Chronic Kidney Disease-Mineral and Bone Disorders (CKD-MBD)
Osama Mosbah*
Nephrology Department, Clinical Medical Division, Theodor Bilharz Research Institute, Giza, Egypt
*Corresponding Author: Dr. Osama Mosbah, Nephrology Department, Clinical Medical Division, Theodor Bilharz Research Institute, El-Nile st, Imbaba, PO box 30, Giza, 12411, Egypt
Received: 29 May 2019; Accepted: 11 June 2019; Published: 17 June 2019
Article Information
Citation: Osama Mosbah. Chronic Kidney Disease-Mineral and Bone Disorders (CKD-MBD). Archives of Nephrology and Urology 2 (2019): 033-051.
DOI: 10.26502/anu.2644-2833008
View / Download Pdf Share at FacebookAbstract
The kidney plays a vital role in the metabolism of minerals and bone health. It is not only the target organ of several regulating hormones such as parathormon (PTH) and fibroblast growth factor-23 (FGF-23), but it is also the main organ that activates vitamin D. CKD-MBD was further expanded to include cardiovascular diseases (CVD), left ventricular hypertrophy (LVH), hypertension, immune dysfunction, inflammation and iron deficiency anemia, and thus its treatment is still a major challenge for the nephrologist that necessitates further pushing for the development of new agents with high specificity to the treatment of CKD induced MBD.
Keywords
<p>Chronic Kidney disease, Metabolic bone diseases</p>
Article Details
1. Introduction
The kidney plays a vital role in the metabolism of minerals and bone health. It is not only the target organ of several regulating hormones such as parathormon (PTH) and fibroblast growth factor-23 (FGF-23), but it is also the main organ that activates vitamin D [1]. Thus, the abnormal mineral metabolism occurs in chronic kidney diseases (CKD) and sequentially affects the bone health. Recently it is renamed chronic kidney disease-mineral and bone disorder (CKD-MBD) as a systemic syndrome (Figure 1) and is called renal osteodystrophy (ROD) (Table 1) if the disease is limited to the bone [2]. CKD-MBD was further expanded to include cardiovascular diseases (CVD), left ventricular hypertrophy (LVH), hypertension, immune dysfunction, inflammation and iron deficiency anemia [3].
4 pathological diseases of bone in CKD [5] are recognized:
- Hyperparathyroid (HPT) bone disease. High bone turnover disease that is attributed to untreated secondary hyperparathyroidism (SHPT). It is represented by bone anomalies such as cortical bone thinning and increased abnormal trabecular bone.
- Adynamic bone disease. Absent or low bone resorption and formation and could be an early finding of CKD. It is often associated with low PTH level and the patients are more vulnerable to develop fractures.
- A slow turnover of bone with an increased unmineralized osteoid matrix that in turn will lead to decrease bone strength. It is often attributed to deficiency of vitamin D, metabolic acidosis and hypocalcemia.
- Mixed renal osteodystrophy. Combined mineralization defects and high bone turnover defects.
Great advances were made in diagnosis, prevention and treatment of CKD-MBD, which is one of a broad spectrum of imbalances [6].
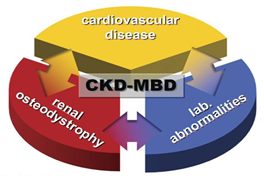
Figure 1: CKD-MBD represents synopsis of 3 1) laboratory abnormalities; 2) indicative of mineral and bone metabolism disturbances and 3) CVD represented by accelerated arteriosclerosis, LVH and abnormal vasculature [4].
|
CKD MBD: Systemic disorder of bone and mineral metabolism manifested by • Abnormalities of Calcium, phosphorus, parathormone and vitamin D. • Abnormalities in bone mineralization and volume. • Vascular or other soft tissue and vascular calcification. |
|
ROD: confined only to CKD bone disease with • Disturbance of bone morphology. • Abnormal skeletal component by bone biopsy histomorphometry. |
Table 1: KDIGO differentiation between CKD-MBD and ROD [1].
2. Incidence and Prevalence
The first descriptions of CKD-MBD (renal rickets or ROD) were nearly 100 years ago, before the modern phenomenon of mass dialysis therapy was remotely envisaged. In 1921, 10 cases of bone deformities caused by chronic nephritis were described [7]. At the beginning of the era of dialysis, in the 1960s, with so many people surviving to some degree with advanced CKD, a wealth of new signs, symptoms and syndromes was described [7]. Stanbury and Lumb [8] were the first who correlated plasma Ca and P values with bone diseases in uremic individuals, e.g. bony pains, resorption of phalanges, myalgia, myopathy, bone fractures, tendon snapping and avulsion, bone deformities due to brown tumours, tumoral calcification and calciphylaxis (Calcific Uremic Arteriolopathy).
Interestingly, it is estimated that 70% to 90% of CKD patients stages III-IV develop alteration in mineral and bone homeostasis [9]. Data from NHANES suggest that bone diseases was twice as common in those with an estimated glomerular filtration rate (eGFR)<60 ml/min/1.73 m2 compared to those with an eGFR>60 ml/min/1.73 m2, with more prevalence in women than men [10]. In general, bone fractures and subsequent mortalities were noticed to be more prevalent in CKD patients [11]. It is reported that a national Egyptian survey showed that renal bone disease prevails among 33.3% of dialysis patients in Egypt with the main factors of pathogenesis include disturbed mineral homeostasis which is important for bone formation and growth “bone modeling” and also for maintenance of bone health in adulthood “bone remodeling” and manifesting as disruption of serum and tissue concentrations of P, Ca and PTH [12].
3. Pathophysiology
It was proved that the kidney activates developmental pathways involved in nephrogenesis and bone health [13]. Theses pathways could be summarized as follows:
3.1 Wingless/integrated/ß-catenin pathways
The wingless/integrated/ß-catenin (Wnt/ß-catenin) pathways, a group of signal transduction pathways, are recognized as the major regulators of bone formation [5]. Wnt/ß-catenin pathway activation stabilizes ß-catenin which is a transcription factor that plays a great role in the production of many osteoblastic factors as Runx2 and osterix that, in turn, stimulate osteoblastic activity and increases bone formation [5]. For activation of these pathways, it is a crucial first for the Wnt ligands, Wnt1, Wnt3a and Wnt10b, to bind with 2 transmembrane proteins, frizzled protein (Fz) and LDL receptor-related protein 5/6 (Lrp5/6), initiating the transcription of genes involved in osteoblastic differentiation [14]. Upon this binding, recruitment of protein disheveled (Dvl) occurs which in turn phosphorylates Lrp5/6, leading to the protection of ß-catenin from degradation by the proteosome via inactivation of its phosphorylation. ß-catenin is then free to translocate to the nucleus and becomes a triggering factor for osteoblastic genes and bone differentiation [15].
