Sequential Use of Hemadsorption Using Cytosorb® and Biosky® Filter-Technology in A COVID-19 Patient Suffering from Severe ARDS
Article Information
Matthias Mezger1,3*, Ingo Eitel1,3, Stephan Ensminger2,3, Dirk Pogorzalek4, Zhipan Huang5, Tobias Graf1,3
1Department of Cardiology, Angiology and Intensive Care Medicine, University Heart Center Lübeck, Lübeck, Germany
2Department of Cardiac and Thoracic Vascular Surgery, University Heart Center Lübeck, Germany
3German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany
4Life Systems, Germany
5Foshan Biosun Medical Technology Co., Ltd. China
*Corresponding Author: Dr. Matthias Mezger, MD, Department of Cardiology, Angiology and Intensive Care Medicine, University Heart Center Lübeck, Lübeck, Germany, Ratzeburger Allee 160, 23538 Lübeck, Germany
Received: 21 August 2020; Accepted: 09 September 2020; Published: 01 October 2020
Citation:
Matthias Mezger, Ingo Eitel, Stephan Ensminger, Dirk Pogorzalek, Zhipan Huang, Tobias Graf. Sequential Use of Hemadsorption Using Cytosorb and Biosky Filter- Technology in A COVID-19 Patient Suffering from Severe ARDS. Archives of Clinical and Medical Case Reports 4 (2020): 969-977.
View / Download Pdf Share at FacebookAbstract
In March 2020, the World Health Organization (WHO) declared the novel coronavirus disease (COVID-19) pandemic. Here, we present the case of a patient who was admitted to our hospital with acute respiratory distress syndrome (ARDS) following infection with COVID-19. After initial stabilization through restrictive fluid management, hemadsorption using Cytosorb? was performed and finally temporary extubation of the patient was possible. However, the patient again clinically deteriorated and needed ventilation and finally veno-venous (VV) extracorporeal membrane oxygenator (ECMO) -support and high catecholamine application. Whilst being on VVECMO, hemadsorption using Biosky? filter was performed. In this manuscript, after a brief overview of the role of hemadsorption in ARDS, a detailed case presentation is followed by a critical discussion of the current literature.
Keywords
COVID-19; Novel coronavirus; SARS CoV-2; Hemadsorption; ECMO
COVID-19 articles, Novel coronavirus articles, SARS CoV-2 articles, Hemadsorption articles, ECMO articles
Article Details
1. Background
Novel SARS-Coronavirus 2 (SARS CoV-2) associated disease (COVID-19) developed into a pandemic health problem during the first quarter of the year 2020. Recommendations for treatment are derived from insights during ARDS treatment or expert opinions [1]. Ongoing inflammation is a major contributing factor to SARS CoV-2 morbidity and mortality and immunomodulation is one novel and innovating approach for treatment of COVID-19 [2]. Immunomodulation might be either achieved through application of immunosuppressive agents, e.g. glucocorticoids or through hemadsorption. During this therapy, blood is perfused through a special filter system. Within the filter, membranes, creating a large surface area, adsorb immune modulating agents that might fuel the vicious circle of inflammation by contributing to vascular leakage and organ failure. Currently there is only limited evidence for filter technology available mostly from small observational studies or case reports [3-5]. On the global marked there are several companies that are offering solutions for hemadsorption (Table 1) and successful use of hemadsorption has been described with respect to sepsis [5], ARDS [6, 7] and endocarditis [8]. So far, results from studies investigating the use of hemadsorption with respect to COVID-19 disease are scarce. We applied CytosorbÒ as well as BioskyÒ filters to treat a patient suffering from severe ARDS due to COVID-19 pneumonia.
2. Case Presentation
A 56 year-old male patient was administered to our tertiary care hospital due to severe ARDS following infection with COVID-19. Previously, he had been treated in another hospital. At the time point of arrival, he was under pressure-controlled ventilation with an FiO2 of 1.0, a PEEP of 16 mbar and 30 mbar peak pressure. The pre-existing conditions documented for the patient were asthma and obesity (body height 188 cm, body weight 110 Kg, BMI 31). Chest x-ray on admission showed bilateral infiltrates. Blood count on administration showed signs of severe infection with leukocytosis (12.700/µl) and highly elevated CRP (319 mg/l). In addition, both D-dimers and LDH were highly increased (> 33 mg/l and 636 U/l, respectively). Troponin levels were slightly increased (16 ng/l), too. However, renal function appeared normal on admission (Creatinine 94 µmol/l). For sufficient blood pressure, administration of norepinephrine (0.48 mg/h) was necessary. Horowitz-index, initially, was 76 mmHg. The following hours after admission, body temperature began to rise, finally reaching 38.8°C and acetaminophen and metamizole were applied for fever control. The following hours, breathing rate had to be increased and we prolonged inspiration time to improve oxygenation.
