Assessment of Haematological Profile Clustering Differences in Brucella-Infected Goats across Urban, Peri-Urban, and Rural Areas of Central Uganda
Hellen Ndagire1*, Kizito M Muwonge 2, Julius Mulindwa3, Charles Kakuhikire Twesigye1
1Kyambogo University, Faculty of Science, Uganda
2Soroti University, School of Medicine & Health Sciences, Uganda
3Makerere University, Department of Biochemistry & Systems Biology, Uganda
*Corresponding Author: Hellen Ndagire, Kyambogo University, Faculty of Science, Uganda.
Received: 07 October 2025; Accepted: 15 October 2025; Published: 06 November 2025
Article Information
Citation:
Hellen Ndagire, Kizito M Muwonge, Julius Mulindwa, Charles K. Twesigye. Assessment of Haematological Profile Clustering Differences in Brucella-Infected Goats across Urban, Peri-Urban, and Rural Areas of Central Uganda. Archives of Veterinary Science and Medicine. 8 (2025): 53-63.
View / Download Pdf Share at FacebookAbstract
Goats provide income to small and medium-scale farmers in developing countries through sale of meat and skins. Brucella is a zoonotic intracellular pathogen with poorly established impact on haematological profiles among goats. The haematological profiles serve as prior indicators of preclinical and para-clinical assessments. Disease and livestock management systems affect their haematological profiles. This study aimed to assess seroprevalence of asymptomatic brucellosis in apparently healthy goats in central Uganda and establish their associated haematological alterations. Haematological profiles of 396 apparently healthy goats from Kassanda [rural, n = 155, 39%], Wakiso [peri-urban, n = 150, 38%] and Kampala [urban, n = 91, 23%] Districts were determined. Body condition scores and farming systems were recorded. Venous blood samples were screened for brucellosis with Rose Bengal plate test. A complete blood count was determined by microscopy. PCV and haemoglobin were determined by haematocrit and colorimetry, respectively. Brucellosis seroprevalence was 3.33% [n = 13/396] among goats, whereas it was 4.5% [n = 7/155], 3.3% [n = 5/150] and 1.09% [n = 1/91] among animals from rural, peri-urban and urban districts, respectively. Principal component analysis based on haematological profiles generated three clusters from brucellosis-positive samples by site of origin. Haematological profiles varied among urban, peri-urban and rural Brucella seropositive apparently healthy goats owing to grazing systems at different sites. These conditions possibly impacted the stress levels and body condition scores, which allowed the goats to respond differently to the infection.
Keywords
<p>Brucellosis; Goats; Haematological profiles; Uganda</p>
Article Details
Introduction
Goats are a source of income, meat and skins and provide spiritual satisfaction requirements to small and medium- scale farmers in low-and middle-income countries [1]. Brucella affects goats and other livestock, reducing their welfare and economic gains [2]. Brucella impacts the welfare and economic benefits of infected wildlife, livestock and human beings [3,4]. Humans are infected through the consumption of contaminated animal products such as meat and milk, direct contact with fluids from infected animals or inhalation of aerosols from animals [5]. Brucella causing bacteria spread by sexual contact among mature animals, contact to aborted placenta, foetus, foetal fluids, or vaginal discharge [6]. Brucella presents with prolonged latent periods, abortion and infertility among animals [4]. Brucellosis impacts the blood elements of infected animals [7]. Haematological and biochemical variations in blood parameters are indicators of the health, nutritional and physiological status of ruminants. Physical or environmental stressors may also affect the haematological and biochemical profiles [8]. The variations also occur in Brucella seropositive animals, with varying body condition scores indicative of stored fat and muscle protein [9,10]. Fat and protein are required for production, reproduction and maintenance. The reserves indicate previous nutritional status and are used in stressful conditions and periods when the animal requires high energy consumption [11]. Farming systems affect the body condition scores of goats. Extensive farming system provides superior welfare compared to intensive or semi-intensive practices [12]. Semi-intensive farming systems generate higher BCSs than does intensive goat management [13]. The Lake Victoria crescent in Uganda is predominantly covered by extensive, semi-intensive and intensive farming systems in rural, peri-urban and urban settings, respectively.
