Curcuma longa Administration Significantly Reduces Acute and Persistent Inflammatory Pain Measures in Male and Female Rats
Mario Alberto Bautista-Carro1,2, Gonzalo Flores3, Tommaso Iannitti3, Gumaro Galindo-Paredes1,4, Julio César Morales-Medina1*
1Centro de Investigación en Reproducción Animal, CINVESTAV-Universidad Autónoma de Tlaxcala, AP 62, CP 90000, Tlaxcala, México
2Maestria en Ciencias Biológicas, Universidad Autónoma, Tlaxcala, México
3Lab. Neuropsiquiatría, Instituto de Fisiología, Benemérita Universidad Autónoma de Puebla, 14 Sur 6301, San Manuel, 72570, Puebla, México
4Doctorado en Ciencias, Departamento de Fisiología, Biofísica y Neurociencias, CINVESTAV, Ciudad de México, México
*Corresponding Author: Julio César Morales-Medina, Km 10.5 carretera San Martin Texmelucan, Ixtacuixtla, Tlaxcala, Mexico
Received: 06 April 2021; Accepted: 14 April 2020; Published: 05 May 2021
Article Information
Citation:
Mario Alberto Bautista-Carro, Gonzalo Flores, Tommaso Iannitti, Gumaro Galindo-Paredes, Julio César Morales-Medina. Curcuma longa Administration Significantly Reduces Acute and Persistent Inflammatory Pain Measures in Male and Female Rats. Archives of Veterinary Science and Medicine 4 (2021): 24-33.
View / Download Pdf Share at FacebookAbstract
Pain management is a worldwide public health concern particularly in women given its high prevalence and severity. Moreover, current pharmaceuticals induce numerous side effects including dependence and tolerance. Therefore, the search for novel therapeutic targets for the treatment of various forms of pain is of foremost importance. In this regard, Curcuma longa (C. longa) or turmeric has shown anti-inflammatory and immunomodulatory effects. Henceforth, in this work, we evaluate the potential anti-nociceptive and anti-inflammatory effects of C. longa in two models of peripheral inflammation in male and female rats. Rats were administered with the inflamogens carrageenan (acute inflammation inducer) and complete Freund’s adjuvant (CFA) (persistent inflammation inducer) in the hindpaw to elicit mechanical allodynia and paw oedema. At the peak of mechanical allodynia, C. longa was administered orally and mechanical thresholds and paw size were monitored until the mechanical thresholds were back to baseline values. C. longa reduced mechanical allodynia in rats with acute or persistent inflammation-induced allodynia in male and female rats to a similar degree. This is the first study to employ a combination of pharmacodynamic models of acute and persistent inflammation to assess efficacy of C. longa on inflammatory pain behavior, also considering sex differences. We found that C. longa reverses inflammation-induced mechanical allodynia in male and female rats therefore showing promise as an effective anti-nociceptive agent. When using the carrageenan and CFA models, both male and female rats can be used without concerns related to sex bias.
Keywords
<p>Allodynia; Curcuma longa; Sex differences; Inflammation; Pain; nociception; inflamogens</p>
Article Details
1. Introduction
Inflammation is known to induce swelling and pain [1]. While acute inflammation is protective, chronic is detrimental, displaying spontaneous pain, allodynia and hyperalgesia [1]. Moreover, there is a major prevalence and intensity of pain in women compared to men [2]. In addition, current therapies for treatment of chronic pain are inadequate and some can induce chronic pain, e.g. opioids [1] and chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) can result in several adverse effects including damage to the gastrointestinal tract [3]. To bridge this gap and find novel therapeutic targets, we rely on animal models that mimic inflammatory processes and have translational value.
Administration of the inflamogens carrageenan or complete Freund’s adjuvant (CFA) results in mechanical allodynia and swelling, therefore, extensively used to test prospective analgesic and anti-inflammatory compounds [4, 5]. For instance, CFA produces a long-lasting inflammatory response accompanied by mechanical allodynia lasting between 1 and 2 weeks with peak observed on day 3 post-administration [6]. Moreover, CFA increases the expression of prostaglandins as well as COX-2 in the spinal cord within hours [7]. Carrageenan induces acute inflammatory pain, which is established already at 1 hr and peaks at 4 hr post-carrageenan administration [5, 6].
