Regenerative Medicine May Mitigate the Need for Amputation in the Setting of a High Society for Vascular Surgery – Wound, Ischemia, Foot Infection (SVS-Wifi) Score
Article Information
Dana M Poloni1*, Christina M Monaco2, Maeghan L Ciampa1, Elizabeth Marie Oliver Coffin1, Joy N Liang1, Eric D Martin1
1Department of Surgery, Dwight D. Eisenhower Army Medical Center, Fort Gordon, GA, USA
2Department of Surgery, Philadelphia College of Osteopathic Medicine, Philadelphia, PA, USA
*Corresponding Author: Dana M Poloni, Department of Surgery, Dwight D. Eisenhower Army Medical Center, Fort Gordon, GA, USA.
Received: 21 October 2022; Accepted: 18 November 2022; Published: 30 November 2022
Citation: Dana M Poloni, Christina M Monaco, Maeghan L Ciampa, Elizabeth Marie Oliver Coffin, Joy N Liang, Eric D Martin. Regenerative Medicine May Mitigate the Need for Amputation in the Setting of a High Society for Vascular Surgery – Wound, Ischemia, Foot Infection (SVS-Wifi) Score. Archives of Clinical and Medical Case Reports 6 (2022): 752-757.
View / Download Pdf Share at FacebookAbstract
Patients with diabetic foot ulcers and peripheral arterial disease are at an increased risk for developing chronic limb threatening ischemia. The Society for Vascular Surgery – Wound, Ischemia, foot Infection (WIfI) classification system assists in stratifying patients that may benefit from revascularization and those that will likely fail. With regenerative medicine, patients with high WIfI scores may have a better chance at limb salvage. This is the case of a 61-year-old male who presented with a high risk foot wound; WIfI 8 (WIfI 323). This case report highlights successful limb salvage after undergoing multiple revascularization procedures and application of regenerative medicine.
Keywords
Chronic Limb Threatening Ischemia; Foot Ulcer; Peripheral Arterial Disease; Regenerative Medicine; SVS-WIfI; Wound Care
Article Details
1. Introduction
Chronic limb threatening ischemia (CTLI) is one of the feared progressions of peripheral arterial disease (PAD). For patients with comorbid diabetes and PAD who present with diabetic foot ulcers (DFU), the potential for wound healing is diminished. When factoring in other comorbidities, 5-year mortality in patients with CTLI approaches 60% [1]. In this setting, the choice to revascularize versus amputate has been a point of contention among vascular surgeons. In 2013, the SVS-WIfI (Society for Vascular Surgery-Wound, Ischemia, foot Infection) classification system was developed to help stratify which patients would benefit from revascularization and which patients would likely fail, ultimately necessitating amputation [1]. Several reports suggest that patients with high WIfI scores undergoing revascularization remain at a high risk for future amputation [1,2,3]. Literature referencing regenerative medicine and PAD is scarce [4,5]. Regenerative medicine is a broad term that is used to describe the placement of stem cells, growth factors, anti-inflammatory cytokines, and extracellular matrix (ECM) components into damaged tissue with the goal of repair or regrowth of damaged cells, tissue, or organs. Here we report a 61-year-old male who presented with a significant and severely infected right lower extremity (RLE) wound with an initial WIfI composite score of 8 (WIfI 323), who after revascularization and placement of regenerative medicine was able to heal his wound and achieve limb salvage. Consent for publication of case content and images was obtained from the patient.
