Correlation Analysis between Netosis Biomarkers and Peripheral Immune Cells in a Preclinical Model of Ischemic Stroke
Junxiang Yin1, Michael Wu1, Saif Ahmad1, Andrew F Ducruet2, Abdullah S Ahmad1, Michael F Waters1,3*
1Departments of Neurobiology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Dignity Health, Phoenix, AZ 85013, USA
2Departments of Neurosurgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Dignity Health, Phoenix, AZ 85013, USA
3Departments of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Dignity Health, Phoenix, AZ 85013, USA
*Corresponding Author: Michael F Waters, Departments of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Dignity Health, Phoenix, AZ 85013, USA.
Received: 15 September 2022; Accepted: 22 September 2022; Published: 26 October 2022
Article Information
Citation: Junxiang Yin, Michael Wu, Saif Ahmad, Andrew F. Ducruet, Abdullah S. Ahmad, Michael F Waters. Correlation Analysis between Netosis Biomarkers and Peripheral Immune Cells in a Preclinical Model of Ischemic Stroke. Fortune Journal of Rheumatology 4 (2022): 17-24.
View / Download Pdf Share at FacebookAbstract
Neutrophil Extracellular Trap Formation (NETosis) facilitates thrombosis and contributes to reperfusion resistance - a major challenge encountered during the treatment of acute ischemic stroke. The effect of acute stroke on plasma NETosis biomarkers remains unclear. In this study, young adult C57BL/6 Wildtype (WT) mice were subjected to acute brain Ischemia-Reperfusion (IR) injury. The IR-subjected mice exhibited a drastic increase in plasma citrullinated histone 3 (CitH3) and Neutrophil Elastase (NE) on day 1 (p<0.05) while Deoxyribonucleic Acid (DNA) and Myeloperoxidase (MPO) reached their peak levels on day 3. IR-subjected mice also showed a significant increase in peripheral neutrophils and decline in peripheral leukocytes, lymphocytes, and monocytes on day 1 and day 2 (p<0.05). The ratios of neutrophil to lymphocyte, neutrophil to leukocyte, and lymphocyte to monocyte dramatically increased on day 1 (p<0.05). Plasma NE, CitH3 and MPO were positively correlated with peripheral neutrophil and the ratio of neutrophil to lymphocyte, but inversely correlated with peripheral lymphocyte (p<0.05). Our data suggest that there are time dependent changes in plasma NETosis biomarkers, reducing these biomarkers before their peak may offer potential therapeutic options to reduce cerebral infarction and prevent functional deterioration after acute ischemic stroke.
Keywords
<p>Stroke; Ischemic-reperfusion; Neutrophil; Neutrophil extracellular traps (NETs); Citrullinated histone 3 (CitH3); Neutrophil elastase (NE); Myeloperoxidase (MPO)</p>
Article Details
1. Introduction
Stroke is the fifth leading cause of death and the leading cause of serious long-term disability in the United States [1]. One component of stroke pathogenesis is a robust inflammatory response and a trafficking of peripheral immune cells into the ischemic brain post -stroke [2]. Of these peripheral immune cells, neutrophils are the first group to infiltrate ischemic tissue. In the process, neutrophils form and release Neutrophil Extracellular Traps (NETs), web-like DNA structures containing histones and cytotoxic proteins, into the brain parenchyma and within cerebral microvasculature [3-6]. Increasing evidence suggests that NETosis (NET formation) facilitates thrombosis and contributes to reperfusion resistance, a major obstacle encountered during the thrombolytic treatment of Acute Ischemic Stroke (AIS) [7, 8]. In fact, plasma Deoxyribonucleic Acid (DNA), a known marker for NETosis, has been used to predict the mortality and morbidity of patients suffering from acute stroke [8-11]. Thus, approaches to minimize or prevent NETosis may have pro-thrombolytic potential in the treatment of AIS and Acute Myocardial Infarction (AMI) [12-18]. Activated neutrophils release nuclear and granular contents to form NETs comprising of DNA and histones, along with associated neutrophil granule proteins, such as Myeloperoxidase (MPO), Neutrophil Elastase (NE), and peptidyl arginine deiminase type IV (PAD4). When activated by calcium binding, PAD4 deiminates histones inside the nucleus of neutrophils, catalyzing the conversion of positively-charged histone H3 arginine into neutral citrulline. Histone citrullination causes the histones to decondense and promote a rapid version of NET formation that is independent of NADPH oxidase (NOX) and is instead dependent on calcium influx. Histone Citrullination (CitH3) is considered the hallmark of calcium-mediated NOX-independent NETosis. On the other hand, NOX-derived reactive oxygen species (ROS) create an optimal environment for the generation of NE and MPO, two factors that are critical components of NETosis. Both NE and MPO are stored in the primary granules of naïve neutrophils and synergize to promote histone decondensation. Although cell-free DNA does not originate exclusively from activated neutrophils, it is an appealing and acceptable NETosis biomarker and has been used as a surrogate given its reliable, simple, and high-throughput ascertainment.
