Clinical Presentation and Complications of Autosomal Dominant Polycystic Kidney Disease: Experience from a Tertiary Care Center
Syed Fazlul Islam1*, Md. Kabir Hossain1, Syed Mahbub Morshed2, Mohammad Mahbub Alam Mazumder3, Ferdous Jahan1, Md. Omar Faroque1, Rana Moharram Hossain4, Md. Rezaul Alam1, Manik Chandra Mondal5, Md. Masudul Karim6, Mohammad Abdus Salam7, A. K. M Shahidur Rahman6
1Associate Professor, Department of Nephrology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
2Assistant Professor, Department of Nephrology, Shaheed Suhrawardy Medical College and Hospital, Dhaka, Bangladesh
3Assistant Professor, Department of Nephrology, Manikganj Medical College, Manikganj, Bangladesh
4Professor, Department of Nephrology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
5Assistant Professor, Department of Nephrology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
6Medical Officer, Department of Nephrology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
7Associate Professor, Department of Urology, Bangladesh Medical University (BMU), Dhaka, Bangladesh
*Corresponding Author: Dr. Syed Fazlul Islam, Associate Professor, Department of Nephrology, Bangladesh Medical University (BMU), Dhaka, Bangladesh.
Received: 10 October 2025; Accepted: 21 October 2025; Published: 13 November 2025
Article Information
Citation: Islam SF, Hossain MK, Morshed SM, Mazumder MMA, Jahan F, Faroque MO, Hossain RM, Alam MR, Mondal MC, Karim MM, Salam MA, Rahman AKMS. Clinical Presentation and Complications of Autosomal Dominant Polycystic Kidney Disease: Experience from a Tertiary Care Center. Archives of Nephrology and Urology 8 (2025): 106-112.
DOI: 10.26502/anu.2644-2833103
View / Download Pdf Share at FacebookAbstract
Background: Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the most prevalent type of genetically inherited kidney diseases. It is one of the leading causes of end-stage renal disease (ESRD) and is responsible for a considerable portion of patients receiving hemodialysis globally. Treatment focuses on symptom management, blood pressure control and delaying the progression of the disease.
Objective: To determine the pattern of clinical presentation and complications of ADPKD at a Tertiary Care Hospital in Bangladesh.
Methods: A total of 100 patients with ADPKD were evaluated at a Tertiary Care Hospital, Dhaka, Bangladesh. Strict clinical criteria (Ravine’s criteria) were used to identify ADPKD patients. Their demographic profile, initial presenting complaints, co-morbidities, family history of ADPKD, any palpable mass on abdominal examination, cardiac assessment for any abnormal findings and usage of antihypertensive medications were all reviewed and documented. Relevant laboratory tests were done accordingly.
Results: Total 100 patients with ADPKD were enrolled, of them 60 were male and 40 were female, a male to female ratio was 1.5:1; their mean age was 36±12 years. We found that cyst burden was rises with increasing age. Hypertension (40%) and pain (45%) were the most common clinical presentation. Ballotable kidneys were found in 30% of ADPKD patients; while dysuria, asymptomatic microscopic hematuria and gross hematuria were present in 30%, 20% and 10% cases respectively. Regarding complications: acute pyelonephritis (12%), acute kidney injury (11%), cyst infection (10%) and salt loosing nephropathy (10%) were the most common. In this study; majority (55%) of ADPKD patients were in early stages of CKD (stage I and stage II); whereas 45% participants were in advanced stages of CKD (stage III to stage V). Among the study participants; diabetes mellitus was the most common (25%) co-morbidities, followed by hypothyroidism, chronic obstructive pulmonary disease, cholelithiasis, congestive heart failure and chronic liver disease. Acute pyelonephritis, cyst infection, septicemia and pyonephrosis were the most frequent infections observed in study cases. Escherichia coli (E-coli) was the major bacterial pathogen for these infections, other pathogens include Pseudomonas, Klebsiella, Enterobacter, Proteus, Acenetobacter and Candida.
Conclusion: This study highlights the clinical heterogeneity and systemic involvement of ADPKD. Hypertension, CKD progression, and cystrelated complications dominate the clinical spectrum. Blood pressure, renal function and relevant clinical evaluations should be performed regularly in ADPKD patients.
