Correlation of Serum Phosphate, Calcium and Parathyroid Hormone Level with Carotid Intimal-Medial Thickness in Patients on Maintenance Hemodialysis
Dr Samira Khatun1*, Dr Madhabi Karmaker2, Dr Khaleda Akhter3, Dr Farnaz Nobi4, Dr Shanjida Sultana Juthy5
1Dialysis Medical Officer, Department of Nephrology, Rajshahi Medical College Hospital, Rajshahi, Bangladesh
2Junior Consultant, Department of Medicine, Dhaka Medical College Hospital, Dhaka, Bangladesh
3Major (Assistant Professor), Combined Military Hospital, Dhaka, Bangladesh, email: shimu.mmch@gmail.com
4Assistant Professor, Department of Nephrology, Kidney Foundation Hospital and Research Institute, Dhaka, Bangladesh
5Assistant Professor, Kidney Foundation Hospital & Research Institute, Dhaka, Bangladesh
*Corresponding Author: Dr Samira Khatun, Dialysis Medical Officer, Department of Nephrology, Rajshahi Medical College Hospital, Rajshahi, Bangladesh
Received: 10 October 2025; Accepted: 21 October 2025; Published: 28 November 2025
Article Information
Citation: Samira Khatun, Madhabi Karmaker, Khaleda Akhter, Farnaz Nobi, Shanjida Sultana Juthy. Correlation of Serum Phosphate, Calcium and Parathyroid Hormone Level with Carotid Intimal-Medial Thickness in Patients on Maintenance Hemodialysis. Archives of Nephrology and Urology 8 (2025): 118-125.
DOI: 10.26502/anu.2644-2833105
View / Download Pdf Share at FacebookAbstract
Background: Cardiovascular disease is a leading cause of mortality and morbidity in hemodialysis patients. Disordered calcium-phosphorus metabolism is a major contributor to vascular calcification. This study investigates the correlation of serum phosphate, calcium, and parathyroid hormone (PTH) levels with carotid intimal-medial thickness (CIMT), a marker of atherosclerosis. Aim of the study: To assess the correlation between serum phosphate, calcium, and PTH levels with CIMT in patients on maintenance hemodialysis. Methods: This cross-sectional study included 80 maintenance hemodialysis patients at the Department of Nephrology in Dhaka Medical College Hospital, Dhaka, Bangladesh, for one year from May 2021 to April 2022. Clinical data were recorded using a structured questionnaire. CIMT was measured via B-mode ultrasonography, and serum phosphate, calcium, and intact PTH levels were analyzed. Data were statistically analyzed using SPSS 26.0. Result: The mean age of participants was 43±11.3 years, with a male predominance (1.42:1). The mean CIMT was 0.59±0.11 mm and was significantly higher in males (p=0.030) and diabetic patients (p<0.001). CIMT positively correlated with age, BMI, serum phosphate, and serum calcium levels but not with iPTH. Conclusion: CIMT in hemodialysis patients positively correlates with serum phosphate, calcium, age, and BMI. Emphasis on correcting hyperphosphatemia may help prevent vascular calcification and progression of arteriosclerosis.