Materials and Methods
Study design and sampling sites
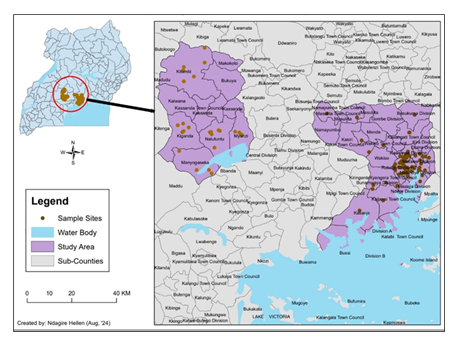
A cross-sectional study was conducted from June to November 2023 by randomly selecting 396 apparently healthy adult goats, without any prior abortion from consenting households from three district sampling sites: Kassanda [rural], Wakiso [peri-urban] and Kampala [urban] in Uganda, East Africa (Figure 1). Body condition scores [BCSs] were recorded and natural non improved pasture grazing with or without supplementation was noted. Goat rearing practices were categorised as extensive, intensive or semi-intensive. Breed descriptors, including coat colour and appearance, presence or absence of horns, size and shape of breed’s ears, presence of a mane, and body size were recorded. Vaccination of the selected goats against brucellosis was noted. Drinking water sources were also recorded.
Sample size
The sample size of the goats was determined according to Daniel [1999]. Three hundred ninety-six goats [396] were sampled from three sites by proportions according to the number of goats as reported by Uganda Bureau of Statistics [26]. One hundred and fifty goats were collected from the peri-urban district sampling sites, one hundred and fifty-five goats from the rural district sampling sites and ninety-one goats from the urban district sampling sites. A random starting point was established at the village level. Every fifteenth household in rural and peri-urban areas was selected. Every fifth household in the urban area was selected. No more than 10% of the flock in the household was sampled. If the targeted homestead did not rare goats or did not consent, then the adjacent household was considered.
Sample collection and analysis
Venous blood was drawn aseptically into EDTA tubes and another without an anticoagulant from each animal and transported to the laboratory at 4°C. Rose Bengal plate test was performed to detect Brucella antibodies. 16S rRNA sequencing confirmed selected Brucella seropositive samples. Haematological parameters were analysed within four to six hours of collection. Microscopy was used to establish Total Red blood cell counts [TRBCs] and leucocyte counts of TWBCs, neutrophils, lymphocytes, eosinophils, monocytes and basophils. Packed cell volume [PCV] was determined by haematocrit [MRC-HCEN]. Haemoglobin levels were determined by colorimetry [MRC-CEN-202]. Mean corpuscular volume [MCV], mean corpusular haemoglobin [MCH] and mean corpusular haemoglobin concentration [MCHC] were calculated from pre-attained values. Red cell indices and leucocyte counts were referenced with MSD manual of Veterinary Medicine [27].
Statistical analysis
Principal component analysis [PCA] was performed on the haematological scores of the samples. Dendrograms were established to illustrate the clustering and interpretation of relationships between variables among the samples. Analysis of variance was used to compare means of haematological profiles with their sites of origin, followed by Least Significant Difference [LSD] - post hoc test using R programming software. Statistical significance was considered at 95% confidence level [p < 0.05].
Study location
Results
Goat breed distribution
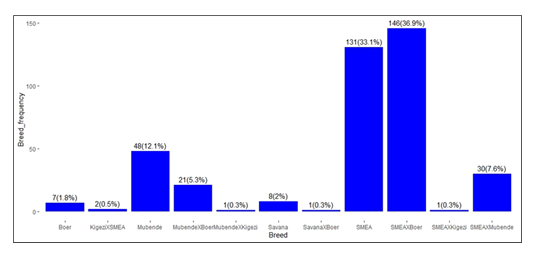
Goats from the rural district were pasture grazing without supplementation under extensive farming. The peri-urban goats were managed mostly through extensive and semi-intensive rearing with limited intensive practices. The urban goats were from farmers who mostly practiced intensive rearing with the following breed ratios sampled: Boer, 1.8% [n = 7/396]; KigeziXSMEA, 1.5% [n = 2/396]; Mubende, 12% [n = 48/396]; MubendeXBoer, 5.3% [n = 21/396]; MubendeXKigezi, 0.3% [n = 1/396]; Savanna, 2% [n = 8/396]; SavannaXBoer, 0.3% [n = 1/396]; SMEA, 33.1% [n = 131/396]; SMEAXBoer, 36.9% [n = 146/396]; SMEAXKigezi, 0.3% [n = 1/396]; and SMEAXMubende, 7.6% [n = 30/396].