Since FiO2 could not be reduced the following hours below 0.9 despite repeated relaxation through administration of rocuronium, we decided to begin prone positioning. In addition, sedation regimen was changed from propofol and remifentanil to sevoflurane and sufentanil. Prone positioning was done for 16 hours daily followed by supine positioning for additional 8 hours. With prone positioning, slowly, improvement of respiratory function was visible and FiO2 could be gradually decreased to 0.4. After 7 days, prone positioning could be stopped after we observed sustained improvement of respiratory function also in supine position.
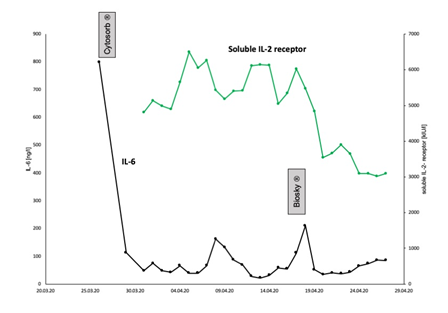
The next days after admission, the patient developed progressive renal failure. Creatinine level increased to 423 µmol/l and urine output almost completely suspended. Therefore, we started continuous renal replacement therapy (CRRT). Here, GeniusÒ 90 system (Fresenius Medical Care, Bad Homburg Germany), was used. Markers of severe systemic inflammation were highly elevated. PCT reached a maximum of 3.7 µg/l, CRP level was 420 mg/l and Leukocytes were elevated (18.000/µl), too. We could also see a highly elevated level of IL-6 (800 ng/l) (Figure 1).
Figure 1: Inflammatory markers. Initially, the levels of all inflammatory markers were highly increased. Here, the time course of IL-6 and soluble IL-2 receptor concentration is illustrated. Soon after admission, the IL-6 concentration was highly increased at 800 ng/l. After three days of CRRT with CytosorbÒ, IL-6 had fallen to 113 ng/l. With ongoing disease course, IL-6 again increased. After change of the antibiotic regimen, finally Caspofungin/Meronem/Linezolid were applied, and after completion of BioskyÒ filter-treatment for additional three days whilst being on VV-ECMO support, IL-6 was as low as 51.9 ng/l. In addition, soluble IL-2-receptor concentration, we measured as well, also decreased tremendously.
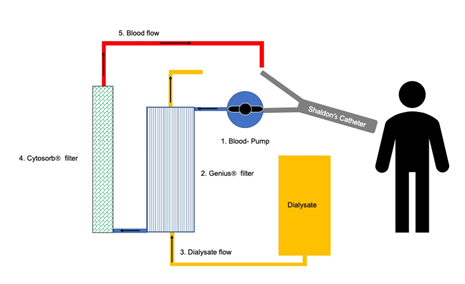
To combat the severe infection, hemadsorption was commenced by using CytosorbÒ filter in line within the CRRT circuit (Figure 2). Treatment with CytosorbÒ filter was performed for a total of three days. After that, blood sampling revealed a significant drop of IL-6, from 800 ng/l to 113 ng/l (Figure 1). All other markers of inflammation also began to decline step by step. The antibiotic therapy with Ampicillin/Sulbactam and Clarithromycin which had been previously started by the colleagues caring for the patient before admission to our university hospital was continued for further 12 days in the case of Ampicillin/Sulbactam and for 8 days in the case of Clarithromycin.
Figure 2: CRRT circuit. We applied the CytosorbÒ system for three days while CRRT (GeniusÒ 90 system, Fresenius Medical Care, Bad Homburg Germany) was performed. Shaldon’s catheter was inserted into the jugular vein. The blood, taken from jugular vein, first was perfused through a blood pump, then through a dialysis filter for renal replacement. Finally, the blood passed the CytosorbÒ filter for hemadsorption before returning to systemic circulation. The CytosorbÒ filter was applied in series to the extracorporeal circuit.