3.2 Wnt/ß-catenin pathway inhibitors
These include 3 inhibitors
3.2.1 Receptor activator of nuclear factor k-B ligand: PTH elevation, is associated with abnormal osteoblastic function and osteocyte stimulation with receptor activator of (NFkß)- ligand (RANK-L) production than anabolic osteoblasts, producing CKD mineralization defect and high bone turnover ROD and bone resorption [16]. Mechanical unloading of the bone stimulates production of RANK-L by the osteoblasts and decreases Wnt/ß-catenin pathway activation that will result in increasing bone resorption. Therefore, the osteocytes regulate osteoblastic production of RANK-L that regulates osteoclasts [5]. It is proved that during CKD with continuous secretion of PTH, there will be upregulation of RANK-L and downregulation of osteoprotegerin (OPG), an inhibitor of osteoclast maturation that protect bone resorption. This will favor bone resorption and increased bone turnover [17].
3.2.2 Sclerostin: Another Wnt/ß-catenin pathway inhibitor that is considered as an osteocytic protein regulating the bone mass [18]. During mechanical loading of the bone, osteocytes produces low amounts of sclerostin leading to activation of Wnt/ß-catenin signaling in pre-osteoblasts increasing bone formation [5]. It is noted that high levels of sclerostin can induce mineralization defects and decreases phosphate-regulating neutral endopeptidase (PHEX), responsible for bone mineralization [19]. It is proved that anti-sclerostin monoclonal antibody treatment increase bone volume in rates suffering from CKD with no improvement of ROD [20].
3.2.3 Dickkop related protein-1: Dickkop related protein-1 (Dkk1) is the only critical Wnt/ß-catenin pathway inhibitor in the kidney. During CKD Dkk1 developed in the kidney and its levels become increased early in disease with tubular epithelial repair then decreases, stimulating renal fibrosis [21]. It is reported that neutralization of Dkk1 elevated in the circulation inhibiting CKD induced VC and ROD with increased ontogenesis and remodeling, increasing bone volume [22].
3.3 Fibroblast growth factor -23/ klotho/calciprotein particles axis
3.3.1 Fibroblast growth factor-23: FGF-23 secreted by osteocytes and osteoblasts, and it represents direct bone-kidney and bone-parathyroid connections involved in CKD-MBD It increases several folds in CKD. It is reported as an early CKD-MBD biomarker and is associated with CKD induced CVDs. Also, FGF-23 elevates accordinf to P homeostasis [23]. It stimulates P excretion by decreasing Na dependent P -II co-transporter (Figure 2) that is presented in proximal renal tubules in the kidney [24].
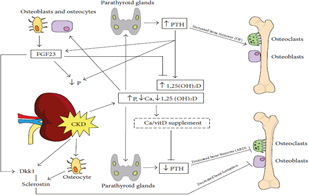
Figure 2: bone and minerals defects in CKD: ↑P, ↓Ca and Vitamin D(↑PTH production by PTG (↑bone turnover and ↑P excretion. ↑PTH and ↑P (upregulation of vitamin D (↑FGF23 production by osteoblasts and osteocytes. As a –ve feedback mechanism FGF23 (↑P excretion and ↓vitamin D [5].
The endocrine action of FGF-23 acts on its binding and activation of its receptor complex (Klotho) [25]. Since the expression of Klotho, declines in the kidney in very early stage CKD, FGF-23 rises (Figure 3) due to resistance to FGF-23 signaling in the kidney inducing urinary P excretion rate decreasing Vit D level as it suppresses renal production of 1, 25(OH) 2 D3 [26]. Therefore the lack of FGF-23, causes hyperphosphatemia increase 1, 25(OH) 2 D3, a situation that produces extra osseous calcification [27]. It is proved that 1, 25(OH) 2 D3 and estrogen upregulate FGF-23 expression by acting on Vit D receptor (VDR) response, an element of FGF-23 promoter [25].
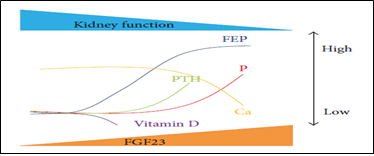
Figure 3: CKD-MBD markers: ↑FEP (factor of P exc), ↑FGF23 levels with ↓1, 25(OH) 2 D3, ↑PTH. WhenFEP fails to respond to ↑FGF23 ESRD à ↑P and ↓Ca [28].
3.3.2 Soluble a-Klotho: Soluble a-Klotho produced from the distal tubular cells of the kidney. Soluble a-Klotho plays a coreceptor of bone, derived protein FGF-23. It is a resulted from shedding of the extracellular domain of the transmembrane-Klotho by two A desintegrin and metalloproteinases (ADAM), ADAM 10 and ADAM 17 [29]. Cleaved Klotho regulates Ca and P excreation and contributes to mineral homeostasis by regulating 1-a-hydroxylase activity, PTH and FGF-23 secretion [30]. Klotho expression is significantly reduced by kidney injuries as AKI, glomerulonephritis, drug abuse as calcinurin inhibitors and chronic allograft injury [31]. The resulting decrease limits the regulation of FGF-23 production and leaves hyperphosphatemia as the main regulator of FGF-23 secretion in CKD [32]. The net result will be inhibition of –ve feedback to FGF-23 secretion and the continuous production and secretion of FGF-23 by the osteocyte, resulting in unique FGF-23 stimulated pathologies as myocyte hypertrophy that is directly linked with CVD [33].
3.3.3 Calciprotein particles CPPs: Calciprotein particles CPPs are complex of Ca, P, Fetuin-A that have an important function in Hydroxyapatite transport to bone [28]. Their levels increase with increase P and Ca inducing atherosclerosis and vascular inflammation in response to CKD. Also CPPs increases in stages I and II of CKD before FGF-23 rise [28].