Farming practices at the district sampling sites
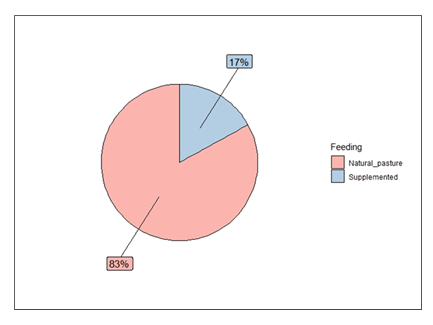
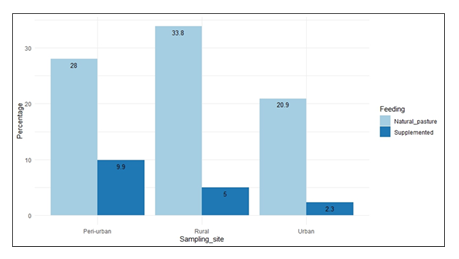
The goats in this study were not vaccinated against brucellosis. Drinking water for animals at the sites in Kassanda District was obtained from shared springs or shallow wells/boreholes, streams, swamps and shorelines of Lake Wamala. Drinking water for animals at the sites in Wakiso and Kampala Districts was obtained from springs or shallow wells/boreholes, streams, swamps and shorelines of Lake Victoria. A few farmers at the Wakiso and Kampala study sites provided chlorinated drinking water to their animals. Chlorination minimises the exposure of animals to disease-causing microorganisms. Most of the goats were fed nonimproved natural pastures [83%], and the rest were on both natural pastures and supplementation with grown pasture like legumes and mineral blocks [17%] [Figure 3]. Most of the feed supplementation among goats occurred in peri-urban settings, whereas the lowest amount occurred in urban areas (Figure 4).
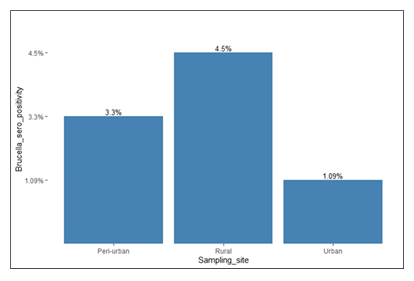
Brucella seropositivity prevalence among the goats
Rose Bengal plate test detected Brucella antibodies in 3.3% [n = 13/396] of the goats. Brucella seropositivity was approximately 4.5% [n = 7/155], 3.3% [n = 5/150] and 1.09% [n = 1/91] at the rural, peri-urban and urban study sites, respectively.
Haematological profiles of the goats
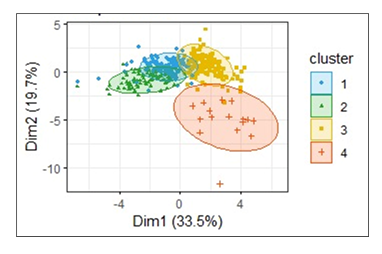
Clustering of the hematological profiles of the goats was performed using principal component analysis. Relationships among variables exhibited distinct patterns of the haematological profiles. The first principal components [Dim1 and Dim2] explained 53.2% of the total variance. The data points displayed as dots [Figure 6] represent the distribution of points that tightly clustered near the origin. Therefore, the majority of the goats did not vary drastically across components. The outliers presented distinct blood profiles. The goats formed four clusters near the origin, indicative of minimal variation in haematological profiles, except for outliers.
Haematological profiles of Brucella-positive goats
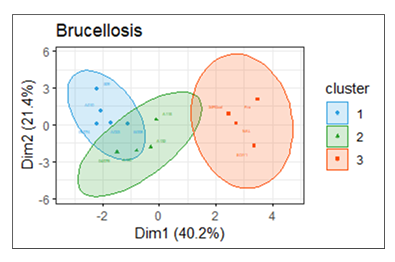
Principal components [Dim1 and Dim2] explained 40.2% and 21.4% of the data, respectively [Figure 7]. Blood pictures were linked to brucellosis among the goats. Haematological profiles reflected the immunological response. Three clusters were formed in reference to haematological profile variations. The clusters indicated the contribution of the haematological parameters to variations in haematological profiles among Brucella-positive goats.