Keywords
<p>ADPKD; Clinical Presentation; Complications; Cyst Infection; Hematuria; Hypertension</p>
Article Details
1. Introduction
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is a genetic condition that causes the formation of multiple fluid-filled cysts in both kidneys and sometimes in other organs like the liver, pancreas and brain coverings (arachnoid membranes) [1]. ADPKD cause a progressive and irreversible decline in kidney function, ultimately leading to renal failure. It affects about 1 in 400 to 1,000 live births and accounts for 5-10% of end-stage renal disease (ESRD) cases worldwide, it is the fourth leading global cause of renal failure [2]. It is a genetic disorder caused by mutations in one of two different genes: PKD1 and PKD2 are located on chromosomes 16p13.3 and 4q21 respectively [3]; and is expressed in an autosomal dominant pattern, with variable expression [4]. Although there has been significant progress in the last several years in the understanding of ADPKD pathogenesis, but little is known about the role of polycystins and the molecular mechanisms driving disease progression. The polycystins constitute a subfamily of protein channels, are believed to control intracellular calcium signaling [2]. Polycystins are expressed in several tissues, such as the pancreatic ducts, hepatic bile ducts, and renal tubular epithelia [2]. Polycystin proteins are located within the cell at the region of primary cilium. Mutated polycystins are one kind of ciliopathy. The cilia carry mechanosensory signals that control cell growth and proliferation. Mutations in polycystins cause cell proliferation and the formation of fluid-filled cysts [5]. The clinical course of ADPKD is highly variable, even among members of the same family. Although the disease is typically asymptomatic in early life, patients often develop symptoms during adulthood, including hypertension, hematuria, abdominal or flank pain, recurrent urinary tract infections (UTIs), and progressive decline in renal function [6]. Functional manifestations include: reduced renal blood flow and concentrating defect. Pain may result from cyst hemorrhage, gross hematuria, nephrolithiasis, infection or renal enlargement [7]. Progression to renal failure may be due to: interstitial inflammation, apoptosis of tubular epithelial cells, hypertensive glomerulosclerosis and compression atrophy [8]. Extra-renal manifestations are also common and may include liver cysts, intracranial aneurysms, cardiac valve abnormalities, and colonic diverticulosis [9]. Hypertension is a major clinical manifestation and predictor of outcome in ADPKD [10-11]. High blood pressure in ADPKD is mainly caused by activation of the kidney’s renin–angiotensin system and reduced blood vessel relaxation [11]. Endothelial vasodilation and constitutive nitric oxide synthase (NOS) activity are reduced in subcutaneous resistance vessels from patients with ADPKD and normal glomerular filtration rate (GFR) [12]. The severity depends on genetic differences: PKD1 mutations usually cause earlier and more severe kidney failure (average age 54 years), while PKD2 mutations are milder (average age 74 years) [13]. The exact mutations vary between families, and some lead to faster progression than others. The disorder typically first appears in people between the ages of 30 and 40. It is characterized by the development of renal cysts, kidney enlargement, and a decline in kidney function, which ultimately results in renal insufficiency [14]. ADPKD is a major hereditary cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide. However, data regarding its clinical presentation, associated complications, and disease progression in the Bangladeshi population remain scarce. This study aimed to delineate the pattern of clinical presentation and complications of ADPKD at a tertiary care center in Bangladesh. This study provides valuable insights into the demographic characteristics, clinical manifestations, and comorbid conditions of ADPKD patients.