Thereafter, no anti-infective therapy was necessary for 6 days. Then, markers of systemic inflammation, again began to rise, so another antibiotic regime using Piperacillin/Tazobactam was started. Microbial diagnostics could only reveal staphylococcus epidermidis in one blood culture drawn from the arterial catheter line, which we considered contamination. Recovery of pulmonary function was complicated by pneumothorax that was successfully treated with drainage, which we could remove later on. We finally tried extubation after a total of 23 days of ventilator support. However, intubation became necessary soon again, after three days off invasive ventilation since the patient developed progressive respiratory failure.
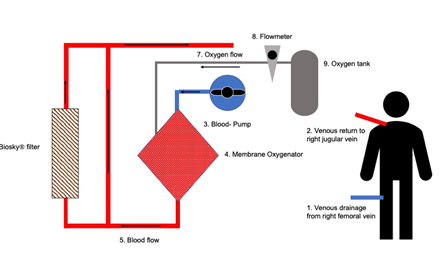
After the second intubation, severe respiratory acidosis was seen in blood gas sampling, despite FiO2 of 1.0. The lung appeared very stiff and tidal volume was 280 ml only, although inspiratory pressure was as high as 40 mbar and PEEP was 14 mbar, too. Both, incorrect position of the endotracheal tube and de novo pneumothorax were excluded. We quickly decided to implant VV-ECMO (Maquet Cardiohelp, Rastatt, Germany). Implantation was done through the right internal jugular vein and right femoral vein. Blood was drawn from the right femoral vein and returned after oxygenation to the right internal jugular vein (Figure 3).
Figure 3: ECMO circuit. We applied the Maquet-CardiohelpÒ system for VV-ECMO. The venous blood was taken from the patients’ right femoral vein. First, the blood went through the pump, then passed the oxygenator and finally was returned to the patient via the right jugular vein. The BioskyÒ filter was incorporated into the ECMO circuit after the oxygenator and the filter was applied in parallel to the blood stream.
After ECMO implantation, respiratory acidosis slowly improved. Both, high norepinephrine support (4,8 mg/h) and intensive fluid therapy were necessary, to achieve sufficient mean arterial pressure. Since blood sampling showed progressive leukocytosis (35.000/µl) and high levels of inflammatory cytokines (IL-6 was 112 ng/l, soluble IL-2 receptor (S-IL2-R) was 6026 kU/l, CRP was 376 mg/l, and PCT was 4,6 µg/l, respectively), we decided to integrate BioskyÒ filter in parallel into the ECMO circuit for cytokine removal (Figure 3). In addition, antibiotic therapy was first escalated to Vancomycin and Meropenem and finally changed to Caspofungin, Linezolid and Meropenem because of an inadequate drop of the inflammatory markers (leukocytes, CRP, IL-6, S-IL2-R). With the combination of a changed antibiotic regimen and BioskyÒ filter, all markers of inflammation, e.g. leukocytes, CRP, PCT, IL-6, soluble interleukin 2-receptor finally started to decrease (Figure 1). Interestingly, neither blood and urine cultures nor tracheal suctioning could reveal microbial infection. Finally, weaning from VV-ECMO was started and ECMO explantation was possible. However, again the patient suffered from very severe respiratory failure and, finally died.
3. Discussion
With this case we want to present our experience using hemadsorption (CytosorbÒ & BioskyÒ filters) in conjunction with antimicrobial therapy in a patient suffering from COVID-19. ARDS might follow infection with SARS-CoV 2, as has been published previously [9]. Since there is only limited evidence regarding the treatment of COVID-19, we first relied on basic principles of ARDS treatment, e.g. adjusting parameters of ventilation for lung protection [1, 10, 11] in line with a restrictive fluid management strategy [11-14]. Here, beneficial effects have already been described with respect to COVID-19, too [15]. As soon as progressive renal failure developed, CRRT was started. To our knowledge, renal disease following infection of COVID-19 is a rare phenomenon [16], which might be associated with cytokine release syndrome [17].