2. Case Report
This patient is a 61-year old male who presented to the Veterans Affairs health care system with a one month history of wet gangrene of his distal right 5th toe in addition to a wound overlying the lateral aspect of his right foot. The patient endorsed worsening pain and purulence associated with his lateral foot wound. His past medical history was significant for diabetes, peripheral arterial disease status post a right external iliac artery angioplasty, history of provoked deep vein thrombosis, a left great toe amputation, and a distal right 4th toe amputation. Additionally, the patient endorsed a former history of heavy tobacco and cocaine use. Prior to onset of foot infection, he was independently ambulatory. On physical examination the right foot was noted to have multiple areas of wet gangrene with exposed bone and expressible purulence. His RLE ankle brachial index (ABI) was 0.51 and the patient met 2 of 4 Systemic Inflammatory Response Syndrome (SIRS) criteria (tachycardia 98 bpm, and leukocytosis 14.0x1,000/uL), meeting criteria for a severe infection (Table 1). An initial WIfI score of 8 (WIfI 323) was assigned to the patient. He was subsequently transferred to a nearby active duty military hospital with access to vascular surgical capabilities. The patient was started on antibiotics and taken to the operating room for surgical debridement where he underwent a right 4th & 5th Ray amputation and 2nd toe amputation to obtain source control. Following his first operation, the patient’s leukocytosis resolved and he was reassigned a WIfI score of 7 (WIfI 322). On postoperative day (POD) 5, the patient underwent bilateral lower extremity angiography with revascularization of the left lower extremity with stenting of left external iliac artery (EIA), left common iliac artery (CIA), and left superficial femoral artery (SFA) (EIA, Viabahn 8x100mm [Gore Medical, Flagstaff, Arizona, USA]; CIA, Viabahn VBX 8x39mm [Gore Medical, Flagstaff, Arizona, USA]; SFA, Lifestent 6x150mm [Bard Peripheral Vascular, Inc., Tempe, Arizona, USA]. Multiple attempts at endovascular cannulation of the right lower extremity were tried, however ultimately the right lower extremity was not amenable to endovascular revascularization. On POD7 from his index procedure, he was taken back to the operating room to re-establish inline flow of the RLE where he underwent a right common femoral artery (CFA) and proximal SFA endarterectomy in addition to a right CFA to posterior tibial artery (PTA) bypass using the ipsilateral greater saphenous vein (GSV) as a reversed autologous graft. Postoperative ABI of the RLE was 0.76 with reassigned WIfI score of 4 (WIfI 310). He returned to the operating room on POD9 for further debridement and resection of nonviable tissue of the right foot. On hospital day 15, the patient’s midfoot wound measured 15x10x3cm and appeared to have no active signs of infection. He was deemed a candidate for placement of regenerative medicine which he underwent successfully at that time (Figure 1). Four days following this procedure, he was discharged home and an updated WIfI score of 1 (WIfI 010) was calculated.
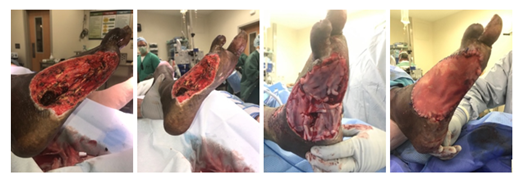
Figure 1: Right foot wound. A) Lateral view and B) Lateroplantar views of the initial right foot wound after mechanical and ultrasonic debridement with successful source control (15x10x3cm). C) Sutured (4) Grafix (6x3cm) stem cell grafts (Osiris Therapeutics, Columbia, Maryland, USA) into the wound bed prior to application of ECM components (Acell Micromatrix, Columbia, Maryland, USA). D) Placement of protective Acell porcine bladder thick wound sheet (Acell, Columbia, Maryland, USA).
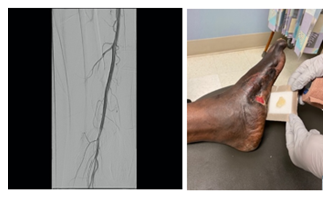
After two months, his wound bed was adequately prepared for closure and the patient underwent placement of an autologous split-thickness skin graft (STSG). Seven days following placement of this STSG, a surveillance duplex ultrasound was performed and revealed decreased flow velocities within the GSV bypass graft. The patient was subsequently taken back to the endovascular suite where multiple areas of proximal stenosis within his right CIA, EIA, and CFA were found, necessitating further angioplasty and stent placement (angioplasty CIA, EIA, and CFA performed with 6x200mm Mustang Balloon [Boston Scientific, Marlborough, Massachusetts, USA]; EIA, 7x120mm Zilver PTX [Cook Medical LLC, Bloomington, Indiana, USA]; CIA, 8x59mm Viabahn VBX [Gore Medical, Flagstaff, Arizona, USA];). Post-procedurally, the RLE ABI = 1.15. On POD6 following STSG placement, the graft was noted to have complete incorporation, and the patient was discharged home. Over the next several months, the patient was seen bi-weekly in wound-care clinic. As the patient progressed in recovery and his wound decreased in size he was seen less frequently. However, approximately 1.5 years following stem cell graft placement, the patient presented to the wound-care clinic with an enlarged wound. At this time the wound was notable for a new area of ulceration measuring 3x3x0.5cm with surrounding erythema and tenderness to palpation along with a decreased ABI of 0.36 in the RLE for which a WIfI score of 5 (WIfI 131) was assigned; Duplex ultrasound and CT angiogram revealed a thrombosed reversed GSV graft. The time of primary assisted patency of the reversed GSV bypass graft was 1.5 years. The patient was readmitted, started on antibiotics, and endovascular interventions were used to re-establish inline flow with stenting of the entire native SFA and P1 segment of the popliteal artery (P1-SFA, 6x150mm Life stent; proximal SFA, 6x200mm Life stent [Bard Peripheral Vascular, Inc, Tempe, Arizona, USA]) (Figure 2A). Post-procedure RLE ABI was 0.75. The following day the patient was taken back to the operating room where he underwent wound debridement and further regenerative medicine placement. Three months after the final procedure the ulcer had decreased in size to 0.5x0.5x0cm; (WIfI composite score 1; WIfI 100). Today the patient is doing well, remains independently ambulatory, and continues to undergo local wound care of his chronic, subcentimeter shallow ulcer (Figure 2B).