2. Methodology
A prospective observational study was carried out in the Department of Nephrology, Bangladesh Medical University (BMU), Dhaka, Bangladesh. A total of 100 cases of Autosomal Dominant Polycystic Kidney Disease (ADPKD) was enrolled in this study over a period of 3 years. Ultrasonogram based Ravine’s criteria were used to diagnose ADPKD [14]. Data collection was done by face-to-face interview: age and gender were identified, family history was obtained- including history of kidney disease, hypertension, diabetes, sudden death, and family history of ADPKD. Intensive history was taken regarding duration of hypertension, recurrent flank pain, any history of recent/recurrent urinary tract infection (UTI) or hematuria. Physical examination to observe pulse rate and blood pressure, ballotability of kidneys, hepatomegaly, ascites, neurological and examination of cardiovascular system were also done accordingly. Relevant investigations like- complete blood count (CBC), urine routine microscopic examination (R/M/E) with culture/sensitivity test, serum creatinine, serum electrolytes, random blood sugar (RBS), ultrasonogram of whole abdomen (with MCC and PVR), CT scan of brain, plain x-Ray of kidney ureter and bladder (KUB) region were done following standard procedure. Serum creatinine levels were used to calculate the estimated glomerular filtration rate (eGFR), which was used to categorize the stages of chronic kidney disease (CKD). Each patient's demographic information with complete medical history, results from their clinical examination and laboratory test reports were taken into account. A structured data collecting sheet was used to document all data. After compilation, all of the data was imported into Microsoft Excel. An windows-based software Statistical Package for the Social Sciences (SPSS) version- 26 was utilized to do statistical analysis. We summarized categorical variables using descriptive statistics, such as frequencies and percentages.
3. Results and Observations
This study was intended to assess the pattern of clinical presentation and complications of Autosomal Dominant Polycystic Kidney Disease (ADPKD) among patients with ADPKD. Total 100 patients with ADPKD were evaluated. Of them 60 were male and 40 were female, a male to female ratio was 1.5:1; their mean age was 36±12 years. Among the study participants 10% had family history of ADPKD and 15% had family history of kidney disease, another 15% had a family history of hypertension (HTN) and only 5% had family history of ESRD on dialysis (Table 1).
Table 1: Basic characteristics of ADPKD patients (N= 100).
|
Characteristics |
Frequency |
Percentage |
|
Age (years) |
36±12 years |
|
|
Age range (minimum-maximum) |
24 - 62 years |
|
|
Sex |
||
|
Male |
60 |
60% |
|
Female |
40 |
40% |
|
Male to female ratio |
1.5:1 |
|
|
Family history of ADPKD |
10 |
10% |
|
Family history of HTN |
15 |
15% |
|
Family history of sudden death |
5 |
5% |
|
Family history of kidney disease |
15 |
15% |
|
Family history of dialysis |
5 |
5% |
|
Family history of intracranial hemorrhage |
3 |
3% |
According to Ravine’s PKD1 diagnosis criteria all study patients were categorized. It was observed that, cyst distribution increases steadily with age. In this study, 21% of patients aged 15-29 years had ≥2 cysts (unilateral or bilateral). In the 30-39 years and 40-59 years age groups, the majority met the criteria of ≥2 cysts in each kidney (23% and 30% respectively). Among patients aged ≥60 years, 26% had ≥4 cysts in each kidney, reflecting disease progression with age. Overall, cyst burden rises with increasing age, consistent with the natural history of ADPKD (Table- 2).
Table 2: Distribution of the ADPKD patients according to ultrasound-based Ravine’s PKD1 diagnostic criteria (N= 100)
|
Age (Years) |
Criteria |
Frequency |
Percentage |
|
15-29 |
≥ 2 cysts, unilateral or bilateral |
21 |
21% |
|
30-39 |
≥ 2 cysts in each kidney |
23 |
23% |
|
40-59 |
≥ 2 cysts in each kidney |
30 |
30% |
|
≥60 |
≥4 cysts in each kidney |
26 |
26% |
Regarding clinical presentation; it was observed that asymptomatic ADPKD comprised 20% of study cases, while 15% had asymptomatic renal impairment detected incidentally. Hypertension (40%) and pain (45%) were the most common clinical presentation. Ballotable kidneys were found in 30% of patients, dysuria was in 30% cases, asymptomatic microscopic hematuria was noted in 20% ADPKD patients and gross hematuria was in 10% patients. Neurological symptoms such as headache (10%) and history of unconsciousness (5%) were less frequent. Dehydration (12%), hypotension (15%) and anemia (8%) were also observed. Extra-renal manifestations included liver cysts (35%) and pancreatic cysts (10%) were found. This spectrum highlights the multisystem involvement and variable clinical manifestations of the disease (Table 3).