Since markers of inflammation, e.g. CRP, PCT, IL-6 all were increased in our patient, we decided together with the consulting nephrologist to add CytosorbÒ filter to CRRT (Figure 2) in order to combat ongoing inflammatory response. IL-6 is known to be increased early after inflammation starts since it is produced directly at the site where inflammation occurs [18]. Further inflammatory responses are exerted through the effect IL-6 has, for example on liver biosynthesis, e.g. leading to CRP release [18]. In COVID-19, IL-6, together with CRP, has been shown to correlate with disease severity [19]. Therefore, modulation of immune responses through targeting IL-6 has been suggested [20, 21], e.g. through administration of the anti-IL-6 antibody tocolizumab [22]. After hemadsorption through application of CytosorbÒ, a significant drop in IL-6 level could be seen in blood specimen of our patient (Figure 1). Since the antibiotic regimen consisting of ampicillin/sulbactam and clarithromycin was already applied before admission to our hospital, it is unlikely that the drop in IL-6 was just a result of the antibiotic therapy. Indeed, we believe that the hemadsorption therapy through CytosorbÒ application had significant effects on the drop in IL-6 levels which we could observe only short time thereafter.
After the patient, again needed invasive ventilation, respiratory function was severely compromised, which was reflected through severe respiratory acidosis despite high peak pressure chosen on the ventilator. To solve that problem VV-ECMO therapy was commenced. ECMO therapy has been suggested for severe ARDS following COVID-19 [23]. Indeed, successful use of VV-ECMO in COVID-19 disease has been reported [24]. Since inflammatory markers again, were dramatically increased in our patient, we decided to integrate BioskyÒ filter in parallel into the ECMO circuit (Figure 3). According to the product information of the manufacturers there are some important differences between the Biosky and the CytosorbÒ filter (Table 1). Both of them have in common a CE certification. BioskyÒ offers different filter sizes between 150 ml and 350 ml. Blood flow can be chosen higher in the CytosorbÒ filter-system (100-700 ml/min) compared to the BioskyÒ system (100-200 ml/min). Anticoagulation is possible with heparin or citrate for CytosorbÒ application. In contrast, BioskyÒ might be only used together with heparin for anticoagulation which makes use difficult for patients with heparin induced thrombocytopenia type II (HIT II).
After application of the BioskyÒ filter system, we could see decreasing markers of inflammation. Of course, the effect of the filter might be also affected from the changes in the antibiotic regimen. However, the drop in the levels of the inflammatory cytokines we saw, is unlikely to be only a result of the different antibiotic regimens we used in short time course. The differences in the drop of the inflammatory markers between CytosorbÒ and BioskyÒ application might be explained with the fact that the CytosorbÒ filter was applied in line with CRRT extracorporeal circuit and the BioskyÒ filter was used in parallel to the VV-ECMO extracorporeal circuit. In addition, the different physicochemical characteristics of the filters just mentioned might be an important factor, too. Despite the fact that we could not save the life of our patient, we believe that hemadsorption might be a valuable tool that can be used in conjunction with established therapies to combat severe inflammation, especially when there is a lack of causal treatment options such as in COVID-19 disease.
|
Biosun medical |
Cytosorbents |
Jafron |
Baxter |
Toray medical |
|
|
Manufacturing |
China |
North America |
China |
Worldwide |
Japan |
|
Sales |
Asia/Pacific |
North America |
Worldwide |
Worldwide |
Worldwide |
|
Name |
MG 150/250/350 |
CytoSorb 300 adsorber |
HA330 disposable hemoperfusion cartridge |
oXiris |
Toramyxin cartridge |
|
Loading Capacity |
n.A |
n.A |
330 +/- 3 ml |
105 ml |
135 +/- 5 ml |
|
Volume |
150 ml/250 ml/350 ml |
150 ml |
185±5 ml |
189 ml |
n.A |
|
Absorbent material |
polystyrene resin |
polystyrene-divinyl-benzene |
styrene divinylbenzene copolymers |
acrylonitrile + sodium sulfonate copolymer |
polystyrene polymyxin B |
|
Housing material |
polycarbonate |
polycarbonate |
polycarbonate |
polycarbonate |
n.A |
|
Sterilization method |
stream sterilization |
irradiation sterilization |
irradiation sterilization |
ethylene oxide |
high pressure stream sterilization |
|
Blood Flow (min/max) |
100-200 ml/min |
100- 700 ml/min |
100-700 ml/min |
100-450 ml/min |
80-120 ml/min |
|
Max treatment duration |
4 h |
24 hours |
12 hours |
61 hours |
2 hours per cartridge |
|
Anticoagulation |
heparin |
possible with heparin or citrate |
Possible with heparin, LMWH or citrate |
possible with heparin or citrate |
heparin |
|
Storage Conditions |
room temperature |
1° to 40° C; upright storage |
Room temperature |
0-30° C |
room temperature |
|
Compatibility |
Dialysis and ECMO |
Dialysis and ECMO |
Dialysis/CRRT/ECMO/CPB |
Prismaflex system |
Dialysis and ECMO |
|
CE |
yes |
yes |
yes |
yes |
yes |
Table 1: Hemadsorption filters on the global market.