3.4 Phosphorus, calcium, vitamin D and parathyroid hormone
3.4.1 Phosphorus: Phosphorus CKD contributes to hyperphosphatemia and VC through inhibition of skeletal function. Bone resorption in turn will increase P production and lowers P deposition inducing hyperphosphatemia [34]. Hyperphosphatemia stimulates transition of osteoblasts in the vessels contributing to extraskeletal mineralization and increasing Ca x P product. In stage IV-V CKD, GFR <30% of normal, this adaptation will be no longer adequate and hyperphosphatemia develops despite high PTH [34].
3.4.2 Calcium and vitamin D: As CKD advances and functioning nephron mass decreases together with hyperphosphatemia (Figure 2) and increased FGF-23, suppression of 1a hydroxylase activity occurs, resulting in calcitrol deficiency with subsequent decreasing intestinal Ca absorption and decreased Ca [35].
3.4.3 Parathormone: Parathormone Calcitrol deficiency also decreases VDRs. In parathyroid gland (PTG), it results in resistance to calcitrol mediated regulation and stimulation of PTH secretion leading to SHPT [36], due to direct stimulation of parathyroid cells contributing to elevation of PTH [1]. SHPT is a prominent hazard of CKD, that will lead to VC VC and CVDs with an increase PTH (Figure 2), leading to Ca and P metabolism imbalance [37]. The estimated costs of healthcare for patients with SHPT increased 3 times higher than patients without SHPT. So SHPT treatment may reduce the economic burden in CKD [38].
3.5 Aluminum toxicity
Aluminum (Al) overload or toxicity was recorded in a large percentage of low bone remodeling lesions among CKD patients. Al is implicated in decreasing levels of calcitrol, thus affecting bone health [12].
3.6 Other factors that contribute to ROD
3.6.1 Uremic metabolic acidosis: Uremic metabolic acidosis may contribute to bone dissolution and bone resorption, inhibiting formation of, and disturb physiological actions of PTH and vitamin D. In patients with CKD, it appears to have a role in developing HPT and ROD [39].
3.6.2 Uremic hypogonadism: Uremic hypogonadism Testosterone (T) deficiency (<10 nmol/l) was present in 44% of the men with renal failure, while 33% showed T insufficiency (10-14 nmol/l), and only 23% had normal T values (> 14 nmol/l) [40]. It was found that free T levels were positively correlated with bone mass density (BMD) while its decrease contributes to increased fracture risk [41].
3.6.3 ß2-microglobulin: Among the expressions of CKD-MBD, the most important are deposits of the protein ß2-microglobulin on tissues, causing joint pain, painful shoulders, carpal tunnel syndrome, trigger finger and arthritis [42]. ß2 microglobulin is demonstrated to be a major constituent of amyloid fibrils especially in patients undergoing HD or CAPD. The incidence of this complication increases with the duration of dialytic therapy and the age of the patient [43].
4. Pathophysiology of Soft Tissue and Vascular Calcification
VC pathogenesis in CKD is complex and instead of happening through a simple precipitation of Ca and P in the vessel wall due to hypersaturation of these ions, it is the result of an active process of transformation of smooth muscle cells into osteoblast like cells [44]. Smooth muscle cells and osteoblasts share a common stem cell. Structures identical to bone tissue, occasionally found in atherosclerotic lesions, suggest that VC is an actively regulated process in which the vascular cell acquires osteoblast like cell functions, secreting osteoid matrix. In other words, this VC pattern equals that of a heterotropic ossification [45]. The smooth muscle cell apoptosis is another mechanism that initiates VC. It is triggered by the interaction of these cells with the inflammatory cells, which expresses surface death ligands and secretes pro-apoptotic cytokines such as tumor necrosis factor-α (TNF-α). The apoptotic bodies of these cells are similar to the matrix vesicles in cells of the epiphyseal cartilage of long bones, which is a part of the physiological process of skeletal ossification [46].
The presence of osteoclastic differentiation factors (RANK/RANK-L system) and precursors in calcified plaques, suggests osteoclastic activity on the arterial wall, The coexistence of osteoblasts and osteoclasts reinforces the hypothesis that the calcification process is similar to that occurring in bone tissue and the imbalance in this process, such as increased differentiation of the osteoblasts and decreased that of the osteoclasts, may lead to calcification, but the exact role played by the reduction in osteoclastic differentiation remains to be investigated. Substances that are involved in the regulation of bone formation are also involved in the process of VC in CKD-BMD, acting as physiological promoters and inhibitors of soft tissue calcification [44].
4.1 Promoters of vascular calcification
P is the most significantly studied VC promoter. The risk factors for VC are divided into traditional, involving advanced age, hypertension, bad glycemic control, smoking, dyslipidemia and others, and the non-traditional ones, including inflammation, oxidative stress and CKD-MBD [44].
4.1.1 Calcium, phosphorus and vitamin D: It was evaluated that cell cultures of human aortas expressed high levels of Ca and P, especially in CKD patients. This substantially increased Ca-P nucleation induces calcification of the matrix vesicles of the viable smooth muscle cells and also in the apoptotic bodies of dead cells. It is worth noting to know that when these cells are exposed to the serum of non-uremic human containing bone morphogenetic proteins (BMPs) and fetuin A, calcification was inhibited [46]. Increasing P within the smooth muscle cells, increases the core binding factor 1 (Cbfa1) expression that induces smooth muscle cell differentiation into osteoblasts, suggesting that other toxins, yet unknown, and hyperphosphatemia, are connected to the upregulation of Cbfa1, with subsequent increased expression of bone matrix in the vascular tissue, resulting in progressive calcification especially in dialysis patients [47].
4.1.2 Parathormone and fibroblast growth factor-23: FGF-23 levels are associated with increased cardiovascular risk in CKD, kidney transplant loss rate and mortality rate. In humans, it was demonstrated that FGF-23 is not only a biomarker associated with cardiovascular risk in CKD, but is also a direct pathogenic factor causing LVH through activation of the calcineurin pathway in cardiac myocytes and promoting Ca reabsorption through stimulation of the apical Ca entry channel in the renal distal tubules which are regulated by Klotho [13]. Recent studies have directly linked Klotho deficiency with CVD, including VC, vascular stiffness and uremic vasculopathy “calciphylaxis” [13]. Elevated FGF-23 levels were independently associated with therapy resistant SHPT and increased cardiovascular risk in CKD patients [48].