Table 3: Clinical presentation of ADPKD patients (N= 100)
|
Clinical presentations* |
Frequency |
Percentage |
|
Asymptomatic |
20 |
20% |
|
Pain |
45 |
45% |
|
Asymptomatic renal impairment |
15 |
15% |
|
Ballotable of kidneys |
30 |
30% |
|
Hypertension |
40 |
40% |
|
Anemia |
8 |
8% |
|
Asymptomatic microscopic hematuria |
20 |
20% |
|
Polycythemia |
15 |
15% |
|
Gross hematuria |
10 |
10% |
|
Fever with chills |
15 |
15% |
|
Dysuria |
30 |
30% |
|
Headache |
10 |
10% |
|
History of unconsciousness |
5 |
5% |
|
Dehydration |
12 |
12% |
|
Jaundice |
6 |
6% |
|
Hypotension |
15 |
15% |
|
Liver cysts |
35 |
35% |
|
Pancreatic cyst |
10 |
10% |
|
Sonologic large kidney size |
90 |
90% |
|
Proteinuria (mild to moderate) |
15 |
15% |
|
Hypertensive retinopathy |
6 |
6% |
|
Left ventricular hypertrophy on Echocardiogram |
10 |
10% |
*Multiple response
In this study among 100 patients with (ADPKD), a range of complications were observed: acute pyelonephritis (12%), acute kidney injury (11%) cyst infection (10%) and salt loosing nephropathy (10%) were the most common. Cyst hemorrhage was notable affecting 8% of ADPKD cases and sub-arachnoid hemorrhage (SAH) occurred in 2% of cases, underscoring vascular involvement. Renal stones (6%), and abdominal hernia (6%) were also found. Less frequent complications such as septicemia, hepatic dysfunction, colonic diverticulitis and malignancy were found in 5%, 3%, 2% and 2% cases respectively (Table 4).
Table 4: Complications among the ADPKD patients (N= 100).
|
Complications |
Frequency |
Percentage |
|
Salt loosing nephropathy |
10 |
10% |
|
Sub-arachnoid hemorrhage |
2 |
2% |
|
Acute pyelonephritis |
12 |
12% |
|
Cyst infections |
10 |
10% |
|
Cyst hemorrhage |
8 |
8% |
|
Renal stones |
6 |
6% |
|
Abdominal hernia |
6 |
6% |
|
Colonic diverticulitis |
2 |
2% |
|
Malignancy |
2 |
2% |
|
Acute pancreatitis |
5 |
5% |
|
Acute kidney injury |
11 |
11% |
|
Hepatic dysfunction |
3 |
3% |
|
Septicemia |
5 |
5% |
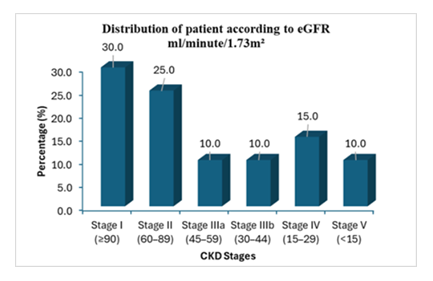
Distribution of patient according to eGFR showed majority of patients were in CKD stage I (30%) and CKD stage II (25%); whereas 10% participants were in CKD stage IIIa and another 10% participants were in CKD stage IIIb. But a notable number of ADPKD patients were in advance CKD stages; 15% in CKD stage IV and 10% in CKD stage-V (Figure 1).
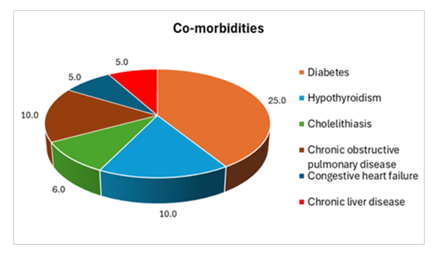
In this study, we found several co-morbidities among the study participants; diabetes mellitus was the most common (25%), followed by hypothyroidism and chronic obstructive pulmonary disease, observed 10% each; but cholelithiasis was present in 6% of cases. While congestive heart failure (5%) and chronic liver disease (5%) were also found in ADPKD patients (Figure 2).
Different types of infection were observed among the study patients: acute pyelonephritis was the most frequent (12%), followed by cyst infection (10%) and septicemia (5%). Pyonephrosis was seen in 3% cases. These findings highlight the high burden of urinary tract and kidney infections in ADPKD patients (Table 5).
