Reticulocyte Haemoglobin Content (CHr) is a Reliable Marker of Iron Deficiency in Pre-dialytic Chronic Kidney Disease (CKD) Patients
Md. Obaidul Huq1*, Rana Mokarram Hossain2, Md. Rezaul Alam3, A. K. M Shahidur Rahman3, Md. Kamrul Hasan4, Tofael Ahammod5, Md. Shariful Haque6, Abu Zafor Md. Salahuddin7, Tahmidul Islam8, Mohammad Kamrul Ahsan9
1Junior Consultant, Department of Nephrology, Shaheed Sheikh Abu Naser Specialized Hospital, Khulna, Bangladesh
2Associate Professor, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
3Medical officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
4Associate Professor, Department of Haematology, Colonel Malek Medical College, Manikganj, Bangladesh
5Assistant Professor, Department of Nephrology, Shaheed Syed Nazrul Islam Medical College, Kishorgonj, Bangladesh
6Associate Professor, Department of Nephrology, M Abdur Rahim Medical College, Dinajpur, Bangladesh
7Registrar, Department of Nephrology, Mymensingh Medical College Hospital (MMCH), Mymensingh, Bangladesh
8Research Assistant, Department of Biochemistry and Molecular Biology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
9Resident Medical Officer Holy Family Red Crescent Medical College Hospital (HFRCMCH), Dhaka, Bangladesh
*Corresponding Author: Dr. Md. Obaidul Huq, Junior Consultant, Department of Nephrology, Shaheed Sheikh Abu Naser Specialized Hospital, Khulna, Bangladesh
Received: 10 March 2022; Accepted: 30 March 2022; Published: 18 April 2022
Article Information
Citation: Huq MO, Hossain RM, Alam MR, Rahman AKMS, Hasan MK, Ahammod T, Haque MS, Salahuddin AZM, Islam T, Ahsan MK. Reticulocyte Haemoglobin Content (CHr) is a Reliable Marker of Iron Deficiency in Pre-dialytic Chronic Kidney Disease (CKD) Patients. Archives of Nephrology and Urology 5 (2022): 34-45.
DOI: 10.26502/anu.2644-2833050
View / Download Pdf Share at FacebookAbstract
Chronic kidney disease (CKD) is a non-communicable epidemic disease. The CKD patients suffer from various types of anemia including iron deficiency anemia. Measurement of reticulocyte hemoglobin content (CHr) has been proposed as a measure of available iron stores. In this study CHr and serum iron profile were compared with stainable iron in the bone marrow to evaluate the CHr as a marker of iron deficiency in pre-dialytic CKD patients. This cross sectional study was conducted at the Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from January 2018 to December 2018. A total of seventy (70) pre-dialytic CKD patients were selected. All participants had undergone bone marrow study for detection of iron deficiency anemia and it was observed that, 60% CKD patients had iron deficiency by bone marrow iron stain. Serum iron profile [serum iron, total iron binding capacity (TIBC), serum ferritin, transferin saturation (TSAT)] and reticulocyte haemoglobin content (CHr) of each patient were determined accordingly. Data analysis reveals that CHr was significantly low in iron deficient group (p<0.001). The sensitivity and specificity of CHr at a cut-off 28 pg/cell was 81% and 75.0% respectively (p<0.001); sensitivity and specificity of serum ferritin at a cut-off 100 ng/ml was 40.6% and 67.9% respectively (p=0.051); while sensitivity and specificity of TSAT at a cut-off 20% was 54.2% and 57.1% respectively (p=0.465). The study proves that reticulocyte haemoglobin content (CHr) is a reliable marker to assess iron status for pre-dialytic CKD patients.
Keywords
<p>Anemia; Chronic Kidney Disease (CKD); Iron Deficiency; Reticulocyte Hemoglobin Content (CHr)</p>
Article Details
1. Introduction
Anemia is a common complication of chronic kidney disease (CKD) with a prevalence of twice (15.4%) as in the general population and it increases with stages of CKD, from 8.4% at stage 1 to 53.4% at stage 5 [1]. Improvement of hemoglobin level in CKD patients leads to improvement of their daily performance status, reduction in the disease progression and ultimately to decrease morbidity and mortality [2]. Along with many other causes, iron deficiency either absolute or functional, is an important cause of anemia in chronic kidney disease (CKD) patients [3]. Iron deficiency anemia (IDA) can be treated either by oral iron or parenteral iron therapy or in some cases by blood transfusion. However, increased tissue iron can aggravate disease progression by precipitation of infection and generation of hyper-reactive free radical mediated tissue injury [4, 5]. Therefore, it is important to determine the exact body iron status in CKD patients for their proper management.