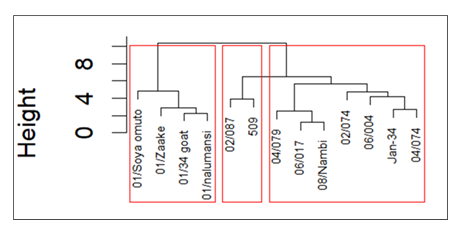
Brucella-positive goats were singled out in a dendrogram [Figure 8]. Clusters and clades formed in response to variations in haematological profiles. Goats in cluster I had higher body condition scores than their counterparts in clusters II and III did [Table 1]. Goats in cluster I were under extensive farming and were either SMEAXMubende or Mubende by breed. Distribution of goats in clusters and clades is indicated in Table 1.
|
Cluster |
Member |
BCS |
Sampling site |
Breed |
Feeding |
|
I |
01/soya omuto |
High |
Rural |
SMEAXMubende |
Pasture |
|
01/Zaake |
High |
Rural |
SMEAXMubende |
Pasture |
|
|
01/34goat |
High |
Rural |
Mubende |
Pasture |
|
|
01/Nalumansi |
High |
Rural |
SMEAXMubende |
Pasture |
|
|
II |
02/087 |
Moderate |
Peri-urban |
SMEAXBoer |
Supplemented |
|
509 |
Modrate |
Rural |
MubendeXBoer |
Pasture |
|
|
III |
04/079 |
Moderate |
Rural |
Mubende |
Pasture |
|
06/017 |
Moderate |
Peri-Urban |
Mubende |
Pasture |
|
|
08/Nambi |
Moderate |
Peri-Urban |
SMEAXBoer |
Pasture |
|
|
02/074 |
Low |
Peri-Urban |
SMEAXBoer |
Supplemented |
|
|
06/004 |
High |
Peri-Urban |
Mubende |
Pasture |
|
|
Jan-34 |
Low |
Urban |
SMEA |
Pasture |
|
|
04/074 |
Moderate |
Rural |
SMEAXBoer |
Pasture |
Table 1: Details of the samples in the different clusters.
Significant differences were observed across the three clusters when haematological parameters of Brucella seropositive samples were analysed [Table 2]. The TWBC, lymphocyte, neutrophil, eosinophil, monocyte and basophil counts were not significantly different across the clusters. Therefore, the goats exhibited similar leucocyte response in brucellosis. However, lymphocyte counts were highest in Cluster II [7.11 ± 1.63]. In contrast, neutrophil counts were lowest in Cluster II [3.20 ± 0.35]. Basophil levels slightly increased in Cluster III [0.21 ± 0.08].
|
Parameter |
Cluster I: Mean± SD |
Cluster II: Mean± SD |
Cluster III: Mean± SD |
|
TWBC X103/µl |
12.68±2.02a |
11.15±2.00a |
10.90±2.70a |
|
Lymphocytes X 103/µl |
6.24±1.37a |
7.11±1.63a |
6.13±1.58a |
|
Neutrophils X 103/ul |
5.46±2.51a |
3.20±0.35a |
3.95±1.12a |
|
Eosinophils X 103/ul |
0.47±0.37a |
0.38±0.03a |
0.40±0.08a |
|
Monocytes X 103/ul |
0.36±0.14a |
0.33±0.08a |
0.22±0.08a |
|
Basophils X 103/ul |
0.14±0.08a |
0.13±0.06a |
0.21±0.08a |
|
TRBC X 106/ul |
13.22±0.55a |
11.20±0.62b |
7.83±0.10c |
|
PCV% |
29.42±1.91a |
22.67±1.15b |
20.78±1.21b |
|
HB, g/dl |
10.06±0.72a |
7.72±0.26b |
7.50±0.54b |
|
MCV, fL |
22.24±1.02b |
20.25±0.52b |
26.54±1.25a |
|
MCH, pg |
7.61±0.4b |
6.90±0.28b |
9.58±0.59a |
|
MCHC% |
34.21±0.95b |
34.06±0.72b |
36.09±1.09a |
Key: a, b &c are hypothetical letters implying significant difference. [LSD, p = .05]
Table 2: Average means of haematological profiles for Brucella seropositive samples.