Table 5: Patterns of infection in ADPKD patients (N= 100)
|
Types of infection |
Frequency |
Percentage |
|
Cyst infection |
10 |
10% |
|
Septicemia |
5 |
5% |
|
Acute pyelonephritis |
12 |
12% |
|
Pyonephrosis |
3 |
3% |
Among 100 ADPKD patient of 40 patients presented with recurrent upper and lower urinary tract infections (UTI). Urine culture and sensitivity tests revealed that, Escherichia coli (E-coli) was the major bacterial pathogen (50%), other pathogens include Pseudomonas, Klebsiella, Enterobacter were 15%, 10% and 2.5% respectively whereas Proteus was found 10% cases but Acenetobacter and Candida were isolated in 2% and 2.5% cases respectively (Table 6).
Table 6: Isolated organisms among ADPKD patients having UTI (n= 40).
|
Organisms |
Frequency |
Percentage |
|
E-coli |
20 |
50% |
|
Pseudomonas |
6 |
15% |
|
Klebsiella |
4 |
10% |
|
Enterobacter |
1 |
2.5% |
|
Acenetobacter |
2 |
5% |
|
Proteus |
4 |
10% |
|
Candida |
1 |
2.5% |
4. Discussion
Autosomal dominant polycystic kidney disease (ADPKD), is a common inherited disorder that affects 1 in 400 to 1 in 1000 persons and is the most frequent cause of end-stage renal disease (ESRD) after diabetes mellitus and hypertension in the United States [15]. ADPKD is a multi-system disease, as evidenced by its various clinical presentations, which include enlarged kidneys due to growing cysts, hypertension, and various extrarenal consequences, such as liver cysts, cerebral aneurysms, and cardiac valvular disease [16]. Due to advancements in gene technology, ADPKD could be diagnosed within first decade of life in the developed countries in contrast to resource limited countries like Bangladesh, where renal ultrasonography and clinical judgment are still the only methods used to diagnose ADPKD. This has hindered our understanding of the disorder. Therefore, this prospective observational study explored the clinical profile and complications of 100 patients with ADPKD at a tertiary care setting in Bangladesh. In this study, the mean age of ADPKD patients was 36 ± 12 years, which is in line with reports that ADPKD commonly manifests early to middle adulthood [17-19]. The male-to-female ratio was 1.5:1, suggesting a slight male predominance and family history of ADPKD was found in only 10% of study patients which is lower than rates reported in developed countries [20-21]. This discrepancy may be due to the limited availability of genetic testing for PKD1 and PKD2 mutations in our setting. Ultrasound criteria (Ravine’s criteria) were effective in diagnosing ADPKD in our population. Which revealed, cyst number increased with age, reflecting the natural history of disease progression [22]. This supports the role of ultrasound as a cost-effective tool for diagnosis and follow-up in resource-limited settings where genetic testing is not available. Pain (45%) was the most common clinical presentation among the study patients. One of the most typical symptoms of ADPKD is pain, which first appears early in the illness and frequently prompts a diagnosis. ADPKD pain can manifest as either an acute pattern such as nephrolithiasis, hemorrhage, cyst infection or urinary tract infection; or as a chronic, disabling pattern [23]. Hypertension (40%) was also a frequent clinical feature among the ADPKD patients of this study. It was reported that hypertension is a common consequence in ADPKD and is slightly less common in females than males [18, 21, 24]. Hypertension in ADPKD is associated with more severe disease, age, PKD1 genotype, male sex, family history renal disease, renal function (eGFR), kidney size and hematuria [18]. Hypertension in ADPKD is primarily linked to activation of the renin–angiotensin–aldosterone system and is a key driver of cardiovascular morbidity and progression of renal dysfunction [25]. The presence of left ventricular hypertrophy (10%) and hypertensive retinopathy (6%) in our study indicates the systemic impact of uncontrolled blood pressure. Other renal manifestations- gross hematuria (10%) and ballotable kidneys (30%) were consistent with cyst-related complications including hemorrhage, rupture, and enlargement. Microscopic hematuria was prominent with 20% of study patients. Liver cysts (35%) represented the most common extra-renal involvement and pancreatic cysts (10%) were less common but clinically relevant systemic nature of ADPKD. These findings were comparable with previous report [24]. The distribution of patients according to CKD stages in our study highlights that majority of the cases (75%) were identified at an early stage (Stage I, II and III). This finding is consistent with a couple of previous studies [26-27]. Early detection of renal impairment is crucial for slowing disease progression