. In contrast, APS is a heterogeneous group of rare disorders characterized by variable degrees of fat loss (partial or generalized), early and accelerated aging process, metabolic alterations, and comorbidities affecting bone, skeletal muscles, peripheral nerves and heart [8]. Among APS patients, those presenting the heterozygous LMNA mutation c.1045 C > T, p. (Arg349Trp) have a very peculiar and recurrent phenotype with clinical features of partial lipodystrophy. The mutation p.R349W, translates arginine into a tryptophan in position 349; this substitution involves the α-helical domain of the lamin A/C protein. Patients with this mutation have progeroid features that include premature graying of the hair, beaked nose, mandibular hypoplasia, high-pitched voice, short stature, thin lips, skin atrophy and alopecia. In addition, they could have micrognathia, acanthosis nigricans, scoliosis, myopathy and sensorineural hearing impairment. Metabolic complications including type 2 diabetes mellitus (T2D), hypertriglyceridemia, and hepatic steatosis are highly prevalent in these patients [6,8]. In addition, this disease is recurrently associated with cardiomyopathy and in rare cases with focal segmental glomerulosclerosis (FSGS) [9]. FSGS has been frequently described associated with generalized, congenital, or acquired lipodystrophy, but rarely in patients with partial lipodystrophy [6,10]. In this paper, we present a case report of an APS patient with partial lipodystrophy associated with heterozygous c.1045 C > T (p.R349W) missense mutation of the LMNA gene and we propose an innovative therapeutic approach for the management of the disease. So far, this mutation has been studied and described in only 10 patients worldwide.
2. Case Description
2.1 Patient Information and Phenotype
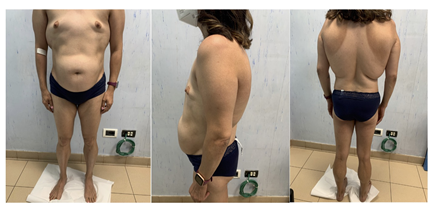
A 31-year-old woman was admitted to the Unit of Endocrinology, Diabetes and Metabolism, S. Giovanni Calibita Fatebenefratelli Hospital of Rome for insulin-resistance, hypertriglyceridemia, and polycystic ovary syndrome (PCOS). The patient is in primary amenorrhea despite the implantation of the subcutaneous device for the administration of the estro-progestin. The maternal grandparents have T2D and her sister has a history of hypothyroidism due to Hashimoto’s thyroiditis. The patient’s body weight was 56 kg, her height was 155 cm, 24.1 kg/m2 of body mass index (BMI), waist circumference (CW) was 80.5 cm and her blood pressure was 110/60 mmHg. On physical examination (figure 1), the patient presented absence of subcutaneous adipose tissue in the lower limbs and gluteal area where the musculature appeared prominent; fat accumulation was observed in the abdomen. Total body dual-energy x-ray absorptiometry (DXA) showed a total fat mass accounting for 28.4% of body weight with a predominance at the level of the trunk; and revealed low bone mineral density (BMD) at the femoral neck (T-score -2.2, Z-score -2.1). The patient presented bird face with beaked nose, thin lips, micrognathia and mandibular hypoplasia, gray hair, mild acanthosis nigricans in axillary and inguinale region, and small breasts due to absence of adipose tissue. In addition, she suffered from bilateral progressive sensorineural hearing impairment from the age of 25 previously investigated without identifying the cause.