3. Discussion
DFUs have a global prevalence of 6.3%; in North America it is even higher at 13.0% [6]. Furthermore, PAD compounds the complications seen in these wounds by preventing healing. Failure to heal wounds in conjunction with inherent properties of diabetes mellitus and PAD lead to increased risk of development of infection and eventual need for amputation in order to obtain source control and promote wound healing. “Life, limb, and eyesight” is an oft-quoted phrase in emergency and military medicine, which highlights the societal importance of limb salvage, especially in previously ambulatory and functionally independent patients. The SVS-WIfI score is a classification system designed to predict major amputation rate in patients with comorbid diabetes mellitus and PAD resulting in diabetic foot wounds and infections, which is a limitation of other PAD classification systems such as Rutherford, Fontaine, and Trans-Atlantic Inter-Society Consensus Document II (TASC-II) [1,2,7]. Rutherford, in particular, is well-known and well-regarded but relies on measurement of ABI, which is often misleading in patients with noncompressible arteries as is common in diabetes mellitus. SVS-WIfI incorporates the measurement of toe pressures or transcutaneous oxygen pressure (TcPO2) for patients with ABI >1.3, which indicates noncompressible arteriopathy. TcPO2 above 30 mmHg is a predictive factor for spontaneous healing with TcPO2 <10 mmHg associated with unfavorable course of wound healing [8]. SVS-WIfI also specifically delineates ulceration versus gangrene, with the latter portending to a worst prognosis [1]. SVS-WIfI addresses the three major components of diabetic foot syndrome: infection status, wound type, and perfusion status1 (Table 1) and is predictive of major amputation risk [2,9]. SVS-WIfI system is used to stratify wounds into clinical stages 1-4 as well as to calculate a WIfI composite score (range 0-9) and a WIfI mean score (range 0-3). WIfI composite and mean scores were consistent predictors of both major amputation risk and mortality following revascularization (endovascular and open bypass) with the mean score being most predictive across all patients [2]. Wounds are classified into 4 stages based on WIfI composite score predictive of 1-year amputation risk as well as likelihood of requirement for revascularization, classifying wounds into very low, low, moderate, and high risk. Based on the patient’s initial presentation with WIfI composite score 8 (WIfI 323), he had a high risk of amputation and requirement for revascularization. His risk of treatment failure and future amputation remained high despite initial treatment and revascularization with 31% 1-year amputation rate and 52% risk of future re-intervention, amputation, or stenosis according to observed outcomes [2]. This risk has been used as an argument for upfront amputation at initial presentation, however this is a point of contention amongst vascular surgeons and requires a careful assessment of preintervention quality of life, comorbid conditions, and patient’s goals of care.