4.2. Inhibitors of vascular calcification
BMPS and some transcription factors such as fetuin-A, osteopontin, OPG and Matrix gamma carboxyglutamic acid (GLA) protein (MGP), which are physiological substances able to inhibit soft tissue calcification, have also been described as inhibitor of vascular calcification. This explains why there is no spontaneous mineralization even in tissues exposed to fluids supersaturated with Ca and P [44].
4.2.1 Wnt/β-catenin pathway inhibitors: It has been hypothesized that Wnt/β-catenin inhibitors e.g. sclerostin, would have the same suppressing effect on VC as on bone formation in CKD, which could suggest a protective effect on the vessels [5]. Other seemingly contradictory findings could reflect a number of pathological mechanisms [49] including:
- Sclerostin inhibits osteoblastic differentiation in bone not in vessels.
- Sclerostin induced decrease of bone turnover may lead to higher circulating Ca and P levels that stimulate VC.
- The circulating sclerostin might indeed delay the progression of VC. The calcified vasculature in CKD could itself be a major source of sclerostin as the disease progresses.
- The role of the Wnt/β-catenin inhibitors during progression of CKD could be different from their role in the healthy population, which would explain the variable results in non-dialyzed versus dialyzed population.
4.2.2 Activin A and activin type 2A receptor: Another factor that increases during the course of CKD is activin A, a known renal developmental factor and circulating hormone that belongs to the transforming growth factor-β superfamily members. Activin type 2A receptor (ActRIIA) ligand trap blocked stimulation of vascular smooth muscle osteoblastic transition, VC and renal fibrosis by CKD [50]. In the skeleton, the ActRIIA ligand trap blocks the CKD stimulation of osteoclastogenesis, bone resorption and remodeling despite no affection of the high PTH levels [51]. In the kidney, the ligand trap inhibits the activin signaling, decreased renal Wnt activation and circulating Dkk1 and increased renal Klotho expression [50].
4.2.3 Osteopontin: Osteopontin is a bone matrix protein that has a physiological inhibitory action on VC. It is a phosphorylated acid glycoprotein that was first discovered in bone. It is not found in most normal tissues; it is abundant at sites of ectopic calcification as CKD-VC. The mechanism by which mineralization is inhibited is not entirely clear, but it is likely to be by inhibition of physical deposition and build-up of hydroxyapatite [52]. Although physiologically it is an inhibitor of calcification, high levels of osteopontin are associated with cardiovascular risk, especially in CKD patients due to a reduced renal excretion caused by renal failure associated with hyperphosphatemia and high Ca-P binding, underscoring its association with VC development [52].
4.2.4 Osteoprotegerin: OPG, a glycoprotein member of TNF-α superfamily, inhibits osteoclasts maturation and protects bone tissue from the resorptive activity [53]. Along with the MGP and fetuin A, it is an important VC inhibitor. Genetic deficiency of OPG was found to cause calcification of the aorta and renal arteries [53].
4.2.5 Matrix gamma carboxyglutamic acid protein: MGP is a matrix protein that inhibits the extracellular matrix mineralization. It is produced by smooth muscle cells and chondrocytes, the 2 cell types which produce non-calcified extra cellular matrix, to the point that MGP could be considered as the first inhibitor of calcification of arteries and cartilage in vivo [44]. The MGP exerts its effect directly by inhibiting the formation of Ca crystals, together with other inhibitors of calcification, such as fetuin-A or, indirectly by influencing the transcription of other factors that inhibit the differentiation of vascular cells into osteoblast like cells [54]. It is proved that MGP precursor production reduced by vitamin D deficiency, leading directly to VC that when abundant would reduce the non carboxylated MGP, the precursor of the active MGP, because of its high affinity for the hydroxyapatite deposition within the vessels in a consumption mechanism [54].
4.2.6 Fetuin-A: Fetuin-A temporarily inhibits the formation and precipitation of hydroxyapatite, being able to inhibit undesirable VC without inhibiting bone mineralization. Reduced serum fetuin A levels in dialysis patients was proved due to a state of chronic micro inflammation that is found in CKD and is indicated by increased C-reactive protein (CRP) level since fetuin A is negatively regulated by inflammation [44]. It was found that reducing calcification inhibitors as fetuin A, BMP, osteopontin and OPG induce smooth muscle cell differentiation into osteoblast like cells acting through transcription factor Cbfa1 which is expressed by mesenchymal precursor cells in the bone marrow, as the key regulator of the differentiation of osteoblasts and the production of bone matrix components such as collagen type I, osteocalcin and osteopontin, making up a pro-mineralization matrix [44].
5. Clinical Picture
Bone and mineral disturbance in CKD patients can be asymptomatic for a long time. Indeed, symptomatic CKD-MBD is much rarer now than was the case 2 or more decades ago, as better detection, better prevention and better treatment options all now exist for the majority of CKD patients. However, occasional patients do present late with advanced CKD with symptomatic bone pathologies. As regards to bone disorders, there are several forms of ROD, including osteitis fibrosa cystica, adynamic bone disease and osteomalacia. In some patients, there is evidence of more than one type, which is called mixed osteodystrophy [7]. The first manifestation of CKD-MBD is bone and muscle pain, weakness and fractures of bone and sometimes avascular necrosis that could be seen in late stages of the disease. The incidence of bone fractures is very high in CKD patients. It is twice as high compared to patients without CKD [1]. These data are alarming because CKD and osteoporosis frequently co localize, and the incidence and prevalence of pre dialysis CKD osteoporosis and fragility fracture are expected to increase exponentially as the population ages progress [55].
Osteoclastomas or brown tumours are caused by localized replacement of bone by vascularized fibrous tissue “osteitis fibrosa cystica” resulting from PTH stimulated osteoclastic activity. They are often painful, and in addition, can cause significant symptoms by local compression. The fibrous tissue contains giant cells and the lesions that may become cystic following necrosis and liquefaction. Brown tumours are frequently solitary, although multiple lesions have been reported. Ribs, pelvis, especially facial bones and femurs are common locations, and the axial skeleton may be involved with neurobiological sequelae. After successful treatment of HPT, typically after parathyroidectomy, Brown tumours may heal with calcification, sclerosis and lesion disappearance, on contrast the lytic area may persist [56]. CKD stimulates VC via intimal calcification in the form of neo-intimal atherosclerotic plaque calcification that is produced by osteoblastic transition of cells in the neo-intima whose origin have been linked to smooth muscle cells and circulating mesenchymal cells [57].