Keywords
<p>Carotid intimal-medial thickness; Serum phosphate; Serum calcium; Parathyroid hormone; Hemodialysis; Cardiovascular disease.</p>
Article Details
1. Introduction
Chronic kidney disease (CKD) significantly predicts cardiovascular disease (CVD), the leading cause of death in end-stage renal disease (ESRD) patients. ESRD patients have a 10–20 times higher mortality risk than the general population. Advanced arteriosclerosis, indicated by increased arterial wall thickness and stiffness, is prevalent in CKD [1]. In the general population, advanced age, hypertension, smoking, and hyperlipidemia are key risk factors for arteriosclerosis [2]. However, in uremic patients, the risk factors for arteriosclerosis are less clearly defined and may differ based on the presence or absence of diabetes. Increased serum phosphate concentration is recognized as a significant risk factor for vascular calcification, an advanced form of atherosclerosis. However, its relationship with arterial wall thickness in CKD patients remains unclear [1,3]. Disorders of calcium and phosphorus metabolism play a pivotal role in the development of secondary hyperparathyroidism in CKD. These disturbances not only lead to renal osteodystrophy but also predispose patients to cardiovascular complications. Abnormalities in calcium and phosphate levels can result in the calcification of cardiac valves and the acceleration of vascular plaque formation [4]. This vascular calcification is associated with poor cardiovascular outcomes and is strongly linked to hyperphosphatemia in CKD patients [5]. CKD-mineral bone disorder (CKD-MBD) is characterized by hyperphosphatemia, which correlates with increased vascular calcification and a heightened risk of mortality. Management of CKD-MBD primarily focuses on controlling systemic phosphate accumulation, but current approaches, such as dietary phosphate restrictions and intestinal phosphate binders, are only partially effective [6]. Two main types of vascular calcifications are observed in hemodialysis patients: common atherosclerosis, characterized by patchy intimal calcifications in atherosclerotic plaques, and media sclerosis, marked by linear medial calcifications linked to mineral metabolism disturbances. Recent studies indicate that media sclerosis is an active cellular process involving vascular smooth muscle cells transforming into osteoblasts, driven by hyperphosphatemia [7,8]. Vascular calcification scores in HD patients have been evaluated using various methods, with B-mode ultrasonography being a commonly used technique [9]. Kidney dysfunction also alters the vascular lumen by inhibiting collagen cross-linking, promoting atherogenesis. Impaired clearance of calcium and phosphorus further contributes to the calcification of major arteries, such as coronary arteries [10]. Hyperphosphatemia, secondary hyperparathyroidism, and metastatic calcification are key contributors to atheromatous plaque formation in carotid vessels, which can be assessed by measuring carotid intima-media thickness (CIMT) [5]. CIMT is a non-invasive, cost-effective method for detecting early CVD and is a reliable marker of atherosclerotic vascular disease. It predicts cardiovascular events independent of age, gender, or other risk factors. A mean CIMT exceeding 1.15 mm is strongly associated with coronary artery disease [11]. Patients on dialysis experience significantly higher mortality due to CVD compared to those with normal kidney function. Vascular calcification, a predictor of all-cause and cardiovascular mortality, is highly prevalent in ESRD patients [12]. Furthermore, mild-to-moderate renal insufficiency also predicts cardiovascular morbidity and mortality [13]. This study evaluates the correlation between serum phosphate, calcium, and parathyroid hormone levels and carotid intimal-medial thickness in patients undergoing maintenance hemodialysis.
2. Methodology and Materials
This rigorously designed cross-sectional study was conducted in the Department of Nephrology at Dhaka Medical College and Hospital (DMCH), Dhaka, Bangladesh. The research conducted over one year, from May 2021 to April 2022, meticulously examined patients undergoing hemodialysis maintenance. Utilizing a purposive sampling technique, 80 patients were systematically recruited, forming a clearly defined and representative study cohort. Strict eligibility criteria guided the inclusion of participants to ensure the findings' reliability, validity, and clinical relevance.
- • Inclusion Criteria:
Individuals aged between 18 and 64 years who were receiving maintenance hemodialysis were included in the study.
- • Exclusion Criteria:
Patients with acute kidney injury superimposed on chronic kidney disease (CKD), a history of carotid artery surgery, those who consumed alcohol, smokers, and pregnant women were excluded.