In a normal healthy individual iron is stored in the liver, muscle and bone marrow. Bone marrow iron is contained mainly in the macrophages that release it to the developing erythron for the synthesis of hemoglobin [5]. Examination of bone marrow iron stores is traditionally considered as the gold standard for the diagnosis of iron deficiency anemia because most of the anemia show normal or increased iron storage in the bone marrow except iron deficiency anemia where it is depleted [6, 7]. But the procedure is cumbersome and not routinely available in most of the laboratories especially in the countries like Bangladesh. Therefore, the easily available tests are used for the diagnosis. A low level of serum iron, serum ferritin, transferrin saturation (TSAT) status and high concentration of serum transferrin indicates iron deficiency [8]. Serum ferritin level and TSAT status are usually considered as the corner stone for the diagnosis of iron deficiency [9]. The World Health Organization (WHO) recommends that a serum ferritin level of <15 μg/l in adult and <12 μg/l in children of less than 5 years is diagnostic of iron deficiency [10]. However, the ferritin is an acute phase reactant protein and its level increases in the inflammatory condition and any chronic disease like CKD [11, 12]. Therefore, the threshold for diagnosis of IDA in CKD is higher. A serum ferritin <100 μg/L or TSAT <20% is considered diagnostic for IDA in CKD and if the serum ferritin is 100–300 μg/L, TSAT <20% is required to confirm iron deficiency, although these two parameters fail to detect functional iron deficiency in all cases of CKD patients [9]. Chronic kidney disease with occult infection may cause increased serum ferritin level irrespective of the actual iron storage [9]. Transferrin saturation (TSAT) may also be misleading since nutritional status and loss in urine can affect transferrin concentration in CKD [13]. The ideal method for detecting initial iron status should be to directly measure the iron content of erythrocytes, particularly in newly produced cells- reticulocytes.
Recently, a novel assay ‘reticulocyte hemoglobin content (CHr)’, a measure of hemoglobin in newly formed reticulocytes, becomes available [14, 15]. This newer marker (CHr) has currently been introduced to guide iron therapy [9]. European best practice guidelines 2005 and Kidney Disease Outcomes Quality Initiative (KDOQI) 2006 guidelines have recommended this newer parameter (CHr) as marker of iron deficiency status [16, 17]. CHr has been reported to identify initial functional iron shortage particularly among CKD patients and has been proposed as a measure of available iron stores that could be superior to the conventional tests in various studies [14-16]. It has the ability to provide a snapshot of the iron available for erythropoiesis, which can be used for early detection of iron deficiency anemia [15]. It is also cheaper than measurement of conventional iron markers such as- TSAT and ferritin. Therefore, CHr can serve as an alternative to conventional markers (TSAT and ferritin) in evaluating iron status in resource poor countries. This study was aimed to evaluate the CHr as a marker of iron deficiency in pre-dialytic CKD patients among Bangladeshi population.
2. Methodology
2.1 Study design
This cross sectional study was conducted in Departement of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from January 2018 to December 2018. This study was approved by the ethical review committee BSMMU, Dhaka, Bangladesh.
2.2 Participants
The study was carried out among seventy (70) pre-dialytic CKD patients who were admitted at Departement of Nephrology, Bangabandhu Sheik Mujib Medical Universiy (BSMMU) hospital in Dhaka, Bangladesh during the study period. Participants were selected by purposive sampling technique following selection criteria. Adult (age ≥ 18 years) pre-dialytic CKD patients of both sexes with chronic anemia were included in this study. CKD patients with active bleeding, bleeding disorder, taking erythropoetin (EPO) or recent blood transfusion (within 120 days) were excluded from the study. Chronic kidney disease (CKD) was defined as a serum creatinine level of >1.3 mg/dl or a creatinine clearance of <60 ml/minute, present for more than 3 months [18].