In severe forms of CKD-MBD, calcific uremic arteriolopathy “calciphylaxis”, a condition in which small cutaneous blood vessels are calcified, is often lethal and is seen in severe SHPT [1]. Calciphylaxis is a rare and often lethal disorder characterized by systemic medial calcification of arterioles, which leads to ischemia and subcutaneous necrosis [7]. Clinical calciphylaxis was first described very early in the dialysis era (1950s to 1960s). Although previously rare, the incidence of this disorder has appeared to be increasing [58]. Calciphylaxis is one of several types of extra osseous calcification, which also includes intimal, medial and valvular calcifications that may occur in patients with end stage renal disease (ESRD). Traditionally, it has been classified as metastatic calcification indicating passive mineralization of Ca and P crystals [7]. Calciphylaxis is typically characterized by areas of excruciatingly painful ischemic necrosis that usually develop on areas with greatest adiposity including abdomen, buttock and thigh [58]. These lesions are represented by violaceous and painful plaques like subcutaneous nodules. The initial purpuric plaques and nodules subsequently progress to ischemic/necrotic ulcers [58]. Furthermore, new roles for high PTH levels in the development of cachexia, sarcopenia, and hyperuricemia have recently been reported in addition to the classic concept of PTH as a uremic toxin [59].
6. Diagnosis
The key measurements used in routine CKD-MBD diagnosis are based on biochemical, radiological, and bone biopsy with subsequent pathological assessment.
6.1 Biochemical parameters
Screening of Ca, P, alkaline phosphatase (ALP) and PTH, based on KDIGO guidelines, is highly recommended. These measurements should be routinely monitored at the early stages of CKD “stage III” [60]. Total Ca, should be checked regularly. However serum Ca cannot be a guide for the underlying CKD-MBD. P should be measured with precautions. There is diurnal and postprandial variation of the P level. Therefore, should be checked on fasting [1]. Total ALP is another bone marker. Although it is not the best marker for bone turnover, an immunoassay for bone specific ALP is a better marker but not in standard practice [1]. However, it does not confer more information regarding bone turnover [61].
In clinical practice, however, measurements of PTH are not always diagnostic of ROD. PTH levels cannot replace bone histology for the diagnosis of ROD; PTH values do not provide any information regarding bone volume and mineralization status. Both skeletal resistance to PTH and variability in PTH assay hinder the predictive value of PTH for bone turnover [61]. There is no doubt that measurement of iPTH (intact assay) is the most important in the diagnosis of CKD-MBD. However, some fragments antagonistic to iPTH are detected. Therefore the assay should be more than three times the upper limit of normal [60].
6.2 Radiological tests
The routine radiological tests used in the diagnosis of CKD-MBD [7] include:
- Imaging by X-ray to assess phalangeal tufts, subperiosteal bone erosion, linear osteosclerosis of the spine and lucent areas of the long bones.
- Ultrasound examination (US) is very useful in detecting PTG hyperplasia and to distinguish diffuse and nodular hyperplasia.
- Computed tomography (CT) and magnetic resonance imaging (MRI) of the skeleton are another tools but relatively insensitive for a diagnosis of CKD-MBD.
- Bone densitometry (DEXA scan) could be done, but its results should also be interpreted carefully as it cannot distinguish between osteoporosis and CKD-MBD.
6.3 Bone biopsy
Bone biopsy is the gold standard for a diagnosis of the pattern of bone disease, but it is not available everywhere, and it is invasive and time consuming. In more recent guidelines, there is new classification system for bone histomorphometry, known as turnover, mineralization and volume (TMV). Instead of the terms of adynamic bone, mixed or mild osteodystrophy, and high turnover bone disease (osteitis fibrosa), the TMV classification is made up of different descriptors: T “from low to high”, M “from normal to abnormal” and V “from low to high” [9]. Usually, bone biopsy (Table 2) in CKD is not contraindicated except in cases of coagulation disorders, soft tissue and/or skin inflammation, and the presence of local infection over the iliac area. The percentage of complications following bone biopsy is very low. Intolerance, allergic reaction, and gastrointestinal disorders have been reported after intake of antibiotics for bone labeling. Bleeding, local bruising, pain, neuropathy, infection, fracture and osteomyelitis have also been reported [62].
|
Clinical indications for bone biopsy in CKD: · Unexplained bone fracture. · Suspicion of osteomalacia. · Before surgical parathyroidectomy, suspicion of aluminum overload. · Evaluation of the histologic effect of several treatments of CKD–MBD. · Before the use of bisphosphonates, Denosumab, and Romosozumab. |
|
Laboratory indications for bone biopsy in CKD: · Discordance between PTH levels and bone-specific ALP levels. · Unexplained hypercalcemia or hypophosphatemia. · Toxicity with aluminum or with other metals imaging. · Extremely increased or decreased BMD. · Unexplained radiologic bone abnormalities. · Progressively rapid cardiovascular calcifications. |
Table 2: Clinical and Laboratory Indications for Bone Biopsy in CKD [9].
Several bone histomorphometric parameters are obtained such as bone mass, static parameters of bone formation and resorption, and dynamic parameters of both bone formation and mineralization are included in the analysis of cancellous bone. The first bone histomorphometric analysis of bone biopsy in CKD patients was based on turnover, the percentage of osteoid and the presence or absence of fibrosis [9]. According to histomorphometric analysis, three types of ROD [63] have been recognized:
- High turnover bone disease including HPT or osteitis fibrosa cystica.
- Low turnover bone disease including adynamic bone disease or osteomalacia.
- Mixed uremic osteodystrophy characterized as high turnover bone disease in association with mineralization defect.
7. Management
The central tenets of treatment of CKD-MBD are correction of hyperphosphatemia, hypocalcaemia and maintaining optimal concentration of calcitrol. Unfortunately, it is still difficult to control mineral disturbance, including increased PTH synthesis and secretion [64].