4. Conclusion
Hemadsorption might be a valuable tool in conjunction with other therapies, especially in circumstances of severe inflammation with no available causal treatment such as COVID-19. Several companies offer solutions for hemadsorption, but individual patient characteristics must be considered when choosing an appropriate filter system.
Disclosures
The authors declare that they have no conflict of interest.
References
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Medicine (2020).
- Schijns V, Lavelle EC. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur J Immunol (2020).
- Zhang Y, Mei CL, Rong S, et al. Effect of the Combination of Hemodialysis and Hemoperfusion on Clearing Advanced Glycation End Products: A Prospective Randomized Two-Stage Crossover Trial in Patients Under Maintenance Hemodialysis. Blood Purification 40 (2015): 127-132.
- Gruda MC, Ruggeberg K-G, O'Sullivan P, et al. Broad adsorption of sepsis-related PAMP and DAMP molecules mycotoxins and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PloS one 13 (2018): e0191676-e0191676.
- Brouwer WP, Duran S, Kuijper M, et al. Ince Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Critical care (London England) 23 (2019): 317-317.
- Träger K, Schütz C, Fischer G, et al. Cytokine Reduction in the Setting of an ARDS-Associated Inflammatory Response with Multiple Organ Failure. Case reports in critical care (2016): 9852073-9852073.
- Lees NJ, Rosenberg AJP, Hurtado-Doce AI, et al. Combination of ECMO and cytokine adsorption therapy for severe sepsis with cardiogenic shock and ARDS due to Panton–Valentine leukocidin—positive Staphylococcus aureus pneumonia and H1N1. Journal of Artificial Organs 19 (2016): 399-402.
- Träger K, Skrabal C, Fischer G, et al. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass -a case series. The International journal of artificial organs 40 (2017): 240-249.
- Wang D, B. Hu C. Hu F. Zhu X. Liu J. Zhang et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan China. JAMA 2020. 323(11): p. 1061-1069.
- Guérin C, Reignier J, Richard J-C, et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. New England Journal of Medicine 368 (2013): 2159-2168.
- Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respiratory Research 6 (2019): e000420.
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354 (2006): 2564-2575.
- Kluge S, Janssens U, Welte T, et al. Karagiannidis German recommendations for critically ill patients with COVID?19. Medizinische Klinik -Intensivmedizin und Notfallmedizin (2020).
- Organization WH. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance 28 January 2020. World Health Organization (2020).
- Kazory A, Ronco C, McCullough PA. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proceedings (Baylor University. Medical Center) (2020): 1-6.
- Guan W-J, Ni Z-Y, Hu W-H, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine (2020).
- Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nature Reviews Nephrology (2020).
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation immunity and disease. Cold Spring Harbor perspectives in biology 6 (2014): a016295-a016295.
- Liu F, Li M L, Xu J, et al. Prognostic value of interleukin-6 C-reactive protein and procalcitonin in patients with COVID-19. Journal of Clinical Virology (2020): 104370.
- Zhang S, Li L, Shen A, et al. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin Drug Investig (2020).
- Ortiz-Martinez Y, Tocilizumab. A new opportunity in the possible therapeutic arsenal against COVID-19. Travel Med Infect Dis (2020): 101678.
- Fontana F, Alfano G, Mori G, et al. Covid-19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine. Am J Transplant (2020).
- Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. The Lancet Respiratory Medicine (2020).
- Hartman ME, Hernandez RA, Patel K, et al. COVID-19 Respiratory Failure: Targeting Inflammation on VV-ECMO Support. Asaio J (2020).


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