A standardized questionnaire collected demographic data, hemodialysis duration and frequency, clinical presentation, associated medical conditions, and findings. Then, the patients were assessed with investigations. Patients were instructed to attend the dialysis centre on the scheduled day of dialysis, and blood was taken from the peripheral vein before the start of hemodialysis for biochemical testing. Patient venous blood (5ml) was collected by sterile disposable syringe with all aseptic precautions and immediately transferred to a dry clean test tube following investigations were done by automated machine (Dimension Xpand plus, Random access biochemistry and immune assay analyzer): Serum phosphate, Serum calcium, iPTH and Fasting lipid profile. All laboratory investigations were carried out in the Clinical pathology department of Dhaka Medical College Hospital, and B-mode ultrasound (Philips Epic-7, Probe- high resolution, US linear probe, frequency 4-18 MHZ) of the carotid artery was done in the Institute of Nuclear Medicine and Allied Sciences, DMCH. Each subject was examined in the supine position. The carotid arteries were investigated bilaterally in longitudinal projections. Three measurements were taken 0.5, 1 and 2 cm below the carotid bifurcation of the common carotid artery on each side. CIMT's normal value is less than 0.8 mm; if it is more than 0.8 mm, it indicates atherosclerosis. The arithmetical averages of these were taken. The IMT of both sides (right and left) was calculated, and averages of these two values were used for statistical analysis. Carotid intimal medial thickness (CIMT) measurement was in plaque-free arterial segments 31, which was always performed by a single-hand sinologist. All data were collected following informed consent from each participant, and the study received prior ethical approval from the institutional ethics committee.
Statistical Analysis:
The collected data were analyzed using SPSS software (version 26). Continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were summarized as frequencies and percentages. Quantitative variables were compared using the unpaired t-test, and the chi-square test was employed for categorical variables. Correlation analyses were conducted using Pearson's correlation coefficient. A p-value of less than 0.05 was considered statistically significant, ensuring robust and reliable interpretations of the results.
3. Results
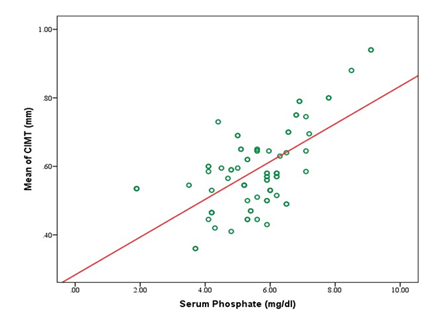
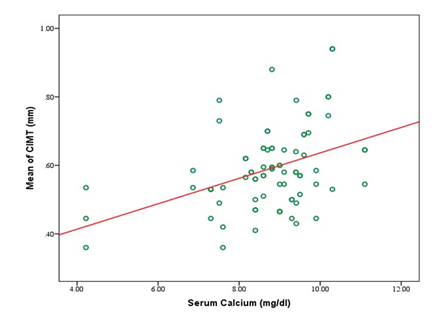
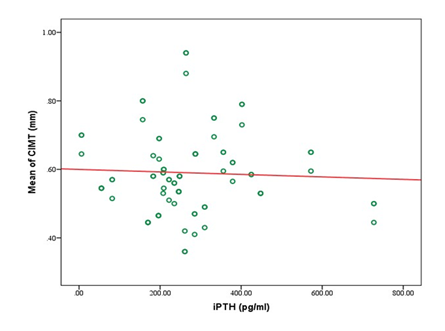
A total of 80 participants were included in the study, with a mean age of 43.1±11.3 years. Most participants were between 31 and 50 years old (61.25%). Males comprised 58.75%, and females 41.25%. Most participants had a normal BMI (67.50%), with 16.25% underweight, 13.75% overweight, and 2.50% obese (Table 1). Table 2 showed that the study cohort had a mean systolic blood pressure of 153.2±22.4 mmHg and diastolic blood pressure of 89.6±10.4 mmHg. The mean carotid intimal-medial thickness (CIMT) was 0.59±0.11 mm, with right-side CIMT at 0.57±0.12 mm and left-side CIMT at 0.61±0.14 mm (Table 3). Significant differences in CIMT were observed in Table 4 based on gender and diabetes status. Males had a higher mean CIMT (0.61±0.12 mm) than females (0.55±0.10 mm, p=0.03). Participants with diabetes had a greater CIMT (0.63±0.12 mm) than those without diabetes (0.54±0.09 mm, p<0.001). However, no significant difference was found between hypertensive and non-hypertensive participants. Biochemical parameters showed a mean serum phosphate level of 5.56±1.36 mg/dL, calcium of 8.74±1.30 mg/dL, and iPTH levels averaging 274.59±150.34 pg/mL (Table 5). The lipid profile indicated a mean total cholesterol level of 189.13±42.50 mg/dL, triglycerides at 188.28±70.01 mg/dL, LDL-C at 121.86±26.67 mg/dL, and HDL-C at 37.93±8.50 mg/dL (Table 6). Correlation analysis revealed several significant associations with CIMT. Age (r=0.432, p<0.001) and BMI (r=0.437, p<0.001) had moderate positive correlations with CIMT. A strong positive correlation was observed between CIMT and serum phosphate (r=0.627, p<0.001) and serum calcium (r=0.404, p<0.001). Duration of dialysis also showed a weak positive correlation (r=0.237, p=0.034). No significant correlations were found between CIMT and iPTH (r=-0.046, p=0.687), total cholesterol (r=0.136, p=0.228), triglycerides (r=0.193, p=0.087), LDL-C (r=0.007, p=0.948), or HDL-C (r=0.081, p=0.473) (Table 7). Figure 1 illustrates the correlation between mean CIMT and serum phosphate levels, with phosphate ranging from 0 to 10 mg/dl and CIMT ranging from 0.3 mm to 1.0 mm. The correlation between mean CIMT and serum calcium levels, ranging from 4.0 to 12.0 mg/dl for calcium and 0.3 mm to 1.0 mm for CIMT, demonstrates a positive trend. The red trend line indicates that the mean CIMT tends to rise as serum calcium levels increase, although some variability is observed in the data points (Figure 2). Figure 3 depicts the correlation between mean CIMT and iPTH levels, with iPTH levels ranging from 0 to 600 pg/ml and CIMT ranging from 0.3 mm to 1.0 mm. The red trend line suggests a weak negative correlation, with higher iPTH levels associated with a slight decrease in mean CIMT, though significant variability is observed around the trend line.
Table 1: Demographic profile of the study subjects (N=80).
|
Variables |
Frequency (n) |
Percentage (%) |
|
Age (years) |
||
|
18-30 |
10 |
12.5 |
|
31-50 |
49 |
61.25 |
|
>50 |
21 |
26.25 |
|
Mean±SD |
43.1±11.3 |
|
|
Gender |
||
|
Male |
47 |
58.75 |
|
Female |
33 |
41.25 |
|
BMI (kg/m2) |
||
|
Underweight (<18.5) |
13 |
16.25 |
|
Normal (18.5-24.9) |
54 |
67.5 |
|
Over weight (25.0-29.9) |
11 |
13.75 |
|
Obese (>30.0) |
2 |
2.5 |
Table 2: Blood pressure of the study subjects (N=80).
|
Blood pressure |
Mean±SD |
Min-Max |
|
Systolic BP (mmHg) |
153.2±22.4 |
120-190 |
|
Diastolic BP (mmHg) |
89.6±10.4 |
60-110 |
Table 3: Mean carotid intimal-medial thickness (CIMT) of the study subjects (N=80).
|
CIMT |
Mean±SD |
Min-max |
|
Right (mm) |
0.57±0.12 |
0.26-0.86 |
|
Left (mm) |
0.61±0.14 |
0.36-1.01 |
|
Average (mm) |
0.59±0.11 |
0.36-0.94 |
Table 4: Carotid intimal-medial thickness (CIMT) by gender and comorbidities (N=80).
|
Variables |
Mean±SD |
P value |
|
Gender |
||
|
Male |
0.61 ± 0.12 |
0.03 |
|
Female |
0.55±0.10 |
|
|
Diabetes mellitus |
||
|
Yes |
0.63±0.12 |
<0.001 |
|
No |
0.54±0.09 |
|
|
Hypertension |
||
|
Yes |
0.59±0.12 |
0.882 |
|
No |
0.60±0.03 |
|
Table 5: Serum phosphate (mg/dl), serum calcium (mg/dl), iPTH (pg/ml) of the study subjects (N=80).