At the site of injection, carrageenan increased the expression of prostaglandins, nitric oxide (NO) and tumor necrosis factor-α (TNF- α) [8, 9] and interleukin (IL)-1β (IL-1β) in serum [10]. Therefore, CFA and carrageenan are two well-stablished models to test potential anti-inflammatory and analgesic compounds.
Plants provide abundant secondary metabolites with possible physiological effects in humans [11, 12]. Curcuma longa (C. longa) or turmeric is a plant member of the Zingiberaceae, native to Asia [11, 12]. C. longa consistently displays anti-inflammatory and anti-oxidant properties [11-13]. In particular, C. longa reduced the expression of IL-6, TNF- α and interferon-γ (IFN- γ) in vitro [14], molecules of the inflammatory soup, in vitro. Therefore, in the current study and, for the first time, we characterized the possible anti-inflammatory and anti-nociceptive effects of C. longa in two pharmacodynamic rat models of peripheral inflammation and pain in male and female rats.
2. Materials and Methods
2.1 Animals and housing
Male (n=61) and female (n=62) Wistar rats 2-3 months of age from our local animal facility at the Centro de Investigacion en Reproduccion Animal (CINVESTAV, Mexico) were housed three to four per cage in a room maintained on a 12-hour light/dark cycle with ad libitum access to food and water. Three cohorts of rats were used for our experiments to test biological properties of C. longa. The first cohort was treated with carrageenan, the second cohort with CFA and a third cohort used for open field test (OFT) experiments.
All procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, within the technical guidelines for the production, care and use of animals in the laboratory issued by SAGARPA Mexico (NOM-062 ZOO-1999) and ARRIVE guidelines for the use of animals [15]. All efforts were made to minimize animal suffering. If animals displayed signs of distress prior or during experimentation, they were immediately assessed by a veterinarian and, if necessary, euthanized.
2.2 Baseline mechanical threshold testing
In brief, rats were acclimated to a stainless steel grid within individual Plexiglas boxes (20 x 13 x 10 cm; three white opaque walls and one clear wall) for 60 min, and then tested for baseline (BL) mechanical thresholds with Von Frey monofilaments (Stoelting Inc., IL, USA) [6, 16]. For each animal, the mid-plantar region of the left hind paw was stimulated with an incremental series of eight monofilaments of logarithmic stiffness. The 50% withdrawal threshold was determined using the up-down method of Dixon, modified by Chaplan et al.(1994) [17] . First, an intermediate Von Frey monofilament (number 4.31 exerting 2.0 g of force) was applied perpendicular to the plantar skin, causing a slight bending. If there was a positive response, consisting of a rapid withdrawal of the paw within 6 seconds, a smaller filament was applied. In contrast, a negative response within six seconds resulted in the use of a larger filament. After BL mechanical thresholds were evaluated, paw thickness was determined with a calliper according to the McCarson method [4]. Then, either CFA or carrageenan was injected.
2.3 Administration with complete freund's adjuvant
After BL mechanical threshold and paw thickness evaluation, an intradermal administration with CFA (Sigma, St. Louis, MO; 50 µl) was administered to rats' ventral mid-plantar left hind paw [5, 6]. Mechanical responses, as previously described for BL mechanical threshold testing, and paw oedema were measured on the left hind paw 3 days post-injection.
2.4 Administration with Carrageenan
A 3% carrageenan solution was prepared by dissolving carrageenan (Sigma, C1867-5G) in saline, heating to 37oC and vortexing [5, 6]. The solution was stored at 4oC overnight. After BL measurements, rats received an intradermal injection of the carrageenan solution (3%; 50 µl) into their ventral mid-plantar left hind paw. Mechanical responses and paw oedema were measured on the left hind paw 4 hours post-injection.