7.1 Management of hyperphosphatemia
Patients with hyperphophatemia must use Ca based P binders but hypercalcemia can be a possible side effect. So the need to manage with non-Ca-based P binder become of great importance e.g. Sevelamer carbonate, a non-Ca-based P binder is effective, with the possibility of decreasing low-density lipoprotein cholesterol levels. Lanthanum carbonate is also an effective P binder, but it is still unavailable in many countries. P binders should be taken at each meal. High pill burden is a major reason for poor adherence by CKD patients [65]. Dietary P restriction <1000 mg/day is recommended. Food with high P food and drinks such as soft drinks, fast and processed foods and coca cola should be avoided. Dietary restriction is insufficient in the majority of patients, particularly those on dialysis. Dialysis adequacy is important in reducing phosphate level, and even more important is more frequent dialysis e.g. short daily or long nocturnal HD [1].
7.2 Management of altered Ca level
A combination of Ca acetate and magnesium carbonate has several advantages. Magnesium can reduce the risk of soft tissue calcification [1]. Bisphosphonates have become a standard treatment for osteoporosis and malignant bone disease. Most are contraindicated in severe renal insufficiency because they are eliminated exclusively by the kidneys. However, the marked impairment of bone metabolism in many dialysis patients provides a rationale for their judicious use in this setting. Animal studies reveal that bisphosphonates inhibit HPT bone changes. Clodronate, Pamidronate and Ibandronate are also readily dialyzable [66].
7.3 Management of altered vitamin D level
Vitamin D or vitamin D analogs are often used in CKD patients to prevent and treat SHPT. For many years, the most commonly used agent was calcitrol. Today, Paricalcitol, a vitamin D analog also called selective VDR activator, is used more frequently [67].
7.4 Management of secondary hyperparathyroidism
The updated KDIGO guidelines suggest that conventional treatment of SHPT in patients with CKD is limited to administration of the vitamin D analog, calcitrol, or dietary P binders to reset the levels of Ca, P and PTH [68]. However, administration of a vitamin D analog increases serum Ca and P levels, therefore, effective therapies for HPT that can control PTH levels without inducing hypercalcemia are needed [38]. Importantly, PTH control by the use of cinacalcet was associated with a lower mortality rate in Japanese dialysis patients with moderate to severe SHPT. Such beneficial effects may in part be explained by decreased FGF23 levels by this drug [69]. Etelcalcetide, a novel calcimimetic agent, is a synthetic peptide agonist of Ca sensing receptor and differs from cinacalcet because it shows mild gastrointestinal symptoms, and could be administered via a HD venous line, thereby decreasing the oral medication load [38]. In some patients, even with new drugs, severe HPT could be present and surgery becomes inevitable. Total or subtotal parathyroidectomy, could be performed. If surgery is indicated, as in patients with severe nodular PTG hyperplasia, it should be done before a kidney transplant [1]. However, rapid decrease in excess PTH after parathyroidectomy causes decrease Ca and P because of their rapid flow into the bone as a result of marked suppression of high turnover bone [28].
7.5 Teriparatide
Anabolic agent, analogue of PTH, is used to treat osteoporosis. In patients with mild to moderate CKD who had normal serum PTH, teriparatide was effective in increasing BMD and reducing the risk of fractures. As increased PTH levels constitutes a contraindication to teriparatide, the use of this drug in CKD, a condition related to HPT, seems inappropriate. Teriparatide might be useful in CKD patients with adynamic bone disease and in those who underwent prior parathyroidectomy [70].
7.6 Management of aluminum toxicity
Use of Al free HD water and non Al containing P binders has dramatically reduced Al toxicity [9]. Use of Al containing oral P binders should be replaced by other binding agents such magnesium hydroxide and calcium carbonate. During dialysis, it is important to eliminate Al from dialysate fluid. It is recommended that Al contents of dialysis fluid should be less than 0.2 mmol/l. HD itself has not been effective in reducing the body burden of Al since more than 80% is tightly bound to protein [71]. Desferrioxamine is an iron chelating agent which after infusion into patients allows substantial Al removal by HD [71].
7.7 Hormone replacement therapy
In addition to ROD, postmenopausal women on dialysis could be at risk of osteoporosis. Hormone replacement therapy (HRT) could have beneficial effects as well as potentially serious risks, especially in uremic women, due to the pharmacokinetics of estradiol in renal failure. Therapeutic alternatives, such as the selective estrogen receptor modulators (SERMs), have shown the benefits of estrogen on bone and serum lipid levels, without its adverse effects on the breast and endometrium, in non-uremic women. So, Raloxifene and other SERMs could represent a good alternative to HRT in postmenopausal uremic women [72].
7.8 Denosumab
Denosumab is a potent anti-resorptive agent acting as a RANK-L antagonist. It is monoclonal antibody against the receptor activator of NFkB ligand, and it inhibits osteoclast proliferation and development. In contrast to bisphosphonates, denosumab is not cleared by the kidney; therefore, there is no risk of over suppressing bone turnover due to drug accumulation in CKD [10].
7.9 Strontium ranelate
This medication rebalances bone remodeling, without a great inhibition of bone resorption, which could reduce the risk of hypocalcemia and adynamic bone disease. However, due to the renal excretion of strontium, it is contraindicated in patients with a GFR ?30 ml/min i.e. CKD stages IV and V. The European Medicines Agency (EMA) recommends using of strontium based on reports of patients with CVD complications using this drug [70].
8. Conclusion
Abnormal mineral metabolism occurs in CKD patients with affection of bone health. Recently, it has been renamed CKD-MBD as a systemic syndrome. A national survey in Egypt showed that renal bone disease prevails among 33.3% of dialysis patients in Egypt. Many factors contribute to CKD-MBD as alteration in Wnt/β-catenin pathway, FGF23/klotho/CPPs axis, P, Ca, vitamin D, PTH, Al homeostasis, metabolic acidosis, hypogonadism and β2-microglobulin. VC pathogenesis in CKD is complex. It is the result of an active process of transformation of smooth muscle cells into osteoblast-like cells. Substances involved in the regulation of bone formation are also involved in the process of VC in CKD-BMD e.g. P, Ca, vitamin D, BMPs, fetuin A, osteopontin, OPG and MGP. The first manifestation of CKD-MBD is biochemical. Bone and muscle pain, weakness and fractures of bone and sometimes avascular necrosis are described. Osteoporosis, osteoclastomas, brown tumours and calciphylaxis frequently occur.