We found significant variation in total red blood cell [TRBC] counts, where Cluster I presented with the highest mean value, whereas Cluster III presented the lowest [Table 2]. Similarly, packed cell volume [PCV] was highest in Cluster I [29.42 ± 1.91] and lowest in Cluster III [20.78 ± 1.21], suggesting a reduction in red blood cell volume, especially in Cluster III. Clusters II and III were not significantly different. Haemoglobin levels were significantly lower in Cluster II [7.72 ± 0.26] and Cluster III [7.50 ± 0.54], compared to Cluster I [10.06 ± 0.72], suggesting brucellosis-induced anaemia. Mean corpuscular volume [MCV] and mean corpuscular haemoglobin [MCH] were highest in Cluster III, indicating macrocytic changes in red blood cells and further supporting severe anaemia in Cluster III. Mean corpuscular haemoglobin concentration [MCHC] was significantly greater in Cluster III [36.09 ± 1.09] compared to Clusters I and II, suggesting a higher haemoglobin concentration in red blood cells in Cluster III. Overall, the haematological profiles of Cluster I goats were least altered among those with brucellosis. Cluster III indicated the most pronounced haematological changes, particularly in the erythrocyte indices. This highlights the varying severity of brucellosis among goats.
Discussion
Asymptomatic latent brucellosis is prevalent among livestock and humans, with limited data concerning their corresponding haematological profiles. There is paucity of data concerning asymptomatic brucellosis among goats in Uganda. Previous studies have reported combined rates of brucellosis among asymptomatic and symptomatic goats in Uganda [19,23]. In contrast, our study investigated the prevalence of asymptomatic brucellosis in apparently healthy goats in central Uganda. The overall rate of asymptomatic latent brucellosis was 3.33% in the three study districts, which was consistent with the 3.92% reported among goats in Karenga District, Uganda [23]. However, our findings of the prevalence of brucellosis among goats in Kassanda, Wakiso and Kampala study sites were inconsistent with the findings of Miller et al. [20], who reported seropositivity of 16% among serum samples from goats in southwestern Uganda. Higher rate of Brucella seropositivity was reported by Mugabi et al. [19] among goats in Kiruhura District, Uganda. Brucella seropositivity in the studies by Miller et al. [20] and Mugabi et al. [19] was irrespective of pre-clinical or clinical manifestations of the disease. In this study, the rate of asymptomatic brucellosis was highest at the rural site [Kassanda, 4.52%], which may be attributable to more frequent sharing and exchange of bucks among rural farmers in Uganda, as reported by Kaumbata et al. [18].
Breed distribution and feeding systems at the study sites
The major indigenous goat breeds in this study were SMEA [33.1%], Mubende [12%] and Kigezi [1.5%], in agreement with previous reports [28,29]. The goat population in Uganda is composed of more than 95% indigenous goat breeds [30]. There is indiscriminate crossbreeding among goats in Uganda [31]. SMEAXBoer was the major cross breed in this study, similar to previous reports [28,31,32].
Majority of the goats [83%] in this study were on nonimproved natural pastures, which is in agreement with Nantongo et al. [28]. Extensive management systems are the main livestock production method in Uganda, where goats are grazed in open noncultivatable areas or fenced farms. Free-range animals have the ability to select their fodder, increasing the quantity and quality of their diet [28,33].
Hierarchical clustering of goats by haematological profiling
In the present study, clustering occurred near the origin, indicative of minimal variation in haematological profiles, similar to the insignificant differences in blood parameters reported by Shamsa et al. [34]. However, the study by Shamsa et al. [34] reported significant differences in lymphocytes. The current study revealed differences in haematological profiles of Brucella seropositive goats, similar to the findings of Mohammed et al. [35], who reported significant differences in haematological parameters among different goat breeds under warm and humid weather conditions.
Hierarchical clustering of samples positive for brucellosis using haematological profiles
Brucella seropositive goat samples clustered into three groups [I, II and III] by haematological profiling. Four indigenous goats constituted cluster I, with three MubendeXSMEA cross breeds and a Mubende goat breed. The goats in cluster I were all from rural sampling sites [Kassanda district] and fed on nonimproved natural pastures under an extensive care system without supplementation. Cluster I was characterised by elevated TWBCs, neutrophils, TRBCs and PCV. Similarly, a previous study by Allam [9] reported increased TWBCs and PCV among Brucella seropositive goats. Elevated TWBCs in Brucella seropositivity may be attributable to recognition of the infection, and the immune system builds up to manage the infection [36].
One of the clades in cluster I consisted of a single leaf [01/soya omuto] with both brucellosis and anaplasmosis. The difference in clade distance from the rest of the candidates in cluster I may be attributed to coinfection. Cluster I was characterised by elevated TWBCs, neutrophils, TRBCs and PCV with high BCSs. Similarly, Allam [9] reported moderately high values of PCV and TWBCs among goats and sheep in Egypt and reported that the body condition score [BCS] was related to the haematological characteristics of Brucella seropositive ruminants.