2.5 OFT
To dissociate the impact of C. longa on antinociception from sedation, locomotor activity was measured using the OFT. The OFT assessed the total locomotion of the animals after treatment with C. longa. In brief, this test was carried out in a square arena (90*90*45 cm) with no top lid [5]. The arena was divided into 25 equal-size squares with 300 LUX of light intensity.
2.6 Administration of C. Longa
- longa (Ayurveda, Valencia, Spain; batch number: 1016-100g) was administered in rats treated with CFA, carrageenan or undergoing OFT. In rats treated with CFA on day 3 and with carrageenan at 4h, C. longa (100 mg/kg) or vehicle (0.5% of gum tragacanth in 0.1M phosphate-buffer saline pH 7.4) were administered orally using a gavage after performing mechanical threshold testing, as previously described in Vidal et al. (2017) [13]. In brief, oral doses were administered by intragastric gavage at 0.5 mL/kg using a syringe connected to a gavage needle (16-gauge with a ball-shaped tip).
Paw oedema measurement and von Frey filament assessment were performed at 15, 30, 60, 90 and 120 minutes post-treatment. For OFT, rats were first habituated to the behavioral room for 60 min, then injected with either C. longa or vehicle, placed in the arena and recorded for a 60-min period. Total locomotion in the arena was evaluated by a subject blind to the testing condition. The arena was cleaned with ethanol solution (70%) prior and after each trial.
2.7 Statistical methods
The data was analysed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). Mechanical threshold, paw oedema, area under the curve (AUC) data and locomotor activity data were analysed using a two-way ANOVA followed by Sidak’s post-hoc test. In all cases, p < 0.05 was considered statistically significant.
3. Results
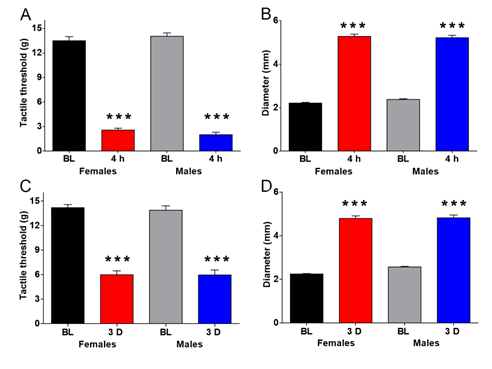
3.1 Carrageenan and CFA decreases mechanical thresholds and increases paw diameter in male and female rats
A significant effect of carrageenan on mechanical thresholds was observed at 4h post-treatment compared to BL (Figure 1A). A significant effect of carrageenan on paw size was observed at 4h post-treatment (Figure 1B). A reduction in mechanical thresholds at 3 days post-CFA treatment compared to BL was observed (Figure 1C). At 3 days post-CFA treatment paw size was also increased (Figure 1D). No differences between sexes were observed.
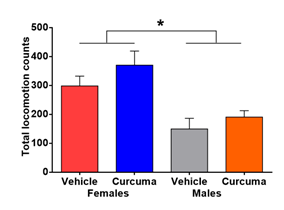
3.2 Curcuma does not modulate locomotor behaviour in rats
- longa does not increase locomotion but there were a difference between sexes was observed with female rats displaying higher locomotion (Figure 2).
Figure 1: Inflamogen administration into the hindpaw induces mechanical allodynia and paw oedema. Carrageenan administration reduced the mechanical thresholds as assessed by two-way ANOVA [Treatment F(1,86)=910.2, p<0.001; Sex F(1, 86)=0.001,p=0.9727; Interaction F(1. 86)=2.158, p=0.1454] (A) and increased the paw size as assessed by two-way ANOVA [Treatment F(1,86)=1407, p<0.001; Sex F(1, 86)=0.4501, p=0.5041; Interaction F(1. 86)=1.971, p=0.1639] (B) in male and female rats. Complete Freund’s adjuvant (CFA) administration resulted in mechanical allodynia [Treatment F(1,88)=260.2, p<0.001; Sex F(1, 88)=0.1148,p=0.7355; Interaction F(1, 88)=0.0838, p=0.7729] (C) and increased the paw size (D) , as assessed by two-way ANOVA [Treatment F(1, 88)=709.9, p<0.001; Sex F(1, 88)=3.860, p=0.0526; Interaction F(1. 88)=2.695, p=0.1042] in male and female rats. The results are presented as mean ± SEM, ***p<0.001, n=22-24 rats per group. BL, baseline; D, days; h, hours.