The key measurements used in routine CKD-MBD diagnosis are based on biochemical, radiological and pathological assessment of bone biopsy which is the gold standard for a diagnosis of the pattern of bone disease. The central tenets of treatment of CKD-MBD are correction of altered Ca and P, calcitrol, manage Al toxicity and control the increased PTH synthesis and secretion. Other treatment lines include using of teriparatide, HRT, denosumab and strontium ranelate. We can, therefore, conclude that the treatment of CKD-MBD is still a major challenge for the nephrologist that necessitates further pushing for the development of new agents with high specificity to the treatment of CKD induced MBD.
References
- Pavlovic D, katicic D, Gulin T, et al. Chronic kidney disease mineral bone disorder. Periodicum Biologorum 117 (2015): 81-85.
- Fukagawa M and Komaba H. Chronic kidney disease-mineral and bone disorder in Asia. Kidney Diseases 3 (2017): 1-7.
- Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney International 90 (2016): 985-996.
- Cozzolino1 M, Urena-Torres P, Vervloet M, et al. Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrology, Dialysis and Transplantation 29 (2014): 1815-1820.
- Bisson S, Ung R and Mac-Way F.Role of the Wnt/β-Catenin pathway in renal osteodystrophy. International Journal of Endocrinology (2018): 1-15.
- Elder J. Pathophysiology of CKD-MBD. Clinical Reviews in Bone and Mineral Metabolism 10 (2012): 128-141.
- Heymann E, Jenkins M and Goldsmith D. Clinical features and manifestations of CKD-MBD. Clinical Reviews in Bone and mineral Metabolism 10 (2012): 142-148.
- Stanbury S and Lumb G. Parathyroid function in chronic renal failure. A statistical survey of the plasma biochemistry in azotaemic renal osteodystrophy. Quarterly Journal of Medicine 35 (1966): 1-23.
- Dousdampanis P and Trigka K. Importance of bone biopsy in chronic kidney disease-mineral bone disorders. Saudi Journal of Kidney Diseases and Transplantation 28 (2017): 992-996.
- Khairallah P and Nickolas T. Management of osteoporosis in CKD. Clinical Journal of the American Society of Nephrology 13 (2018): 1-8.
- Kim S, Long J, Montez-Rath M, et al. Hip fracture in patients in non-dialysis requiring chronic kidney disease. Journal of Bone and Mineral Research 31 (2016): 1803-1809.
- Ahmed H, Elzorkany K, Yasein Y, et al. Prevalence of mineral bone disorders among hemodialysis patients in Menoufia Governorate, Egypt. Menoufia Medical Journal 30 (2017): 687-692.
- Hruska K, Sugatani T, Agapova O, et al.. The chronic kidney disease-mineral bone disorder (CKD-MBD): advances in pathophysiology. Bone 100 (2017): 80-86.
- Krishnan V, Bryant H and Macdougald O. Regulation of bone mass by Wnt signaling. Journal of Clinical Investigation 116 (2006): 1202-1209.
- Bernkopf D, Hadjihannas M and Behrens J. Negative feedback regulation of the Wnt pathway by conductinaxin2 involves insensitivity to upstream signaling. Journal of Cell Science 128 (2015): 33-39.
- Meir T, Durlacher K and Pan Z. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney International 86(2014): 1106-1115.
- Shimizu M, Noda H and Joyashiki E. The optimal duration of PTH (1-34) infusion is one hour per day to increase bone mass in rats. Biological and Pharmaceutical Bulletin 39 (2016): 625-630.
- Asamiya Y, Tsuchiya K and Nitta K. Role of sclerostin in the pathogenesis of chronic kidney disease-mineral bone disorder. Advances in Renal Replacement Therapy 2 (2016): 1-8.
- Tu X, Delgado-Calle J and Condon K. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proceedings of the National Academy of Sciences of USA 112 (2015): 478-486.
- Moe S, Chen Nand Newman C. Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. Journal of Bone and Mineral Research 30 (2015a): 499-509.
- Ren S, Johnson B and Kida Y. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proceedings of the National Academy of Sciences of USA 110 (2013): 1440-1445.
- Fang Y, Ginsberg C and Seifert M. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-MBD. Journal of the American Society of Nephrology 25 (2014): 1760-1763.
- Humalda J, Heerspink L and Kwakernaak A. Fibroblast growth factor- 23 and the antiproteinuric response to dietary sodium restriction during renin angiotensin-aldosterone system blockade. American Journal of Kidney Diseases 65 (2015): 259-266.
- Hori M, Kinoshita Y, Taguchi M, et al. Phosphate enhances FGF-23 expression through reactive oxygen species in UMR-106 cells. Journal of Bone and Mineral Metabolism 34 (2016): 132-139.
- Rodriguez M, Lopez I, Munoz J, et al. FGF23 and mineral metabolism, implications in CKD-MBD. Nefrologia 32 (2012): 275-278.
- Kimura T, Shiiizaki K, Kuro M, et al. FGF23 initially plays an important role on phosphate homeostasis in chronic kidney disease. Journal of the American Society of Nephrology 27 (2016): 221.
- Prie D, Urena T and Friedlander G. Latest findings in phosphate homeostasis. Kidney International 75 (2009): 882-889.
- Akiyama K, Kimura T and Shiizaki K. Biological and clinical effects of calciprotein particles on chronic kidney disease-mineral and bone disorder. International Journal of Endocrinology (2018): 1-6.
- Abdallah E, Mosbah O, Khalifa G, et al. Assessment of the relationship between serum soluble Klotho and carotid intima-media thickness and left ventricular dysfunction in hemodialysis patients. Kidney Research and Clinical Practice 35 (2016): 42-49.
- Sakan H, Asai O, Imura A, et al. Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. Public Library of Science One 9 (2014): e86301.
- Lu X and Hu M. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Disease 3 (2017): 15-23.
- Grabner A, Ansel P and Schramm K. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metabolism 22 (2015): 1020-1032.
- Cianciolo G, Galassi A, Capelli I, et al. Klotho-FGF23, cardiovascular disease, and vascular calcification: black or white? Current Vascular Pharmacology Journal 16 (2018): 143-156.
- Silver J, Rodriguez M and Slatopolsky E. FGF23 and PTH-double agents at the heart of CKD. Nephrology, Dialysis and Transplantation 27 (2012): 1715-1720.
- Malluche H, Porter D and Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nature Reviews in Nephrology 9 (2013): 671-680.