2.2 Clinical and Genetic Investigations
A 75 gr glucose tolerance test (OGTT) revealed T2D with marked hyperinsulinemia (table 1A). The OGTT was used to assess insulin secretion and insulin sensitivity through modelling analysis [11,12]. In spite of a diabetic OGTT, insulin secretion was markedly increased compared to a reference population of nondiabetic subjects [13]. In particular, fasting and total insulin secretion in the first 2 h of the OGTT, and early insulin secretion (represented by the parameter denoted as rate sensitivity), were 3-4 fold greater than the reference values (table 1B). Glucose sensitivity, i.e. the slope of the relationship between insulin secretion and glucose concentration, which is markedly depressed in diabetic patients [14], was not abnormal. In contrast, insulin sensitivity, calculated as the 2h-OGIS index (11), was strongly reduced, even below the values observed in morbidly obese patients [15,16] (table 1B). Laboratory investigations (table 1C) showed hypertransaminasemia, hypertriglyceridemia, elevated creatine kinase (CK) and aldolase levels, reduced adiponectin levels and normal leptin levels. The glycated hemoglobin A1c (HbA1c) detected highlighted a good glycemic control. A urine collection showed proteinuria and microalbuminuria with normal estimate glomerular filtration rate (eGFR). The patient had urinary tract infection, therefore we decided with the nephrologist not to start angiotensin-converting enzyme (ACE) inhibitors therapy and to re-evaluate the patient’s condition after the resolution of the urinary tract infection. The hormonal tests related to the function of thyroid, hypophysis, adrenal and reproductive system, did not report alterations. An abdominal ultrasound examination reported hepatic steatosis with hepatomegaly in line with elevated transaminase levels; a single cystic formation of 5 cm of ovarian origin, externally to the left ovary was also observed. The elevated CK and aldolase levels and muscle weakness reported by the patient suggested a myopathy. Neurological tests showed no evidence of peripheral or autonomic diabetic neuropathy. No abnormalities were found during holter 24-hour electrocardiogram and echocardiogram. The ophthalmological examinations did not report alterations. The clinical and laboratory findings outlined above, suggested a FPLD with progeroid features [6,8]. Genetic analysis was carried out with sanger sequencing and revealed the heterozygous c.1045 C > T (p.R349W) missense mutation in exon 6 of the LMNA gene that established diagnosis of APS with clinical features of partial lipodystrophy. In addition, the genetic analysis was also performed for the patient’s parents who did not report the LMNA gene mutation.
|
Time (min) |
0 |
30 |
60 |
120 |
180 |
240 |
|
Glucose (mg/dl) |
108 |
201 |
175 |
263 |
324 |
293 |
|
Insulin (mUI/mL) |
76.3 |
207.2 |
259 |
576 |
565 |
754 |
|
C-Peptide (ng/ml) |
9.7 |
16 |
16 |
27 |
27 |
29 |
Plasma glucose, insulin and C-peptide levels at time 0, 30, 60, 120, 180 and 240 min.
|
Parameter |
Patient’s value |
Reference1 |
|
Fasting insulin secretion (pmol min-1m-2) |
476 |
106 (40) |
|
Total insulin secretion over 2 h (nmol m-2) |
144 |
52 (18) |
|
Rate sensitivity (pmol m-2mmol-1L) |
3508 |
921 (699) |
|
Glucose sensitivity (pmol min-1m-2mmol-1L) |
81 |
113 (55) |
|
OGIS (mL min-1m-2) |
171 |
381 (59) |
Insulin secretion and insulin sensitivity assessments.
1Mean (SD) values in a nondiabetic cohort of more than 2100 subjects (from Koivula 2019).