Cluster II consisted of two goats, Mubende X Boer and SMEA X Boer cross breeds from the rural and peri-urban [Wakiso] sampling sites, respectively. Lymphocytes and monocytes were slightly increased among goats in cluster II while the other haematological parameters did not differ from those in clusters I and III. Elevated lymphocytes and monocytes in brucellosis are attributed to the presence of Brucella organisms, regardless of disease symptoms or asymptomatic state [9].
Cluster III was characterised by increased basophils and lower TWBCs, TRBCs and PCVs. Allam [9] reported low HB, reduced RBC and lower PCV values among animals with low BCSs, which is in agreement with the findings of this study. According to Allam’s report, [9] increased monocytes and basophils were proportional to high BCS among Brucella seropositive animals. This study did not reveal any significant differences in lymphocyte counts among all the clusters, which differed from the findings of Allam et al. [9], who reported the presence of lymphocytosis in clinical or subclinical cases irrespective of the BCS. This study also revealed that brucellosis affected the haematological profiles of goats, which contrasts with the findings of Skider [37], who reported that Brucella infection cannot be attributed to significant changes of haematological profiles of animals.
In this study, the body condition scores were high for the goats in the extensive care farming systems, which were practiced predominantly in the rural sampling site. Body condition scores are linked to health status, production and welfare of goats [38]. An animal’s body condition indicates the amount of lipid [fat] and protein [muscle] reserves that are available for maintenance, reproduction and production [10]. Fat reserves are utilised during high energy demand periods, such as stress, or under conditions of under nutrition [10]. Intensive and semi-intensive farming practices require herding with frequent human handling. Low BCSs have been previously reported in intensive care systems among goats with frequent human handling, which harms the appetite center of the hypothalamus in the brain. Disruption of the hypothalamus causes reduced feed intake and thus decreased weight [39]. However, Kharrat [40] reported a decreasing BCS with low nutritive value pastures in Lebanon. Similarly, Bushara et al. [41] reported reduced BCS among goats during drought conditions associated with poor natural pastures in extensive farming systems in Sudan. In the present study, the low BCS may be attributable to frequent human handling in the intensive care system [39].
Variation in haematological profiles of goat samples
Cluster I presented insignificant differences in haematological profiles of Brucella seropositive goats. However, cluster I exhibited elevated TWBCs, similar to the findings of a previous report by Muayard [42] and their study reported elevated TWBCs among extensively managed goats in Malaysia. Extensive system in goat farming subjects the animals to numerous microbial challenges [43], which is in agreement with our findings of elevated neutrophils, eosinophils, and monocytes. The elevated values in cluster I, although not significant, simulated the findings of Olayemi et al. [44], reported significantly higher values of TWBCs, neutrophils, and eosinophils among extensively reared West African dwarf goats in Nigeria.
Goats in cluster I on an extensive management system had significantly higher TRBC, PCV and HB in contrast with reduced TRBC, PCV and HB values, as previously reported by Olayemi et al. [44]. However, our findings were obtained during a rainy season, whereas Olayemi et al. [44] reported contrasting results for a study in a dry season with poor pastures. Our findings of elevated TRBC, PCV and HB in goats with brucellosis are in agreement with those of a previous study by Elsa and Onyeyili [45] during the rainy season in southern Nigeria.
Conclusion
The effects of brucellosis on haematological profiles varied among urban, peri-urban and rural apparently healthy goats owing to the different grazing systems in the different areas, which resulted in different stress levels for the animals and differences in body condition, which allowed the goats to respond differently to the impact of the infection. We recommend a larger sample size of apparently healthy indigenous, crossbred and exotic goats to affirm the impact of brucellosis on animals in the different ecological zones and grazing systems in Uganda.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Ethical approval was obtained from the Institutional Animal Care and Use Committee of the School of Veterinary Medicine and Animal Resources, Makerere University [Reference number: SVAR-IACUC/97/2021].
Informed consent
All the farmers provided written consent to participate in this study.
Consent for publication
Not applicable
Source of funding
No external funding was received.
Author contributions
HN conceptualised the research design, performed the laboratory activities and analysis, and drafted the initial manuscript. KMM conducted the laboratory analysis. JM and CTK supervised the research activities. KMM, JM and CTK revised the manuscript. All the authors read and approved the final manuscript.