and implementing lifestyle modifications and pharmacological interventions that can preserve renal function. However, a considerable proportion of ADPKD patients in our cohort (15% Stage IV, and 10% Stage V) had already progressed to moderate-to-severe CKD at the time of diagnosis. This is consistent with previous studies from low- and middle-income countries, where late presentation remains a challenge due to lack of routine screening, limited access to nephrology care, and low public awareness [28-29]. Advanced CKD stages in ADPKD are associated with higher morbidity, mortality, and healthcare costs, underscoring the importance of early identification and timely referral. The study participants had a number of co-morbidities, with diabetes mellitus being the most prevalent at 25%, followed by hypothyroidism and chronic obstructive pulmonary disease at 10% each. Cholelithiasis was found in 6% of ADPKD cases. Chronic liver disease (5%) and congestive heart failure (5%) were also observed in ADPKD patients. These findings were supported by recent previous reports as indicated that ADPKD is associated with a number of co-morbidities [30-31]. Although current study observed a high prevalence of diabetes in ADPKD cases, but it was hypothesized that metabolic abnormalities in polycystic kidneys inhibit endogenous glucose production and decrease renal breakdown of insulin, thus there is a lower prevalence of diabetes among patients with ADPKD [32].
In this study, various forms of infection were found among the study participants: acute pyelonephritis was the most common (12%), followed by cyst infection (10%) and septicemia (5%). Pyonephrosis was detected in 3% of ADPKD patients. These data emphasize the significant burden of urinary tract and kidney infections in ADPKD patients. The bacteriological profile revealed Escherichia coli as the predominant pathogen (50%), followed by Pseudomonas and Proteus. This is consistent with prior studies, which describe E. coli as the leading cause of UTIs in ADPKD, although the frequency of resistant organisms like Pseudomonas highlights the need for careful antimicrobial stewardship in this population [33]. This highlights the importance of urine culture and sensitivity testing for guiding antibiotic therapy in ADPKD [34].
5. Conclusion
This study highlights the clinical heterogeneity and systemic involvement of ADPKD in a Bangladeshi cohort. Pain, hypertension, CKD progression, and cyst-related complications dominate the clinical spectrum. The absence of molecular diagnostics remains a barrier to comprehensive care. Early identification through family screening, regular imaging, and blood pressure control are essential to delay disease progression. Future initiatives should focus on expanding access to genetic testing, enhancing public awareness, and establishing longitudinal registries to better characterize disease trajectory in South Asian populations.
Limitation of Study
It was a single center study with a relatively small sample size.
Recommendation
A large population based multicenter study should be done to validate the findings of this study.
Conflicts of Interest
No conflict of interest was claimed regarding this publication.
References
- Guay-Woodford LM. Autosomal recessive polycystic kidney disease: the prototype of the hepato-renal fibrocystic diseases. Journal of pediatric genetics 3 (2014): 89-101.
- Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. American journal of kidney diseases: the official journal of the National Kidney Foundation 67 (2015): 792-810.
- Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1812 (2011): 1202-1212.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. The Lancet 369 (2007): 1287-1301.
- Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Current opinion in nephrology and hypertension 18 (2009): 99-106.
- Bennett WM. Autosomal dominant polycystic kidney disease: 2009 update for internists. The Korean journal of internal medicine 24 (2009): 165-168.
- Harris PC, Torres VE. Polycystic Kidney Disease, Autosomal Dominant. In: Gene Reviews®. University of Washington, Seattle, Seattle (2002).
- Ghata J, Cowley Jr BD. Polycystic kidney disease. Comprehensive Physiology 7 (2017): 945-975.
- Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrology Dialysis Transplantation 29 (2014): 247-254.
- Sans-Atxer L, Torra R, Fernández-Llama P. Hypertension in autosomal-dominant polycystic kidney disease (ADPKD). Clinical kidney journal 6 (2013): 457-463.
- Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Advances in chronic kidney disease 17 (2010): 153-163.
- Wang D, Iversen J, Wilcox CS, et al. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney international 64 (2003): 1381-1388.