|
Parameters |
Patient’s values |
Reference range |
|
Glucose (mg/dl) |
108 |
70-100 |
|
HbA1c (%) |
6.7 |
04-Jun |
|
Total cholesterol (mg/dl) |
250 |
<200 |
|
LDL cholesterol (mg/dl) |
69 |
<100 |
|
HDL cholesterol (mg/dl) |
45 |
40-60 |
|
Triglycerides (mg/dl) |
682 |
<150 |
|
Leptin (ng/ml) |
7.7 |
3.63-11.09 |
|
Adiponectin (mg/ml) |
0.99 |
4-19.4 |
|
AST (U/L) |
61 |
<35 |
|
ALT (U/L) |
78 |
<35 |
|
GGT (U/L) |
55 |
<38 |
|
Total bilirubin (mg/dl) |
0.58 |
0.30-1.20 |
|
Direct bilirubin (mg/dl) |
0.1 |
0-0.20 |
|
Indirect bilirubin (mg/dl) |
0.48 |
0.10-0.80 |
|
α-Amilase (U/L) |
86 |
28-100 |
|
LDH (U/L) |
207 |
<247 |
|
Aldolase (U/I) |
9.5 |
<7.6 |
|
CK (U/L) |
183 |
<145 |
|
Creatinine (mg/dl) |
0.5 |
0.51-0.95 |
|
Azotemia (mg/dl) |
24 |
17-43 |
|
eGFR (ml/min) |
129.38 |
>90 |
|
Microalbuminuria/Creatininuria (mg/g) |
59 |
<30 |
|
Proteinuria (mg/dl) |
92.9 |
<15 |
|
TSH (mUI/mL) |
2.7 |
0.340-5.600 |
|
FT3 (pg/dL) |
3.97 |
2.50-3.90 |
|
FT4 (ng/dl) |
0.96 |
0.61-1.12 |
|
Anti-Thyroglobulin antibodies |
0.4 |
<4 |
|
Anti-Thyroperoxidase antibodies |
0.8 |
<9 |
|
PTH (pg/ml) |
17.4 |
Dec-88 |
|
ACTH (pg/ml) |
23.2 |
<60 |
|
Prolactin 0’ |
9.5 |
3.3-26.7 |
|
Prolactin 15’ |
8.4 |
3.3-26.7 |
|
Prolactin 30’ |
8.4 |
3.3-26.7 |
|
Cortisol (ng/ml) |
218.7 |
43-224 |
|
DHEA-S (mmol/L) |
1 |
0.62-7.22 |
|
FSH (mUI/mL) |
6.8 |
3.85-8.78 |
|
LH (mUI/mL) |
9.6 |
2.12-10.89 |
|
Estradiol E2 (pg/mL) |
37 |
27-122 |
|
17-a-hydroxyprogesterone (ng/mL) |
0.723 |
0.1-0.8 |
|
4-androstestenedione (ng/mL) |
1.81 |
0.3-3.3 |
|
Testosterone (ng/mL) |
0.5 |
0.1-0.75 |
|
Free testosterone (pg/mL) |
3.84 |
<4.2 |
|
5-a-dihydrotestosterone (pg/mL) |
215 |
24-368 |
|
SHBG (nMol/L) |
15.1 |
18.2-135.5 |
|
Vitamin D (ng/ml) |
25.4 |
>30 |
|
Calcium (mg/dl) |
9.9 |
8.8-10.6 |
- Patient’s initial biochemical and hormonal investigations.
Table 1: 75 gr Oral Glucose Tolerance Test and laboratory investigations at the diagnosis.
2.3 Therapeutic Intervention
The patient was initially treated with metformin (850 mg 3 times per day), fenofibrate (145 mg per day), omega-3 (1000 mg 3 times per day) and low carb and low fat diet; later, liraglutide (a Glucagon-Like Peptide-1 analog, GLP-1) (1.8 mg injected subcutaneously once daily) therapy was added with the purpose to improve body composition, distribution of fat mass and liver volume.
2.4 Follow up and Outcomes
The patient showed a good adherence to the therapeutic intervention, the therapy was well tolerated and no severe side effects were observed. After 3 months from the diagnosis, triglycerides levels were reduced (682 mg/dl to 135 mg/dl), transaminases, aldolase and CK levels returned to normal levels and proteinuria and microalbuminuria were slightly improved. After 6 months of liraglutide therapy the HbA1c of the patient showed an improved glycemic control, and we observed a relevant improvement of anthropometric parameters, body composition and distribution of fat mass with a reduction of visceral fat (table 2). In addition, abdominal ultrasound examination reported a reduced liver volume. The patient’s condition remained stable over one-year period. After the resolution of the urinary tract infection, the proteinuria and microalbuminuria were still observed, therefore ACE inhibitors treatment was added. In the following two years, the patient continued to show optimal glycemic control, however increased triglycerides values were detected (263 mg/dl and 385 mg/dl) and a renal biopsy was performed following the onset of marked macroalbuminuria (ratio microalbuminuria/creatininuria 2480 mg/g) and proteinuria (228.20 mg/dl) which was consistent with FSGS but with normal eGFR (125 ml/min). The fibroblast growth factor 23 (FGF-23), a marker of kidney function, resulted normal compared to reference values (45.7 ng/L, reference range 23.2-95.4 ng/L). In addition, the neurological tests showed signs and symptoms related to sensorimotor peripheral polyneuropathy: vibration perception value on dorsal side of right hallux was 20.5, normal values (n.v) <11.6, vibratory perception value on dorsal side of left hallux was 22, n.v <10.4; absence of ankles reflexes; DNI (Diabetic Neuropathy Index) was 5, n.v <2; MNSI (Michigan Neuropathy Screening Instrument) was 7, n.v <1; DN4 (Douleur Neuropathique 4) was 5, n.v <3.