Acknowledgement
The authors acknowledge the participants and research assistants involved in this study.
References
- Girma M. Production system of indigenous goat population reared in pastoral and agro-pastoral districts of South Omo, Ethiopia. Int J Agric Res Innov Technol 13 (2023): 51–59.
- Kumar R, Eipers P, Little RB, et al. Getting started with microbiome analysis: Sample acquisition to bioinformatics. Curr Protoc Hum Genet 2014 (2014): 18.8.1–18.8.29.
- Brunetti R, Ottaiano M, Fordellone M, et al. Risk factors for the spread of brucellosis in sheep and goats in the Campania region in the years 2015–2020. Microorganisms 11 (2023): 1–14.
- Elrashedy A, Gaafar M, Mousa W, et al. Immune response and recent advances in diagnosis and control of brucellosis. Ger J Vet Res 2 (2022): 10–24.
- Bushoborozi B, Agwu E, Obeagu EI. Burden of human brucellosis in Uganda: A review. 2023 (October).
- Munsi MN, Akther S, Rahman MH, et al. Seroprevalence of brucellosis in goats in some selected areas of Bangladesh. J Adv Vet Anim Res 8 (2021): 123–128.
- Darwish AA, Mahmoud MA, El-Kattan AM. Clinicopathological studies on brucellosis in sheep and goat at Matrouh Governorate, Egypt. J Anim Health Prod 11 (2023): 25–33.
- Zaher HA, Mesalam A, Al Bloushi AI, et al. Hematological and biochemical indices, growth performance, and puberty of goats fed with Mombasa and blue panic as salt-tolerant alternatives to alfalfa under arid conditions. Front Vet Sci 9 (2022).
- Allam N, Allam T, Abdelsalam ME, et al. Comprehensive epidemiological evaluation of ruminant brucellosis and associated risk factors in some Egyptian Governorates. 17 (2024): 2780–2796.
- Ghosh CP, Mandal D, Roy DC, et al. Body condition scoring in goat: Impact and significance. J Entomol Zool Stud 7 (2019): 554–560.
- Caldeira RM, Portugal AV. Relationships of body composition and fat partition with body condition score in Serra da Estrela ewes. Asian-Australas J Anim Sci 20 (2007): 1108–1114.
- Temple D, Manteca X. Animal welfare in extensive production systems is still an area of concern. Front Sustain Food Syst 4 (2020).
- Silva Salas MÁ, Mondragón-Ancelmo J, Jiménez Badillo M del R, et al. Assessing dairy goat welfare in intensive or semi-intensive farming conditions in Mexico. J Dairy Sci 104 (2021): 6175–6184.
- Kabagambe EK, Elzer PH, Geaghan JP, et al. Risk factors for Brucella seropositivity in goat herds in eastern and western Uganda. Prev Vet Med 52 (2001): 91–108.
- Kansiime C, Atuyambe LM, Asiimwe BB, et al. Community perceptions on integrating animal vaccination and health education by veterinary and public health workers in the prevention of brucellosis among pastoral communities of South Western Uganda. 2015: 1–15.
- Naseer A, Mo S, Olsen SC. Brucella melitensis vaccines: A systematic review. 2023.
- Makita K, Fèvre EM, Waiswa C, et al. Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. 2011.
- Kaumbata W, Nakimbugwe H, Nandolo W, et al. Experiences from the implementation of community-based goat breeding programs in Malawi and Uganda: A potential approach for conservation and improvement of indigenous small ruminants in smallholder farms. Sustain 13 (2021): 1–16.
- Mugabi R, Khaitsa M, Miller R, et al. Seroprevalence of brucellosis in selected herds of cattle and goats in Kiruhura district, Uganda. Afr J Anim Biomed Sci 7 (2012).
- Miller R, Nakavuma JL, Ssajjakambwe P, et al. The prevalence of brucellosis in cattle, goats and humans in rural Uganda: A comparative study. 2015: 1–14.
- Kakooza S, Watuwa J, Ipola PA, et al. Seromonitoring of brucellosis in goats and sheep slaughtered at an abattoir in Kampala, Uganda. J Vet Diagn Invest 34 (2022): 964–967.
- Bugeza J, Roesel K, Moriyon I, et al. Sero-prevalence and factors associated with anti-Brucella antibodies in slaughter livestock in Uganda. Front Epidemiol 3 (2023): 1–10.