|
SVS-WIfI Classification System |
|
|
Wound |
|
|
0 |
Ischemic rest pain without presence of wound or ulceration |
|
1 |
Minor tissue loss without gangrene, includes small shallow ulcers on distal leg or feet or exposure of distal phalanx only |
|
2 |
Deeper ulcer with exposed bone, tendon, or joint or gangrene limited to digits or shallow heel ulcer without calcaneal involve |
|
3 |
Extensive deep ulcer involving forefoot or midfoot, deep or full-thickness heel ulcer with or without calcaneal involvement, or extensive gangrene involving forefoot, midfoot, or heel |
|
Ischemia |
|
|
0 |
ABI >0.8, ankle systolic pressure >100 mmHg, or transcutaneous oxygen pressure (TcPO2) >60 mmHg |
|
1 |
ABI 0.6-0.79, ankle systolic pressure 70-100 mmHg, or transcutaneous oxygen pressure (TcPO2) 40-59 mmHg |
|
2 |
ABI 0.4-0.59, ankle systolic pressure 50-70 mmHg, or transcutaneous oxygen pressure (TcPO2) 30-39 mmHg |
|
3 |
ABI <0.39, ankle systolic pressure <50 mmHg, or transcutaneous oxygen pressure (TcPO2) <30 mmHg |
|
Infection |
|
|
0 |
No symptoms or signs of infection |
|
1 |
Mild infection: local infection (local swelling or induration, erythema 0.5-2cm of around ulcer, local tenderness or pain, local warmth, purulent discharge) without systemic symptoms or SIRS criteria |
|
2 |
Moderate infection: location infection with >3cm of erythema involving deeper structures without systemic symptoms or SIRS criteria |
|
3 |
Severe of life-threatening infection: involves systemic symptoms or at least 2 SIRS criteria (temperature >38 C or <36 C, heart rate >90 bpm, respiratory rate >20 breaths/min or PaCO2 <32 mmHg, white blood cell count >12,000 or <4,000 cu/mm or 10% immature) |
Table 1: Society of Vascular Surgery: Wound, Ischemia, and foot Infection (SVS-WIfI) classification system.
If a patient with comorbid diabetes and CTLI develops a high risk DFU and is able to undergo revascularization, the patient still possesses a 3-year major amputation rate of 21-54% [2,3,9]. The SVS-WIfI scoring system is a useful clinical tool in stratifying DFUs, however it fails to factor in the potential benefit of regenerative medicine after successful revascularization. Recently, regenerative medicine has made many advancements in wound healing [10-14]. The approach taken at our institution with regenerative medicine is elementary in ideology and straightforward in application. Our initial priority is debridement of all non-viable or infected tissue. After source control is obtained, revascularization using an open, endovascular, or hybrid approach is then performed to prepare the extremity for wound healing. After adequate source control and revascularization, preparation of the wound bed is then performed by application of regenerative medicine with placement of stem cell grafts, followed by extracellular matrix components, and then followed by a wound matrix. Finally, the wound closure is performed with delayed application of split-thickness skin graft (Table 2).
|
Key Elements of Limb Salvage in Infected CLTI |
|
Source control of infected limb |
|
Proximal revascularization of affected extremity |
|
Endovascular |
|
Open |
|
Hybrid |
|
Preparation of wound bed |
|
Wound closure |
Table 2: Algorithm for surgical management of infected DFU with concomitant CTLI.
Despite the overall success in limb salvage in this patient, this case especially highlights the importance of a multidepartment interdisciplinary approach to these patients. Limb salvage in the setting of diabetic foot infections and CLTI requires a prolonged course of intensive care, requiring podiatrists for prevention and early wound care, vascular or general surgeons for debridement and eventual revascularization, infectious disease providers for antibiotic management and stewardship, wound care nurses and providers for intervening wound care before and between staged surgical interventions, and primary care providers for medical management and optimization of comorbid conditions. Regenerative medicine is an emerging discipline that incorporates the use of biologic materials stem cells, growth factors, anti-inflammatory cytokines and extracellular matrix in order to restore impaired function by stimulating regeneration of cells, tissues, or organs [4,5,8,10-14]. Normal wound healing occurs with multiple overlapping phases including early inflammation followed by the proliferative phase, which includes granulation tissue formation, epithelialization and angiogenesis, followed by remodeling [16,17]. Regenerative medicine has been found to have a niche role in the healing of chronic wounds, which present as a result of an interruption of the normal healing process. While early evidence points to the benefit of regenerative medicine in the healing of chronic wounds, the results are limited by the lack of standardization in practice. At our facility, we use a combination of stem cell grafts, extracellular matrix components, and wound matrix applied in a specific order to maximally prepare the wound bed. The goals for optimizing the wound for healing include producing a well-vascularized bed controlling infection and inflammation resulting in a minimal amount of exudate [8]. In this patient, the wound bed was prepared with ultrasonic debridement which uses low-frequency ultrasound waves to produce atraumatic selective tissue debridement and decrease inflammatory cytokines [15]. We followed this with placement of a cryopreserved placental membrane containing mesenchymal stem cells, collagen matrix, and growth factors. The cryopreservation allows for preservation of viable endogenous cells and improved anti-inflammatory, chemoattractive, and angiogenic activities in in vitro studies compared to devitalized placental products, with >80% cell viability post-thaw [16]. This is followed by extracellular matrix components (micromatrix, derived from porcine bladder) which provide both structural integrity and scaffolding as well as growth factors and cytokines [17]. Finally, we use amniotic and chorionic membrane allografts in order to provide a wound matrix. Protection of the prepared wound bed and regenerative medicine is critical in order to allow for incorporation of its components. Therefore, a thick wound sheet or “housing” is sutured into place around the periphery of the wound edge which is set to overly the wound bed and stem cell grafts. Following this, negative pressure wound therapy is typically employed for the purpose of moisture control as well as minimization of the need for frequent dressing changes.