|
Time |
Baseline |
6 months |
|
Hemoglobin A1c (HbA1c %) |
6.7 |
5.2 |
|
Weight (kg) |
56 |
52 |
|
BMI (kg/m2) |
23.3 |
21.6 |
|
Waist circumference (cm) |
80.5 |
73.5 |
|
Total fat mass (kg) |
15.7 |
12.1 |
|
Trunk fat (kg) |
8 |
5.7 |
|
Trunk fat/limbs fat |
1.3 |
1 |
|
% trunk fat/% legs fat |
3 |
2.5 |
Table 2: Glycemic control, anthropometric parameters, body composition and distribution of fat mass at the diagnosis and after 6 months of liraglutide therapy.
3. Discussion
We described the clinical features and the short and long-term follow-up of an APS patient with partial lipodystrophy turned out to carry a pathogenic heterozygous p. (Arg349Trp) variant in LMNA gene and we illustrated an optimal therapeutic approach for the management of the disease. Her clinical manifestations revealed many distinguishing progeroid features such as premature graying of hair, mandibular hypoplasia and beaked nose. Additional features, expanding the phenotype known to be associated to this variant included acanthosis nigricans, micrognathia, myopathy, skeletal abnormalities and sensorineural hearing impairment that represent the key pathological hallmarks of this subtype of APS [6]. Conversely, she did not display any cardiovascular features frequently reported in association to this variant [8]. The presence of partial lipodystrophy was characterized by a reduction in subcutaneous adipose tissue, mainly over the extremities, fat accumulation in the abdomen and reduced levels of adiponectin but normal leptin. In line with this, she showed marked metabolic abnormalities that include T2D, hypertriglyceridemia, and hepatic steatosis, present in 75% of patients with partial lipodystrophy and progeria [8]. The baseline insulin sensitivity and secretion assessment showed strong insulin-resistance with hyperglycemia and elevated insulin secretion. These results show that the patient has strong insulin-resistance but preserved beta cells function despite having T2D. Therefore, this condition diverges from the classical T2D, which prevalently occurs when insulin-resistance is associated with a deficit of insulin secretion [17]. Management of lipodystrophy is currently difficult, due to the underlying impaired insulin sensitivity, usually severe, and its manifestations. Fibrates, especially fenofibrate decreases hypertriglyceridemia [18]. Regarding T2D, there are no specific guidelines for patients with lipodystrophy. Since insulin-resistance is at the base of T2D in lipodystrophy, insulin sensitizers are a rational approach. The data in literature reported moderate results associated with metformin treatment in patient with lipodystrophy [19]. GLP-1 analogues have been shown to amplify insulin signaling in peripheral skeletal muscle, liver and adipose tissue [20]. According to previous studies, these drugs significantly influence fat distribution, and even a short course of treatment results in a significant decrease of visceral fat deposits in patients with T2D [21]. In the presented case, the patient was treated with fenofibrate, metformin and liraglutide and after short course of treatment we observed improvements in metabolic and anthropometric parameters, liver volume, body composition and distribution of fat mass with a decrease of visceral fat. After 3 years from the diagnosis, despite a good glycemic control, she showed worsening of the lipid profile and developed macroalbuminuria, proteinuria and FSGS on kidney biopsy. Although multiple organs are affected in LMNA mutations, the association with kidney disease is very rare in patients with partial lipodystrophy [9]. FSGS is a podocyte injury associated with proteinuria and is a major cause of end-stage renal disease [10]. It has been suggested that LMNA mutation may lead to an excess of Transforming Growth Factor Beta 1 (TGFb1) production from podocytes, promoting glomerulosclerosis in patients with partial lipodystrophy [22]. Despite the