|
Variables |
Mean±SD |
Min-max |
|
Serum phosphate (mg/dl) |
5.56±1.36 |
1.89–9.10 |
|
Serum calcium (mg/dl) |
8.74±1.30 |
4.22–11.10 |
|
iPTH (pg/ml) |
274.59±150.34 |
5.96–727.50 |
Table 6: Lipid profile of the study subjects (N=80).
|
Variables |
Mean±SD |
Min-max |
|
Total cholesterol (mg/dl) |
189.13±42.50 |
136-287 |
|
Triglyceride (mg/dl) |
188.28±70.01 |
88-34 |
|
LDL-C (mg/dl) |
121.86±26.67 |
84-176 |
|
HDL-C (mg/dl) |
37.93±8.50 |
25-55 |
Table 7: Correlation of CIMT with different parameters (N=80).
|
Variables |
r |
P value |
|
Age |
0.432 |
<0.001 |
|
BMI |
0.437 |
<0.001 |
|
Serum Phosphate |
0.627 |
<0.001 |
|
Serum Calcium |
0.404 |
<0.001 |
|
iPTH |
-0.046 |
0.687 |
|
Duration of dialysis |
0.237 |
0.034 |
|
Total Cholesterol |
0.136 |
0.228 |
|
Triglyceride |
0.193 |
0.087 |
|
LDL-C |
0.007 |
0.948 |
|
HDL-C |
0.081 |
0.473 |
4. Discussion
This cross-sectional study evaluated serum phosphate, calcium, and parathyroid hormone (PTH) levels and their correlation with carotid intimal-medial thickness (CIMT) in patients undergoing maintenance hemodialysis. The study included participants with a mean age of 43.1±11.3 years; the majority (61.25%) were between 31 and 50. CIMT showed a significant positive correlation with age (r=0.432, p<0.001). These findings align with the study by Kiykim et al. (2004), which reported a mean age of 42.8±13.3 among 92 hemodialysis patients and a significant correlation between CIMT and age (r=0.48, p<0.001) [14]. Similarly, Brzosko et al. (2005) found a mean age of 49.6±16.7 with a stronger correlation (r=0.68, p=0.001) [15]. Kuswardhani et al. (2018) reported a mean age of 56.28±11.79 among 68 hemodialysis patients, with CIMT correlating significantly with age (r=0.607, p<0.001) [11]. These associations suggest that ageing exacerbates vascular endothelial degeneration, likely due to risk factors such as hypertension, oxidative stress, and hyperglycemia [16]. Male participants (58.75%) were more prevalent than females (41.25%), and CIMT was significantly higher in males than in females (p=0.030). Kuswardhani et al. (2019) similarly observed male predominance (60.29%) and higher CIMT values in males (p=0.003) [11]. Ayub et al. (2020) also reported a higher proportion of males (56.4%) compared to females (43.2%) [5]. Men’s greater vulnerability to atherosclerosis may be attributed to lower circulating estrogen levels, which play a role in vasodilation [16]. Regarding body mass index (BMI), most patients (67.50%) had normal BMI, while 16.25% were underweight, 13.75% overweight, and 2.50% obese. CIMT was positively correlated with BMI (r=0.432, p<0.001). Chhajed et al. (2014) similarly reported a significant positive correlation between CIMT and BMI (r=0.377, p<0.001) [17]. Brzosko et al. (2005) also found a positive correlation (r=0.50, p=0.02) [15]. Obesity is associated with metabolic changes that accelerate atherosclerosis and cardiovascular disease progression [18]. The mean duration of dialysis in this study was 3.56±2.27 years, with a significant positive correlation between CIMT and dialysis duration (r=0.237, p=0.034). Briese et al. (2006) reported similar findings, indicating that prolonged dialysis correlates with structural alterations in the heart and arteries [19]. Blood pressure analysis showed a mean systolic BP of 153.2±22.4 mmHg and a diastolic BP of 89.6±10.4 mmHg. No significant difference in CIMT was observed between hypertensive and non-hypertensive patients (p=0.882). Ishimura et al. (2005) reported comparable mean systolic (153±17 mmHg) and diastolic BP (80±9 mmHg) [12]. Afolabi et al. (2018) found no association between CIMT and blood pressure parameters, possibly due to long-term