- Akwongo CJ, Kakooza S. Exposure to Brucella spp. in goats and sheep in Karenga District, Uganda diagnosed by modified Rose Bengal method. Zoonotic Dis 2 (2022): 163–171.
- Vudriko P, Ekiri AB, Endacott I, et al. A survey of priority livestock diseases and laboratory diagnostic needs of animal health professionals and farmers in Uganda. Front Vet Sci 8 (2021): 1–20.
- González-Gordon L, Porphyre T, Muwonge A, et al. Identifying target areas for risk-based surveillance and control of transboundary animal diseases: A seasonal analysis of slaughter and live-trade cattle movements in Uganda. Sci Rep 13 (2023): 1–16.
- Uganda Bureau of Statistics (UBOS). Uganda annual agricultural survey 2018. National Digital Archives. Kampala; 2020.
- Kahn CM. The Merck Veterinary Manual. 9th ed. Whitehouse Station, NJ: Merck & Co; 2005.
- Nantongo Z, Agaba M, Shirima G, et al. Variability in body weight and morphology of Uganda’s indigenous goat breeds across agroecological zones. PLoS One 19 (2024): 1–14.
- Ssewannyana E. Strategies for management of animal genetic resources in Uganda. Uganda J Agric Sci 9 (2004): 888–892.
- Onzima RB, Gizaw S, Kugonza DR, et al. Production system and participatory identification of breeding objective traits for indigenous goat breeds of Uganda. Small Rumin Res 163 (2018): 51–59.
- Muwanika BV, Masembe C, Nampanzira DK, et al. Enhancing productivity of traditional goat phenotypes among smallholder farmers of Uganda: The role of research. 14 (2016): 17–21.
- Ssewannyana E, Oluka J, Masaba JK. Growth and performance of indigenous and crossbred goats. 2004: 537–542.
- Muir JP, Jordao C, Massaete ES. Comparative growth characteristics of goats tethered on native pasture and free-ranged on cultivated pasture. Small Rumin Res 17 (1995): 111–116.
- Shamsa MT. Study of hematological changes in local goats (Capra aegagrus) at Al-Najaf Province, Iraq. Indian J Forensic Med Toxicol 14 (2020): 7973–7978.
- Mohammed MTA, Dhuha JM, Al-Bakri SA, et al. Hematological parameters of goat breeds in warm and humid weather. Indian J Ecol 48 (2021): 47–50.
- Ali B, Naeem M, Ullah S, et al. Molecular detection, seasonality, epidemiology and effect of Brucella melitensis infection on the hematological profile of cattle breeds. Sci Rep 15 (2025): 1–11.
- Sikder S, Mushfiqur SM, Md R, et al. Haematological variations in Brucella abortus antibody positive cross-bred cattle at Chittagong, Bangladesh. 23 (2012): 125–128.
- Sejian V, Silpa MV, Nair MRR, et al. When other species struggle to live, sheep and goats can withstand periods of extreme heat stress and lack of water and feed better. Production Considerations 11 (2021): 1–24.
- So-in C. Influence of goat management systems on hematological, oxidative stress profiles, and parasitic gastrointestinal infection. 16 (2023): 483–490.
- Kharrat M, Bocquier F. Adaptive responses at the whole lactation scale of Baladi dairy goats according to feed supply and level of body reserves in agro-pastoral feeding system. Small Rumin Res 90 (2010): 120–126.
- Abdelhadi O, Bushara I, Elemam MB, et al. Effect of parity number on the productivity of Taggar goats under dry land farming in Western Sudan. J Agric Environ Sci 10 (2011): 515–518.
- Mohammed Muayad TA, Haniza MZH, Husni I, et al. Haematological values of apparently healthy indigenous goats in Malaysia: A comparative study. Indian J Anim Res 52 (2018): 1701–1704.
- Josefina A, Pizetti M, Sarmiento RO, et al. Haematological and protein profile of goat rodeo in extensive productions of different regions in the province of Salta, Argentina. 2021.
- Olayemi FO, Oboye OO, Azeez IO, et al. Influence of management systems and sex on haematology of West African dwarf goat. Afr J Agric Res 4 (2009): 1199–1202.
- Elsa AT, Onyeyili PA. Haem profiles in extensive goats. 2002.

 Impact Factor: * 1.1
Impact Factor: * 1.1 Acceptance Rate: 80.20%
Acceptance Rate: 80.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 