2.3. Definition of chronic kidney disease (CKD)
According to KDIGO* chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function, present for more than three (3) months [18].
[*KDIGO = Kidney Disease Improving Global Outcomes].
Criteria for chronic kidney disease (CKD)
[Either of the following present for more than 3 months]
- a) Markers of kidney damage (one or more) [18]
- Albuminuria [Urine albumin excretion rate (AER)] ≥ 30 mg/24 hours; Urine albumin to creatinine ratio (ACR) ≥ 30 mg/gm.
- Urine sediment abnormalities
- Electrolyte and other abnormalities due to tubular disorders
- Abnormalities detected by histology
- Structural abnormalities detected by imaging
- History of kidney transplantation
- b) Decreased glomerular filtration rate (GFR) [18]
Glomerular filtration rate (GFR) < 60 ml/minute/1.73 m2 (GFR categories G3a–G5).
2.4. Procedure
After taking informed written consent from the study participants, their bone marrow was aspirated from the posterior iliac crest under local anaesthesia with all aseptic precaution. Bone marrow materials were examined for presence or absence of stainable iron deposits. According to the bone marrow aspiration report study patients were divided into two groups; iron deficient group and iron present group. At the same time, serum iron profile [that includes- serum iron, total iron binding capacity (TIBC), serum ferritin and transferin saturation (TSAT)] and reticulocyte haemoglobin content (CHr) of each patient were determined accordingly. Serum iron, TIBC, serum ferritin levels were measured by automated analyzer. Transferin saturation (TSAT) was calculated from the ‘serum iron level’ and ‘TIBC’ in each case. Reticulocyte haemoglobin content (CHr) was determined by Sysmex XT-4000 automated hematology analyzer. All data were collected in a data collection sheet by taking history, examining the patients clinically and from the laboratory findings.
2.5 Statistical analysis
Data were analyzed using Statistical Package for Social Science (SPSS) software version 23. Quantitative data were expressed as mean and standard deviation. Categorical data were expressed as frequency and percentage. Association between categorical variables was seen by chi-square test. Comparison of continuous variables was done by independent sample ‘t’ test. A receiver operator characteristic curve (ROC curve) was used to evaluate the accuracy of the parameters. For all statistical tests, p-values less than 0.05 was considered as significant.
3. Results
A total of seventy (70) pre-dialytic chronic kidney disease (CKD) patients due to various causes of CKD with chronic anemia were studied. Of them 48.6% had diabetic nephropathy (DN), 41.4% chronic glomerulonephritis (CGN), 7.1% hypertensive nephropathy (HTN) and 2.9% obstructive nephropathy (Figure 1).
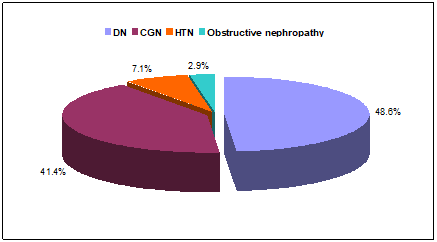
Figure 1: Pie diagram displaying the causes of CKD among the study population (N=70).
Among total 70 pre-dialytic CKD patients 46 (65.7%) were male and 24 (34.3%) were female with a male to female ratio was 1.9:1. The mean age of the study patients was 43.6±11.13 years (ranged 24 - 65 years), their mean haemoglobin level and mean serum creatinine level were 9.73±0.93 gm/dl (ranged 7 - 11.3 gm/dl) and 3.55±0.92 mg/dl (ranged 2.20 - 5.90 mg/dl) respectively (Table 1).
|
Characteristics |
Mean±SD (Range) |
Frequency (%) |
|
Age |
43.6±11.13 (24 - 65) years |
|
|
Gender |
||
|
Male |
46(65.7%) |
|
|
Female |
24(34.3%) |
|
|
Ratio |
1.9:1 |
|
|
Haemoglobin (gm/dl) |
9.73±0.93 (7.0 - 11.3 gm/dl) |
|
|
Serum creatinine (mg/dl) |
3.55±0.92 (2.20 - 5.90 mg/dl) |
|
Table 1: Basic data of the study patients (N=70).