antihypertensive treatment in CKD patients [20]. The mean CIMT values were 0.57±0.12 mm (right), 0.61±0.14 mm (left), and 0.59±0.11 mm (average). Kiykim et al. (2004) found higher mean CIMT values (right: 0.78±0.12 mm, left: 0.79±0.16 mm) [14], while Kuswardhani et al. (2018) reported a mean CIMT of 0.67±0.13 mm [11]. Ayub et al. (2020) observed a lower mean CIMT (0.45±0.09 mm) [5]. Patients with diabetes mellitus (DM) had significantly higher CIMT values (0.63±0.12 mm) compared to nondiabetic patients (0.54±0.09 mm, p<0.001). Sharma et al. (2014) found a similar trend, with CIMT significantly elevated in diabetic patients (0.78±0.25 mm vs. 0.66±0.18 mm, p<0.001) [1]. Afolabi et al. (2018) also reported a positive correlation between DM and CIMT (r=0.338, p=0.003) [20]. The mean serum phosphate and calcium levels were 5.56±1.36 mg/dL and 8.74±1.30 mg/dL, respectively. CIMT positively correlated with both serum phosphate (r=0.627, p<0.001) and calcium (r=0.404, p<0.001). Yonata et al. (2020) observed a significant correlation between CIMT and serum phosphate (r=0.290, p=0.032) but not with calcium [21]. Sharma et al. (2014) found strong correlations with serum phosphate (r=0.911, p<0.001) and calcium (r=0.398, p<0.001) [1]. Ishimura et al. (2005) also reported a correlation with phosphate (r=0.093, p=0.0127) but not calcium [12]. Elevated phosphate and calcium levels may impair endothelial integrity via oxidative stress and mitochondrial disruption, contributing to atherosclerosis [22]. The mean iPTH level was 274.59±150.34 pg/mL, with no significant correlation between iPTH and CIMT (r= -0.046, p=0.687). Yonata et al. (2020) and Ishimura et al. (2005) similarly found no correlation [12,21]. This could be attributed to variations in dietary compliance and intermittent vitamin D3 therapy, which can suppress PTH release [23]. Regarding lipid profiles, mean total cholesterol was 189.13±42.50 mg/dL, triglycerides 188.28±70.01 mg/dL, LDL-C 121.86±26.67 mg/dL, and HDL-C 37.93±8.50 mg/dL. No significant correlation was observed between CIMT and lipid parameters. However, Ishimura et al. (2005) found a significant association between CIMT and non-HDL cholesterol (r=0.102, p=0.0063) [12]. Afolabi et al. (2018) reported a significant correlation between CIMT and triglycerides (r=0.332, p=0.004) but not with other lipid measures [20].
Limitations of the study: This study has several limitations. It lacked a control group; all participants were from a single dialysis center undergoing twice-weekly hemodialysis with the same dialysis solution, which may limit generalizability. The adequacy of dialysis for individual patients was not assessed. Carotid intima-media thickness (CIMT) was used as a morphological marker of atherosclerosis; however, measuring arterial wall stiffness could have provided additional insights into the functional vascular changes associated with renal failure in maintenance hemodialysis patients.
5. Conclusion and Recommendations
This study highlights carotid intima-media thickness (CIMT) as a valuable non-invasive tool for the early detection of cardiovascular disease in hemodialysis patients. Elevated serum phosphate levels, along with age, BMI, and serum calcium levels, were identified as significant and independent factors associated with increased CIMT. Among these, serum phosphate emerged as a common independent predictor of increased CIMT. Further multicenter studies with larger sample sizes are needed to validate these findings and provide deeper insights.
References
- Sharma VK, Dwivedi P, Dubey AK. Correlation of serum phosphate with carotid intimal-medial thickness in chronic kidney disease patients. Indian J Nephrol 24 (2014): 15-19.