- Yang B, Wang Q, Wang R, et al. Clinical manifestation, management and prognosis of acute myocardial infarction in autosomal dominant polycystic kidney disease. Kidney and Blood Pressure Research 43 (2018): 1806-1812.
- Gradzik M, Niemczyk M, Gołębiowski M, et al. Diagnostic imaging of autosomal dominant polycystic kidney disease. Polish journal of radiology 81(2016): 441-453.
- Helal I, Lassoued F, Maiz HB, et al. Clinical presentation and outcomes of autosomal dominant polycystic kidney disease in the elderly. Am J Med Sci and Med 1 (2013): 18-20.
- Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. The Lancet 393 (2019): 919-935.
- Milutinovic J, Phillips L, Bryant J, et al. Autosomal dominant polycystic kidney disease: Early diagnosis and data for genetic counselling. The Lancet 315 (1980): 1203-1206.
- Martínez V, Furlano M, Sans L, et al. Autosomal dominant polycystic kidney disease in young adults. Clinical Kidney Journal 16 (2023): 985-995.
- Wigerinck S, Schellekens P, Smith BH, et al. Characteristics of patients with autosomal polycystic kidney disease reaching kidney failure by age 40. Pediatric Nephrology 40 (2025): 1997-2007.
- Hajji M, Barbouch S, Harzallah A, et al. Clinical study on autosomal dominant polycystic kidney disease among North Tunisians. Saudi Journal of Kidney Diseases and Transplantation 30 (2019): 175-184.
- Solazzo A, Testa F, Giovanella S, et al. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): A meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS On 13 (2018): e0190430.
- Ravine D, Sheffield LJ, Danks DM, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. The Lancet 343 (1994): 824-827.
- Hogan MC, Norby SM. Evaluation and management of pain in autosomal dominant polycystic kidney disease. Advances in chronic kidney disease 17 (2010): e1-6.
- Rabbani MA, Ali SS, Murtaza G, et al. Clinical presentation and outcome of autosomal dominant polycystic kidney disease in Pakistan: a single center experience. JPMA. The Journal of the Pakistan Medical Association 58 (2008): 305-309.
- Lawson CR, Doulton TW, MacGregor GA. Autosomal dominant polycystic kidney disease: role of the renin-angiotensin system in raised blood pressure in progression of renal and cardiovascular disease. Journal of the Renin-Angiotensin-Aldosterone System 7 (2006): 139-145.
- Meijer E, Rook M, Tent H, et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clinical Journal of the American Society of Nephrology 5 (2010): 1091-1098.
- Higashihara E, Horie S, Muto S, et al. Renal disease progression in autosomal dominant polycystic kidney disease. Clinical and experimental nephrology 16 (2012): 622-628.
- Nobakht N, Hanna RM, Al-Baghdadi M, et al. Advances in autosomal dominant polycystic kidney disease: a clinical review. Kidney medicine 2 (2020): 196-208.
- George C, Mogueo A, Okpechi I, et al. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ global health 2 (2017): e000256.
- Chen LC, Chu YC, Lu T, et al. Cardiometabolic comorbidities in autosomal dominant polycystic kidney disease: a 16-year retrospective cohort study. BMC nephrology 24 (2023): 333.
- Gallini JW, Jasien CL, Mrug M, et al. US Veterans Administration Autosomal Dominant Polycystic Kidney Disease Cohort: Demographic, Comorbidity, and Key Laboratory Data Characteristics. Kidney360 5 (2024): 529-537.
- Pietrzak-Nowacka M, Rózanski J, Safranow K, et al. Autosomal dominant polycystic kidney disease reduces the risk of diabetes mellitus. Archives of medical research 37 (2006): 360-364.
- Gao C, Zhang L, Zhang Y, et al. Insights into cellular and molecular basis for urinary tract infection in autosomal-dominant polycystic kidney disease. American Journal of Physiology-Renal Physiology 313 (2017): 1077-1083.
- Alkhawaldeh R, Abu Farha R, Abu Hammour K, et al. Optimizing antimicrobial therapy in urinary tract infections: A focus on urine culture and sensitivity testing. Frontiers in Pharmacology 13 (2022): 1058669.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 73.59%
Acceptance Rate: 73.59%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 