According to the bone marrow aspiration report; out of 70 study patients, 42(60%) patients were iron deficient group and 28(40%) patients were iron present group. The mean(±SD) haemoglobin (Hb) level, reticulocyte haemoglobin content (CHr), serum ferritin level, and transferin saturation (TSAT) status in iron present group and iron deficient group were 10.55±0.32 mg/dl, 29.32±1.25 pg/cell, 279.1±171.1 ng/ml, 25.3±10.4 (%) and 9.18±0.79 mg/dl, 26.57±1.82 pg/cell, 192.8±136.2 ng/ml, 23.5±11.4 (%) respectively. The different parameters among each subgroup were compared. It was observed that, parameters like haemoglobin (Hb), reticulocyte haemoglobin content (CHr), serum ferritin were significantly different among the groups (p<0.05), while difference in transferin saturation (TSAT) was not significant between the groups (p=0.517) (Table 2).
|
Parameters |
Bone marrow aspiration report: Stainable iron |
p-value |
|
|
Present (n=28) Mean±SD |
Absent (n=42) Mean±SD |
||
|
Hb (gm/dl) |
10.55±0.32 |
9.18±0.79 |
<0.001 |
|
CHr (pg/cell) |
29.32±1.25 |
26.57±1.82 |
<0.001 |
|
Serum Ferritin (ng/ml) |
279.1±171.1 |
192.8±136.2 |
0.022 |
|
TSAT (%) |
25.3±10.4 |
23.5±11.4 |
0.517 |
saturation (TSAT) status in iron
Unpaired t-test was done
Table 2: Values of different parameters in iron present and iron deficient group (N=70).
Table 3 shows comparison of reticulocyte haemoglobin content (CHr), serum ferritin and transferin saturation (TSAT) with bone marrow iron status at different cut off values [19]. Data analysis revealed that, among 28 patients with stainable iron deposits in the marrow; 7 patients had CHr £28 pg/cell, 21 paients had CHr >28 pg/cell and out of the 42 patients with no stainable iron in the marrow; 34 patients had CHr £28 pg /cell, 8 patients had CHr >28 pg/cell. On the other hand serum ferritin level <100 (ng/ml), 100-500 (ng/ml), >500 (ng/ml) were found respectively in 10, 15 and 3 patients of detectable iron present in the marrow group, while serum ferritin <100 (ng/ml), 100-500 (ng/ml), >500 (ng/ml) were found respectively in 20, 21 and 1 patients having no detectable iron deposits in the marrow group. In this series transferin saturation (TSAT) £20% and >20% were found in 12 and 16 patients respectively among detectable iron present in the marrow group, while TSAT £20% and >20% were found in 23 and 19 patients respectively among no detectable iron deposits in the marrow group. The difference between two groups was statistically significant only regarding reticulocyte haemoglobin content level (p<0.001) (Table 3).
|
Parameters |
Bone marrow aspiration report: Stainable iron |
p-value |
|
|
Present (n=28) No. (%) |
Absent (n=42) No. (%) |
||
|
Reticulocyte haemoglobin content (CHr) |
|||
|
£28 pg/cell |
7(25.0) |
34(81.0) |
<0.001 |
|
>28 pg/cell |
21(75.0) |
8(19.0) |
|
|
Serum Ferritin |
|||
|
<100 (ng/ml) |
10(35.7) |
20(47.6) |
|
|
100-500 (ng/ml) |
15(53.6) |
21(50.0) |
0.267 |
|
>500 (ng/ml) |
3(10.7) |
1(2.4) |
|
|
Transferin saturation (TSAT) |
|||
|
£ 20 |
12(42.9) |
23(54.8) |
0.329 |
|
> 20 |
16(57.1) |
19(45.2) |
|
Chi-square test was done; Values in the parentheses denote the corresponding percentage
Table 3: Comparison of reticulocyte haemoglobin content (CHr), serum ferritin and transferin saturation (TSAT) with bone marrow iron status at different cut off values (N=70).
The ability of reticulocyte haemoglobin content (CHr) to predict bone marrow iron stores was compared with that of serum ferritin and transferin saturation (TSAT) by receiver operator characteristic (ROC) curve analysis. It was observed that the best cut off point of CHr (blue color) was 28.0 pg/cell for iron deficiency in pre-dialytic CKD patients (Figure 2).