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. New England J Med 344 (2001):1608-1621.
- Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int 60 (2001): 472-479.
- Stevens LA, Djurdjev O, Cardew S, et al. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 15 (2004): 770-779.
- Ayub H, Ghulam MF, Hashmi MF. Correlation Between Calcium Phosphorus Product and Carotid Intimal-Medial Thickness in Patients of Chronic Kidney Disease Presenting to a Tertiary Care Hospital. Medical Forum Monthly 31 (2020).
- Hum JM, O’Bryan LM, Tatiparthi AK, et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. J Am Soc Nephrol 28 (2017): 1162-1174.
- Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Research 87 (2009): e10-e17.
- Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 15 (2004): 2959-2964.
- Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 19 (2004): 1480-1488.
- Tushar D, Pratik S, Atul S, Atul M, Charan B, Pavan W. Study of Correlation of Serum Phosphorus with Carotid Intima Media Thickness in Chronic Kidney Disease. IOSR J Dental Med Sci 18 (2019): 22-26.
- Kuswardhani RT, Wiradharma KG, Kandarini Y, et al. Factors associated with carotid intima-media thickness in patients on maintenance hemodialysis. International J Gen Med 18 (2016): 1-6.
- Ishimura E, Taniwaki H, Tabata T, et al. Cross-sectional association of serum phosphate with carotid intima-medial thickness in hemodialysis patients. Am J Kidney Dis 45 (2005): 859-865.
- Hinderliter A, Padilla RL, Gillespie BW, et al. Association of carotid intima-media thickness with cardiovascular risk factors and patient outcomes in advanced chronic kidney disease: the RRI-CKD study. Clin Nephrol 84 (2015): 10-20.
- Kiykim AA, Camsarı A, Kahraman S, et al. Increased incidence of carotid artery wall changes and associated variables in hemodialysis patients without symptomatic cardiovascular disease. Yonsei Med J 45 (2004): 247-254.
- Brzosko S, Lebkowska U, Malyszko J, et al. Intima media thickness of common carotid arteries is associated with traditional risk factors and presence of ischemic heart disease in hemodialysis patients. Physiol Res 54 (2005): 497-504.
- Gaspar A, Watanabe R, Montebello M, et al. Evaluation of intima-media thickness in patients with chronic kidney disease not on dialysis: A prospective study of 24 months. J Bras Nefrol 36 (2014): 35-41.
- Chhajed N, Chandra BS, Shetty MS, et al. Correlation of carotid intimal-medial thickness with estimated glomerular filtration rate and cardiovascular risk factors in chronic kidney disease. Saudi J Kidney Dis Transplant 25 (2014): 572-576.
- Singh AS, Atam V, Patel ML, et al. Carotid intima media thickness as a reflection of generalized atherosclerosis is related to body mass index in ischemic stroke patients. North Am J Med Sci 5 (2013): 228-234.
- Briese S, Wiesner S, Will JC, et al. Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease—impact of calcium and vitamin D therapy. Nephrol Dial Transplant 21 (2006): 1906-1914.
- Afolabi OF, Ibewuike CU, Ulasi II, et al. Carotid Intima Media Thickness Measurements in Nigerian Pre Dialysis CKD Patients. J Med Sci Res 6 (2018): 1-7.
- Yonata A, Ali Z, Indrajaya T, et al. Factors Influence Carotid Intima Thickening Media Thickness in Chronic Kidney Disease Patients. Biomed J Indonesia 6 (2020): 9-17.
- Calò LA, Savica V, Piccoli A, et al. Reduction of hyperphosphatemia is related with the reduction of C-reactive protein in dialysis patients. Study in sevelamer-resistant dialysis patients treated with chitosan chewing gum as salivary phosphate binder. Renal Failure 33 (2011): 11-14.
- Vikrant S, Parashar A. Prevalence and severity of disordered mineral metabolism in patients with chronic kidney disease: A study from a tertiary care hospital in India. Ind J Endocrinol Metabolism 20 (2016): 460-467.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 73.59%
Acceptance Rate: 73.59%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 