4. Conclusion
The validation and application of the SVS-WIfI scoring system has proven to be of great benefit in diabetic patients afflicted by PAD who suffer from both CTLI and distal lower extremity wounds. However, with the recent advances made in regenerative medicine techniques, stronger consideration should be given to limb salvage options. This case study highlights the idea that in a suitable candidate with good wound healing potential, regenerative medicine in conjunction with revascularization may obviate the need for amputation in high risk patients.
References
- Mills JL, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity threatened limb classification system: Risk stratification based on wound, ischemia, and foot infection (WIFI). J Vasc Surg 59 (2014): 220-234.e2.
- Darling JD, McCallum JC, Soden PA, et al. Predictive ability of the Society for Vascular Surgery Wound, ischemia, and foot infection (WIFI) classification system after first-time lower extremity revascularizations. J Vasc Surg 65 (2017): 695-704.
- Darling JD, McCallum JC, Soden PA, et al. Predictive ability of the Society for Vascular Surgery Wound, ischemia, and foot infection (WIFI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg 64 (2016): 616-622.
- Sneider EB, Nowicki PT, Messina LM. Regenerative medicine in the treatment of peripheral arterial disease. J Cell Biochem 108 (2009): 753-761.
- Fujita Y, Kawamoto A. Stem cell-based peripheral vascular regeneration. Adv Drug Deliv Rev 120 (2017): 25-40.
- Zhang P, Lu J, Jing Y, et al. Global Epidemiology of Diabetic foot ulceration: A systematic review and meta-analysis. Ann Med 49 (2016): 106-116.
- Hardman R, Jazaeri O, Yi J, et al. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol 31 (2014): 378-388
- Fife C, Smart D, Sheffield P, et al. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med 36 (2009): 43-53.
- Mills JL. Update and validation of the Society for Vascular Surgery Wound, ischemia, and foot infection threatened limb classification system. Semin Vasc Surg 27 (2014): 16–22.
- Mora C, Serzanti M, Consiglio A, et al. Clinical potentials of human pluripotent stem cells. Cell Biol Toxicol 33 (2014): 351-360.
- Zarrintaj P, Moghaddam AS, Manouchehri S, et al. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine (Lond) 12 (2017): 2403-2422.
- Cable J, Fuchs E, Weissman I, et al. Adult Stem Cells and regenerative medicine - a symposium report. Ann N Y Acad Sci 1462 (2019): 27-36.
- Fuchs E, Blau HM. Tissue stem cells: Architects of their niches. Cell Stem Cell 27 (2020): 532-556.
- Ciccocioppo R, Cantore A, Chaimov D, et al. Regenerative medicine: The red planet for clinicians. Intern Emerg Med 14 (2019): 911-921.
- Kataoka Y, Kunimitsu M, Nakagami G, et al. Effectiveness of ultrasonic debridement on reduction of bacteria and biofilm in patients with chronic wounds: a scoping review. Int Wound J 18 (2020): 176-186
- Gibbons G. Grafix®, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv Wound Care (New Rochelle) 4 (2015): 534-544
- Xue M, Jackson C. Extracellular matrix reorganization during wound healing and its impact on future scarring. Adv Wound Care (New Rochelle) 4 (2015): 119-136


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