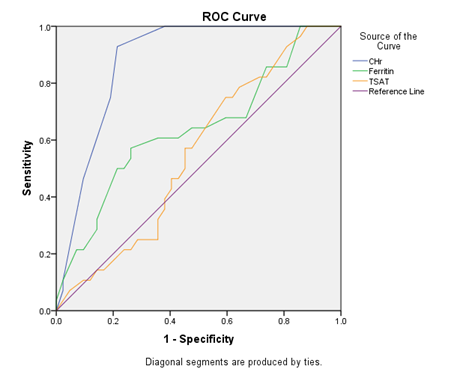
Figure 2: Receiver operator characteristic (ROC) curve showing comparison of reticulocyte haemoglobin content (CHr), serum ferritin and transferin saturation (TSAT) for the detection of absent bone marrow iron stores.
ROC curve analysis revealed that sensitivity and specificity of reticulocyte haemoglobin content (CHr) at a cut off 28 pg/cell were 81.0% and 75.0% respectively with an area under curve (AUC) 0.879±0.04 [95% confidence interval (CI); 0.785-0.954, p<0.001]. Serum ferritin at a cut off 100 ng/ml had sensitivity and specificity were 40.6% and 67.9% respectively with an AUC 0.639±0.69 (95% CI; 0.503-0.775, p=0.051). Transferin saturation (TSAT) at a cut off 20% had sensitivity and specificity was 54.2% and 57.1% respectively with an AUC 0.552±0.069 (95% CI; 0.417-0.687, p=0.465) (Table 4).
|
Iron measured |
AUC* |
Cut off value |
Sensitivity (%) |
Specificity (%) |
p-value |
95% CI |
|
CHr (pg/cell) |
0.879±0.04 |
28.0 |
81.0% |
75.0% |
0.001 |
0.785-0.954 |
|
Ferritin (ng/ml) |
0.639±0.69 |
100.0 |
40.6% |
67.9% |
0.051 |
0.503-0.775 |
|
TSAT (%) |
0.552±0.069 |
20.0 |
54.2% |
57.1% |
0.465 |
0.417-0.687 |
*AUC= Area under curve
Table 4: Sensitivity and specificity of iron measures for detecting iron deficiency in pre-dialytic CKD patients (N=70).
4. Discussion
Iron is an important mineral needed for many essential functions in our body including transport of oxygen from lung to tissues. The body iron is mainly stored in the red blood cells (RBC) and is transported in the circulation by transferrin [20]. Patients with chronic kidney disease (CKD) have lower intestinal iron absorption [21]. Hence the conventional iron markers are not always adequate to diagnose iron deficiency in CKD patients. Reticulocyte haemoglobin content (CHr) appears to be a better tool to predict iron deficiency anemia among pre-dialytic CKD patients [14-16]. In this background current study aimed to evaluate the effectiveness of reticulocyte haemoglobin content (CHr) as a marker of iron deficiency in pre-dialytic CKD patients among Bangladeshi population.
In this study, a total of 70 pre-dialytic CKD patients with chronic anemia were included. Regarding causes of CKD it was found that; 48.6% study patient had diabetic nephropathy (DN), 41.4% had chronic glomerulonephritis (CGN), 7.1% had hypertensive nephropathy (HTN) and 2.9% had obstructive nephropathy. Among the study population; stainable iron deposits were detected in 28 (40%) patients and the rest 42(60%) patients had no stainable iron in the marrow. Out of 28 patients who had stainable iron deposits in the marrow; 7 patients had CHr £28 pg/cell, 21 paients had CHr >28 pg/cell and out of 42 patients with no stainable iron in the marrow; 34 patients had CHr £28 pg /cell, 8 patients had CHr >28 pg/cell. In this series; serum ferritin level <100 (ng/ml), 100-500 (ng/ml), >500 (ng/ml) were found respectively in 10, 15 and 3 patients of detectable iron present in the marrow group, while serum ferritin <100 (ng/ml), 100-500 (ng/ml), >500 (ng/ml) were found respectively in 20, 21 and 1 patients having no detectable iron deposits in the marrow group. In this current study transferin saturation (TSAT) £20% and >20% were found in 12 and 16 patients respectively among detectable iron present in the marrow group, on the other hand TSAT £20% and >20% were found in 23 and 19 patients respectively among no detectable iron deposits in the marrow group. Values of various parameters [haemoglobin (Hb), reticulocyte haemoglobin content (CHr), serum ferritin and transferin saturation (TSAT)] among bone marrow iron deficient and iron present groups shows reticulocyte haemoglobin content (CHr) and serum ferritin were statistically significant between the groups (p<0.05). In this context, Vidyashankar et al. in their study found reticulocyte haemoglobin content (CHr) and serum ferritin were statistically significant which was similar to our findings [22].