kidney damage, the patient had a normal glomerular filtration and the FGF-23 levels were normal. The FGF-23 regulates phosphate homeostasis; when kidney function is altered, less phosphate is filtered and reabsorbed and FGF-23 increases. Therefore, FGF-23 increases when glomerular filtration decreases [23]. In our case reported, the presence of a nephropathy, with normal eGFR explains why FGF-23 is normal. Concurrently to the FSGS, the patient developed peripheral neuropathy. The peripheral nerves conditions observed in the third year of follow-up cannot originate from diabetic neuropathy because the patient always had optimal glycemic control during the 3 years. The abnormalities in peripheral nerves were described as possible comorbidities associated to LMNA mutations in patient with lipodystrophy [6]. In addition, it has been reported that elevated triglycerides levels correlate with the progression of neuropathy in diabetic patients [24], therefore the worsening of the lipid profile may have contributed to the development and progression of peripheral neuropathy.
4. Conclusion
To the best of our knowledge this is the first time that an in vivo human diabetes model characterized with only insulin-resistance without deficit of insulin secretion has been described. Treatment with liraglutide in association with metformin and fenofibrate showed a significant improvement in the disease management of the APS case patient with partial lipodystrophy presenting heterozygous p.(Arg349Trp) missense LMNA mutation. To the best of our knowledge, the data existing on the literature report two cases of patients with partial lipodystrophy that have been treated with liraglutide therapy in order to achieve a good glycemic control [25]. In our case reported, the use of liraglutide not only allowed further improvement of the glycemic control but also favored the reduction of visceral fat and liver volume. We therefore propose the treatment with liraglutide, in association with metformin and fenofibrate, as innovative therapeutic approach to optimally manage patients with this rare lipodystrophy disorder. However, with disease progression the patient’s lipid profile worsened and FSGS and peripheral neuropathy developed. In the last years, the efficacy of metreleptin treatment in patients with partial lipodystrophy caused by LMNA pathogenic variants has been demonstrated [26]; the use of metreleptin resulted in improvements in glycemic control, hypertriglyceridemia and liver volume [27]. Possible future therapeutic strategy include treatment with metreleptin in order to better manage the progression of the disease.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Russo wrote the manuscript and was involved in the patient’s care. M. Menduni reviewed the manuscript and was involved in the care of the patient. A. Mari performed the modelling analysis, reviewed and edited the manuscript. C. Pelosini reviewed the manuscript and was involved in the biochemical and hormonal investigations. F. Brancati and M.R.D’ Apice reviewed and edited the manuscript and performed the genetic analysis. F. Picconi reviewed and edited the manuscript and was involved in the care of the patient. S. Frontoni reviewed and edited the manuscript and supervised the management of the patient’s disease. I. Malandrucco supervised the drafting of the paper, conducted the study of the case reported and managed the patient’s disease.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Garg A. Lipodystrophies. Am. J. Med 108 (2000):143-152.
- Hussain I. Lipodystrophy Syndromes. Endocrinol. Metab. Clin. North. Am 45 (2016): 783-797.
- Peters JM, Barnes R, Bennett L, et al. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q2122. Nat. Genet 18 (1998): 292-295.
- Hegele RA, Cao H, Anderson CM, et al. Heterogeneity of nuclear lamin a mutations in Dunnigan-type familial partial lipodystrophy. J. Clin. Endocrinol. Metab 85 (2000): 3431-3435.