Figure 2: Effect of C. longa on spontaneous locomotor activity in rats. This figure shows the total locomotion in the arena for 60 min with a difference between sexes in locomotor behaviour [Treatment F(1,28)=2.379, p=0.1342; Sex F(1,28)=20.13, p<0.001; Interaction F(1,28)=0.1809, p=0.6739] . The results are presented as mean ± SEM, n=8 rats for all groups.
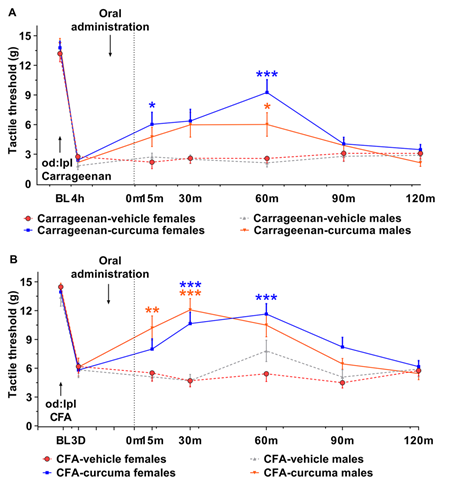
Figure 3: Time-course of the effect of C. longa post inflamogen administration on mechanical allodynia and paw oedema in rats. (A) shows mechanical response threshold at baseline (BL), at 4 h post-carrageenan administration and at 15, 30, 60, 90 and 190 min post C longa administration. A two-wayANOVA analysis showed a significant effects increasing the mechanical thresholds at 15 and 60 minutes post-carrageenan between C. longa and vehicle in females [Treatment F(1,120)=32.34, p<0.001; Time F(5,120)=4.409, p<0.001; Interaction F(5,120)=5.939, p<0.00 ] and males [Treatment F(1,126)=16.34, p<0.001; Time F(5,126)=3.068, p<0.01; Interaction F(5,126)=3.15, p<0.01] with significant difference between C. longa and vehicle at 60 minutes post-carrageenan. (B) depicts the mechanical response threshold at baseline (BL), at 4 h post- Complete Freund’s adjuvant (CFA) administration and at 15, 30, 60, 90 and 190 min post C longa administration. A two-way ANOVA indicated an overall effect in females treated with CFA [Treatment F(1,132)=40.64, p<0.001; Time F(5, 132)=3.036, p<0.01; Interaction F(5, 132)=5.427, p<0.001] with significant difference between C. longa and vehicle at 15 and 60 minutes post-treatment and males [Treatment F(1,120)=27.14, p<0.001; Time F(5, 120)=5.474, p<0.001; Interaction F(5, 120)=5.510, p<0.001] with significant differences between C. longa and vehicle at 30 and 60 minutes post-treatment (B).
The results are presented as mean ± SEM, n=11-12 rats per group. *p<0.05 compared to vehicle-treated female rats, ***p<0. 001 compared to vehicle-treated female rats, *p<0.05 compared to vehicle-treated male rats, **p<0. 01 compared to vehicle-treated male rats, ***p<0. 001 compared to vehicle-treated male rats. BL, baseline.