In this study comparison of reticulocyte haemoglobin content (CHr), serum ferritin, and TSAT at different cut off values with bone marrow iron status was done accordingly [19]. It was revealed that only reticulocyte haemoglobin content (CHr) was statistically significant (p<0.001). This finding was consistent with a couple of previous study [23, 24].
We found that the sensitivity and specificity of CHr at a cut-off 28 pg/cell was 81.0% and 75.0% respectively. The sensitivity and specificity of CHr in this present study was almost similar to the related previous studies [23-25]. We observed sensitivity and specificity of serum ferritin at a cut-off 100 ng/ml was 40.6% and 67.9% respectively which was comparable with a previous study [26]. In this study TSAT at a cut-off 20% had diagnostic sensitivity and specificity of 54.2% and 57.1% respectively. In accordance Chuang et al. in their study on CKD patients found that TSAT <19 had diagnostic sensitivity and specificity of 58.8% and 78.4% respectively [26]. The sensitivity of TSAT at a cut-off 20% of our findings had similarity but specificity was different from the study mentioned which may be due to different cut off value of TSAT or due to chronic inflammation or may be other causes.
In this current study, area under the ROC curve of CHr at a cut-off 28 pg/cell was 0.879±0.04 (p<0.001), while that of serum ferritin at a cut-off 100 ng/ml was 0.639±0.69 (p=0.051) and TSAT at a cut-off 20% was 0.552±0.069 (p=0.465). So the area under the ROC curve of CHr exceeded that of ferritin and TSAT, indicating that it had the best overall sensitivity and specificity for diagnosing iron deficiency in the population tested. Mast et al. found that the area under the ROC curve was greater for CHr than for serum ferritin and TSAT which was an agreement to our findings [25].
This study demonstrated a strong association of reticulocyte haemoglobin content (CHr) with iron deficiency in pre-dialytic CKD patients. An early detection of iron deficiency among pre-dialytic CKD patients is important for specific management to correct anemia of these patients. Conventionally iron status of CKD patients is assessed by serum ferritin and transferrin saturation (TSAT). Serum ferritin is an acute phase protein and could be raised among individuals with any infection. On the other hand transferrin saturation (TSAT) is measured by indirect method and hence is a poor indicator of body iron load. Therefore a novel biomarker is needed which is not influenced by any other physiological or inflammatory conditionsr. Reticulocyte haemoglobin content (CHr) could be a better option to predict iron deficiency in pre-dialytic CKD patients.
5. Conclusion
This study concluded that the reticulocyte haemoglobin content (CHr) is an ideal test to assess iron status for pre-dialytic CKD patients. The CHr, at a cut-off value of 28 pg/cell could be an accurate predictor of iron deficiency in pre-dialytic CKD patients among Bangladeshi adults.
Limitations
It was a single centre study with a relatively small sample size.
Recommendation
A population based prospective study with large sample size should be done to establish the reticulocyte haemoglobin content (CHr) as a marker of iron deficiency in pre-dialytic CKD patients.
Conflicts of Interest
The authors declare that they had no conflicts of interest regarding the publication of this paper.
References
- Stauffer ME, Fan T. Prevalence of Anemia in chronic kidney disease in the United States. PloS one. 9 (2014): e84943.
- Gianella P, Martin PY, Stucker F. Management of renal Anemia in 2013. Revue Medicale Suisse. 9 (2013): 462-464.
- Eschbach JW, Cook JD, Scribner BH, et al. Iron balance in hemodialysis patients. Annals of internal medicine 87 (1977): 710-713.