- Eldin AJ, Akinci B, da Rocha AM, et al. Cardiac phenotype in familial partial lipodystrophy. Clin Endocrinol 94 (2021): 1043-1053.
- Magno S, Ceccarini G, Pelosini C, et al. Atypical Progeroid Syndrome and Partial Lipodystrophy Due to LMNA Gene p.R349W Mutation. J. Endocr. Soc 4 (2020): bvaa108.
- Chen L, Lee L, Kudlow BA, et al. LMNA mutations in atypical Werner’s syndrome. Lancet 362 (2003): 440-445.
- Hussain I, Jin RR, Baum HBA, et al. Multisystem Progeroid Syndrome With Lipodystrophy, Cardiomyopathy, and Nephropathy Due to an LMNA p.R349W Variant. J. Endocr. Soc 4 (2020):10.
- Thong KM, Xu Y, Cook J, et al. Cosegregation of focal segmental glomerulosclerosis in a family with familial partial lipodystrophy due to a mutation in LMNA. Nephron. Clin. Pract 124 (2013): 31-37.
- Javor ED, Moran SA, Young JR, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J. Clin. Endocrinol. Metab 89 (2004): 3199-3207.
- Mari A, Pacini G, Murphy E, et al. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diab. Care 24 (2001): 539-548.
- Mari A, Tura A, Gastaldelli A, et al. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 5 (2002): S221-S226.
- Koivula RW, Forgie IM, Kurbasic A, et al. Discovery of biomarkers for glycaemic deterioration before and after the onset of type 2 diabetes: descriptive characteristics of the epidemiological studies within the IMI DIRECT Consortium. Diabetologia 62 (2019): 1601-1615.
- Ferrannini E, Gastaldelli A, Miyazaki Y, et al. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J. Clin. Endocrinol. Metab 90 (2005): 493-500.
- Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia 49 (2006): 2136-2143.
- Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J. Clin. Endocrinol. Metab 96 (2011): E1372-1379.
- Cersosimo E, Triplitt C, Mandarino LJ, et al. Pathogenesis of Type 2 Diabetes Mellitus. Endotext (2015).
- Polyzos SA, Kountouras J, Zavos C, et al. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes. Obes. Metab 12 (2010): 365-383.
- Polyzos SA, Mantzoros CS. Lipodystrophy: Time for a global registry and randomized clinical trials to assess efficacy, safety and cost-effectiveness of established and novel medications. Metabolism 72 (2017): A4-A10.
- Jinnouchi H, Sugiyama S, Yoshida A, et al. Liraglutide, a Glucagon-Like Peptide-1 Analog, Increased Insulin Sensitivity Assessed by Hyperinsulinemic-Euglycemic Clamp Examination in Patients with Uncontrolled Type 2 Diabetes Mellitus. J. Diabetes. Res 2015 (2015): 706416.
- Morano S, Romagnoli E, Filardi T, et al. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: an ultrasonography study. Acta Diabetol 52 (2015): 727-732.
- Jacob KN, Garg A. Laminopathies: multisystem dystrophy syndromes. Mol. Genet. Metab 87 (2006): 289-302.
- Vervloet MG. FGF23 measurement in chronic kidney disease: What is it really reflecting? Clin. Chim. Acta 505 (2020): 160-166.
- Wiggin TD, Sullivan KA, Pop-Busui R, et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 58 (2009): 1634-1640.
- Oliveira J, Lau E, Carvalho D, et al. Glucagon-like peptide-1 analogues - an efficient therapeutic option for the severe insulin resistance of lipodystrophic syndromes: two case reports. Journal of medical case reports 11 (2017): 12.
- Sekizkardes H, Cochran E, Malandrino N, et al. Efficacy of Metreleptin Treatment in Familial Partial Lipodystrophy Due to PPARG vs LMNA Pathogenic Variants. J. Clin. Endocrinol. Metab 104 (2019): 3068-3076.
- Oral EA, Gorden P, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine 64 (2019): 500-511.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