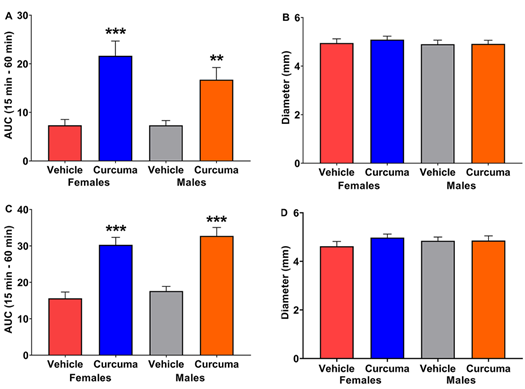
Figure 4: Area under the curve (AUC) of the effect of C. longa post inflamogen administration on mechanical allodynia and paw oedema in rats. (A) presents the AUC for the carrageenan model between 15 and 60 min post C. longa or vehicle administration as shown by two-way ANOVA [Treatment F(1,41)=30.81, p<0.001; Sex F(1,41)=1.329, Interaction F(1,41)=1.315, p=0.2581]. (B) shows the mean diameter of the left hindpaw 4 h post-carrageenan administration and 120 min post C longa administration. (C) shows the AUC for the Complete Freund’s adjuvant (CFA) model between 15 and 60 min post C. longa or vehicle administration as indicated by a two-way ANOVA [Treatment F(1,42)=62.40, p<0.001; Sex F(1, 42)=1.385, p=0.2459; Interaction F(1. 42)=0.01509, p=0.9028]. (D) shows the mean diameter of the left hindpaw 3 days post-CFA administration and 120 min post C longa administration. The results are presented as mean ± SEM, n=11-12 rats per group **p<0.01, ***p<0.001
- longa increased the mechanical thresholds compared to vehicle in carrageenan-treated rats, as assessed by AUC analysis, (Figure 4A). No modifications in paw diameter were observed after C. longa treatment in carrageenan-treated rats (Figure 4B).
In CFA-treated rats, the AUC analysis revealed an effect of C. longa treatment (Figure 4C). No changes in paw diameter were observed after C. longa treatment (Figure 4D).
4. Discussion
As expected, administration of carrageenan or CFA induced oedema and mechanical allodynia ipsilateral to the site of injection. Moreover, this is the first study that evaluates the anti-nociceptive effects of C. longa in two models of peripheral inflammation and pain in male and female rats. Administration of C. longa transiently reversed the inflammation-induced mechanical allodynia in both sexes in rats. First, we evaluated the effect of C. longa, administered at 100 mg/Kg in rats undergoing nociception and locomotor behavioural tests. Previously, Zhu et al. (2014) showed that curcumin dosed at 10-40 mg/Kg, did not affect spontaneous locomotor activity. Similarly, the present study showed no changes in locomotor activity suggesting an analgesic rather than a sedative effect.
The present results show that C. longa displays anti-nociceptive effects on carrageenan- and CFA-induced mechanical allodynia in male and female rats. Previous studies have shown that curcumin, display anti-nociceptive properties in other models of pain. For example, in rats treated with CFA in the joint, curcumin reduced behavioural signs of pain [18]. Acute administration of curcumin also transiently reversed the mechanical allodynia in a male rat model of postoperative pain [19]. A limitation of both studies is they were carried out only in male rodents. The present study show an anti-nociceptive role of C. longa in male and female rats. Moreover,, co-administration of C. longa with Allium hookeri supressed the expression of cytokines namely IFN- γ, IL-1β, IL-6, IL-13 and IL-17, known to regulate inflammation and pain [14]. Therefore, we hypothesize that C. longa induces anti-nociceptive effects by modulating the inflammatory soup.
Most of the studies using preclinical models of pain are usually only run in males although women present higher prevalence of pain [2, 20]. Oddly, no more than 10% of preclinical research is undertaken evaluating sex differences [20]. Preclinical studies show that female rodents have greater sensitivity to pain while male rodents are more sensitive to analgesic compounds [2]. In the present study, we observed that inflamogen induced mechanical allodynia generates a similar response in male and female rats, adding to previously published literature. Moreover, C. longa presented similar anti-nociceptive effects in male and female rats in both the models assayed. In contrast, there is cumulative evidence of pharmaceutical compounds displaying greater benefits in male rats [2]. Our group recently showed that cerebrolysin, a multimodal neuropeptide preparation, was more effective in female rats treated with CFA or carrageenan, suggesting that these models of inflammation-induced pain can be used to determine sex differences [6]. These models will be useful given the need for further research to assess sex differences in the mode of action of bio-pharmaceutical preparations.