- Gutteridge JM, Rowley DA, Griffiths E, et al. Low-molecular-weight iron complexes and oxygen radical reactions in idiopathic haemochromatosis. Clinical science (London, England: 1979). 68 (1985): 463-467.
- Kuvibidila S. Iron deficiency, cell-mediated immunity and resistance against infections: present knowledge and controversies. Nutrition Research. 7 (1987): 989-1003.
- Saxena R, Pati HP, Mahapatra M. de Gruchy’s Clinical Haematology in Medical Practice, 6th adapted ed. India: The Magic International Pvt. Ltd (2013).
- Fishbane S, Mittal SK, Maesaka JK. Beneficial effects of iron therapy in renal failure patients on hemodialysis. Kidney International. 55 (1999): S67-S70.
- Jeremiah ZA, Buseri FI, Uko EK. Iron deficiency Anemia and evaluation of the utility of iron deficiency indicators among healthy Nigerian children. Hematology. 12 (2007): 249-253.
- Kidney Disease Outcomes Quality Initiative. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for Anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 50 (2007): 471-530.
- World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. World Health Organization (2011).
- Domrongkitchaiporn S, Jirakranont B, Atamasrikul K, et al. Indices of iron status in continuous ambulatory peritoneal dialysis patients. American journal of kidney diseases. 34 (1999): 29-35.
- Streetz KL, Wüstefeld T, Klein C, et al. Mediators of inflammation and acute phase response in the liver. Cellular and molecular biology (Noisy-le-Grand, France). 47 (2001): 661-673.
- Rifkind D, Kravetz HM, Knight V, et al. Urinary excretion of iron-binding protein in the nephrotic syndrome. New England Journal of Medicine. 265 (1961): 115-118.
- Tsuchiya K, Saito M, Okano-Sugiyama Het al. Monitoring the content of reticulocyte hemoglobin (CHr) as the progression of Anemia in nondialysis chronic renal failure (CRF) patients. Renal failure. 27 (2005): 59-65.
- Fishbane S, Galgano C, Langley Jr RC, Canfield W, et al. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney international. 52 (1997): 217-222.
- Cullen P, Söffker J, Höpfl M, et al. Hypochromic red cells and reticulocyte haemglobin content as markers of iron-deficient erythropoiesis in patients undergoing chronic haemodialysis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association-European Renal Association. 14 (1999): 659-665.
- Gilmore J. KDOQI clinical practice guidelines and clinical practice recommendations--2006 updates. Nephrology Nursing Journal 33 (2006): 487-489.
- Wheeler DC, Winkelmayer WC. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) foreword. Kidney International Supplements 7 (2017): 1-59.
- Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clinical Journal of the American Society of Nephrology. 1 (2006): S4-S8.
- Knutson MD. Iron transport proteins: Gateways of cellular and systemic iron homeostasis. J Biol Chem 292 (2017): 12735-12743.
- Rostoker G, Vaziri ND, Fishbane S. Iatrogenic Iron Overload in Dialysis Patients at the Beginning of the 21st Century. Drugs 76 (2016): 741-757.
- Vidyashankar P, Almeida AF, Hase NK, et al. Diagnosis of iron deficiency of chronic kidney disease: validity of iron parameters, reticulocyte hemoglobin content (CHr) and hypochromic red cells in inflammatory state. International Journal of Current Research and Review 5 (2013): 83.
- Schapkaitz E, Mahlangu JN, Buldeo S. Diagnosis of iron deficiency Anemia in hospital patients: use of the reticulocyte haemoglobin content to differentiate iron deficiency Anemia from Anemia ofchronic disease: in practice. South African Medical Journal. 106 (2016): 53-54.
- Tessitore N, Solero GP, Lippi G, et al. The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrology Dialysis Transplantation. 16 (2001): 1416-1423.
- Mast AE, Blinder MA, Lu Q, et al. Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood, The Journal of the American Society of Hematology. 99 (2002): 1489-1491.
- Chuang CL, Liu RS, Wei YH, et al. Early prediction of response to intravenous iron supplementation by reticulocyte haemoglobin content and high-fluorescence reticulocyte count in haemodialysis patients. Nephrology Dialysis Transplantation. 18 (2003): 370-377.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 73.59%
Acceptance Rate: 73.59%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 