- Prie D and Friedlander G. Reciprocal control of 1, 25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clinical Journal of the American Society of Nephrology 5 (2010): 1717-1722.
- Papademetriou V, Lovato L and Doumas M. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney International 87 (2015): 649-659.
- Mima A, Tansho K, Nagahara D, et al.. Treatment of secondary hyperparathyroidism in patients on hemodialysis using a novel synthetic peptide alcimimetic, etelcalcetide: a short-term clinical study. Journal of International medical Research (2018): 1-8.
- Kraut J. The role of metabolic acidosis in the pathogenesis of renal osteodystrophy. Advances in Renal Replacement Therapy 2 (1995): 40-51.
- Thirumavalavan N, Wilken N and Ramasamy R. Hypogonadism and renal failure: An update, Indian Journal of Urology 31 (2015): 89-93.
- Golds G, Houdek D and Arnason T. Male hypogonadism and osteoporosis: the effects, clinical consequences, and treatment of testosterone deficiency in bone health, International Journal of Endocrinology (2017): 1-15.
- Vides M and Martins M. Bone pain assessment in patients with chronic kidney disease undergoing hemodialysis, Revista Dor 18 (2017): 245-249.
- Wada T, Miyata T, Sakai H, et al. Beta2-microglobulin and renal bone disease, Peritoneal Dialysis International 2 (1999): 413-416.
- Peres L and Percio P. Mineral and bone disorder and vascular calcification in patients with chronic kidney disease. Journal Brasileiro de Nefrologia 36 (2014): 201-207.
- Lewis R. Mineral and bone disorders in chronic kidney disease: new insights into mechanism and management. Annals of Clinical Biochemistry 49 (2012): 432-440.
- Reynolds J, Joannides A, Skepper J, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular Ca and P concentrations: a potential mechanism for accelerated vascular calcification in ESRD. Journal of the American Society of Nephrology 15 (2004): 2857-2867.
- Jono S, McKee M, Murry C, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circulation Research 87 (2000): 7-10.
- Raafat M, Madkour M, Metwaly A, et al. Clinical significance of FGF-23 in Chronic Kidney Disease Patients. Scholars Journal of Applied Medical Sciences 3 (2015): 741-750.
- Qureshi A, Olauson H and Witasp A. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney International 88 (2015): 1356-1364.
- Agapova O, Fang Y, Sugatani T, et al. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney International 89 (2016): 1231-1243.
- Sugatani T, Fang Y, Alycia G, et al. A ligand of the activin receptor type IIA mediates osteoclast stimulation of bone remodeling in diabetic mice with CKD. Kidney International 91 (2016): 86-95.
- Giachelli C, Speer M, Li X, et al. Regulation of vascular calcification: roles of phosphate and osteopontin. Circulation Research 96 (2005): 717-722.
- Ossareh S. Vascular calcification in chronic kidney disease: mechanisms and clinical implications. Iranian Journal of Kidney Disease 5 (2011): 285-299.
- Parker B, Ix J, Cranenburg E, et al. Association of kidney function and uncarboxylated matrix Gla protein: data from the Heart and Soul Study. Nephrology, Dialysis and Transplantation 24 (2009): 2095-2101.
- Toussaint N, Elder G and Kerr P. A rational guide to reducing fracture risk in dialysis patients. Seminars in Dialysis 23 (2010): 43-54.
- Klawansky S, Komaroff E, Cavanaugh P, et al. Relationship between age, renal function and bone mineral density in the US population. Osteoporosis International 14 (2003): 570-576.
- Zhu D, Mackenzie N, Shanahan C, et al. BMP-9 regulates the osteoblastic differentiation and calcification of vascular smooth muscle cells through an ALK1 mediated pathway. Journal of Cellular and Molecular Medicine 19 (2015): 165-174.
- Brandenburg V, Cozzolino M and Ketteler M. Calciphylaxis: a still unmet challenge. Journal of Nephrology 24 (2011): 142-148.
- Sugimoto R, Watanabe H, Ikegami K, et al. The down-regulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney International 91 (2017): 658-670.
- Chen W and Bushisky D. KDIGO CKD-MBD guideline update: evolution of the face of uncertainty, Nature Reviews Nephrology 13 (2017): 600-602.
- El-Husseini A and Sawaya B. What is the role of bone biopsy in the management of adult dialysis patients? Seminars in Dialysis 27 (2014): 266-269.
- Trueba D, Sawaya B, Mawad H, et al. Bone biopsy: Indications, techniques, and complications. Seminars in Dialysis 16 (2003): 341-345.
- Torres P, Bover J, Mazzaferro S, et al. When, how, and why a bone biopsy should be performed in patients with chronic kidney disease. Seminars in Nephrology 34 (2014): 612-625.
- Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Therapeutic Apheresis and Dialysis 17 (2013): 247-288.
- Pavlovic D, Katicic D and Josipovic J. Chronic kidney disease-disturbance of minerals and bone metabolism: Why and how to control phosphorus? Acta Medica Croatica 66 (2012): 64-67.
- Bergner R. Bisphosphonate therapy in renal osteodystrophy. Journal of Nephrology 26 (2013): 450-455.
- Cuningham J, Locatelli F and Rodriguez M. Secondary HPT: pathogenesis, disease progression, and therapeutic options. Clinical Journal of the American Society of Nephrology 6 (2011): 913-921.
- Ketteler M, Block G and Evenepoel P. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney International 92 (2017): 26-36.
- Moe S, Chertow G, Parfrey P, et al. Evaluation of cinacalcet HCL therapy to lower cardiovascular events (EVOLVE) trial investigators: cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCL Therapy to lower cardiovascular events (EVOLVE) trial. Circulation 132 (2015b): 27-39.
- Lima G, Paranhos-Neto F, Pereira G, et al. Osteoporosis management in patient with renal function impairment, Arquivos Brasileiros de Endocrinologia et Metabologia 58 (2014): 530-539.
- Moshtaghie A. Aluminum toxicity: a review in relation to chronic renal failure patients maintained on regular hemodialysis, Medical journal of the Islamic Republic of Iran 7 (1993): 63-72.
- Weisinger J, Heilberg I, Hernandez E, et al. Selective estrogen receptor modulators in chronic renal failure, Kidney International 63 (2003): 62-65.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 73.59%
Acceptance Rate: 73.59%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 