The results presented in this study are novel as we are the first to demonstrate analgesic properties of C. longa in two models of inflammatory pain and, importantly, the first to show such analgesic properties in both sexes in rats, which will permit further studies to be conducted in either sex, without concerns of bias. These findings extend our knowledge of the effect of C. longa on pain and inflammation, signalling the need for further clinical studies to confirm translational relevance.
Conflict-of-Interest Statement
The authors have no known conflicts of interest in regards to the materials discussed in this manuscript.
Acknowledgments
JCMM and GF recognize the National Research System of Mexico (CONACyT) for membership. MABC and GGP thanks CONACyT for scholarships (No. 775675 and 2019-000037-02NACF) respectively.. This work was funded by CINVESTAV.
References
- Ji RJ, Nackley A, Huh Y, et al. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129 (2018): 343-366.
- Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nature reviews. Neuroscience (2020).
- Sostres C, Gargallo CJ, Arroyo MT, et al. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best practice & research. Clinical gastroenterology 24 (2010): 121-132.
- McCarson KE. Models of Inflammation: Carrageenan- or Complete Freund's Adjuvant (CFA)-Induced Edema and Hypersensitivity in the Rat. Current protocols in pharmacology 70 (2015): 541-549.
- Iannitti T, Di Cerbo A, Loschi A, et al. Repeated administration of a flavonoid-based formulated extract from citrus peels significantly reduces peripheral inflammation-induced pain in the rat. Food Sci Nutr., In press (2020).
- Morales-Medina JC, Flores G, Vallelunga A, et al. Cerebrolysin improves peripheral inflammatory pain: Sex differences in two models of acute and chronic mechanical hypersensitivity. Drug Dev Res (2019): 513-518.
- Fang JF, Liang Y, Du JY, et al. Transcutaneous electrical nerve stimulation attenuates CFA-induced hyperalgesia and inhibits spinal ERK1/2-COX-2 pathway activation in rats. BMC Complement Altern Med 13 (2013): 134.
- Vinegar R, Schreiber W, et al. Biphasic development of carrageenin edema in rats. The Journal of pharmacology and experimental therapeutics 166 (1969): 96-103.
- Ialenti A, Ianaro A, Moncada S, . et al. Modulation of acute inflammation by endogenous nitric oxide. European journal of pharmacology 211 (1992): 177-182.
- Huber JD, Campos CR, Mark KS, et al. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 290 (2006): H732-H740.
- Flores G. Curcuma longa L. extract improves the cortical neural connectivity during the aging process. Neural regeneration research 12 (2017): 875-880.
- Sun J, Chen F, Braun C, et al. Role of curcumin in the management of pathological pain. Phytomedicine : international journal of phytotherapy and phytopharmacology 48 (2018): 129-140.
- Vidal B, Vazquez-Roque RA, Gnecco D, et al. Curcuma treatment prevents cognitive deficit and alteration of neuronal morphology in the limbic system of aging rats. Synapse 71 (2017).
- Lee SY, Cho SS, Li Y, et al. Anti-inflammatory Effect of Curcuma longa and Allium hookeri Co-treatment via NF-kappaB and COX-2 Pathways. Scientific reports 10 (2020): 5718.
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology 8 (2010): e1000412.
- Morales-Medina JC, Rastogi A, Mintz E, et al. Increased immediate early gene activation in the basolateral amygdala following persistent peripheral inflammation. Neuroreport, In press (2020).
- Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 53 (1994): 55-63.
- Arora R, Kuhad A, Kaur IP, et al. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. European journal of pain 19 (2015): 940-952.
- Zhu Q, Sun Y, Yun X, et al. Antinociceptive effects of curcumin in a rat model of postoperative pain. Scientific reports 4 (2014): 4932.
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature reviews. Neuroscience 13 (2012): 859-866.

 Impact Factor: * 1.1
Impact Factor: * 1.1 Acceptance Rate: 80.20%
Acceptance Rate: 80.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 