Recent studies have shown that there are strong relationships between the severity of stroke and the ratios of neutrophil to lymphocyte, neutrophil to leukocyte, and platelet to neutrophil. Changes in these ratios may also be used as an independent predictor of stroke outcomes [19, 20]. How stroke affects NETosis biomarkers in peripheral blood and whether there is a relationship between NETosis biomarkers and cellular ratios remains unclear. The goal of this study is to detail the change in NETosis biomarkers (NE, MPO, DNA, and CitH3) in mouse plasma over time, to characterize the profiles of peripheral blood cells in mice after IR, and analyze the relationship between plasma NETosis biomarkers and peripheral blood cells’ profiles.
2. Materials and Methods
2.1 Animals
Wildtype (WT) C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All experiments involving animals were performed following the protocols in accordance with the Revised Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Barrow Neurological Institute. The study was carried out in compliance with the ARRIVE guidelines. All methods were performed in accordance with the relevant guidelines and regulations.
2.2 Middle cerebral artery occlusion
Adult male mice (aged 12 weeks) were exposed to middle cerebral artery occlusion (MCAO) using an intraluminal filament method as described previously [30]. All surgical manipulations were performed under aseptic conditions. Briefly, mice were anesthetized with 3% isoflurane followed by maintenance with 1.5% isoflurane. Mice were placed in the supine position on a heating pad, and the body temperature was monitored using the small animal anesthesia system (Kent Scientific Cor. FL, USA). Under a stereo dissecting microscope (AmScope, CA, USA), the right Common Carotid Artery (CCA), External Carotid Artery (ECA), and Internal Carotid Artery (ICA) were exposed and isolated. To induce MCAO, a nylon monofilament coated with silicon (Doccol, MA, USA) was introduced into the internal carotid artery through the external carotid artery, then further advanced to the origin of MCA. Successful MCAO was confirmed by >80% decrease in cerebral blood flow monitored by laser doppler flowmeter (MoorVMS-LDF2, Moor Instruments, UK).The incision was sutured, and the mouse was put into a portable animal intensive unit (ThermoCare, CT, USA) to recover from anesthesia. Mice were re-anesthetized and the filament was withdrawn for reperfusion at 60 minutes of MCAO (Ischemia-Reperfusion, IR). The incision was re-sutured, and the mouse was put into a portable animal intensive unit to recover then transferred to the home cage.
2.3 Whole blood cell count
Mice were anesthetized on day 1 (n=8), day 2 (n=16), day 3 (n=9), day 5 (n=5), and day 7 (n=5) after brain IR and whole peripheral blood was collected into a MiniCollect tube (Fisher Scientific, MA, USA). Samples were loaded on Element HT5 hematology analyzer (Heska, CO, USA) for whole blood cell counts.
2.4 Quantification of NETosis biomarkers concentration in plasma
After the blood count, the remaining whole blood was centrifuged at 1500g for 15 minutes at 4°C to isolate and collect plasma. Plasma levels of cell free DNA were measured using a fluorometric assay for double-stranded DNA by Quant-iT PicoGreen dsDNA Assay kit (ThermoFisher, #P11496) following the manufacturer’s instructions. Plasma levels of CitH3, MPO, and NE were determined by ELISA with CitH3 (Clone 11D3) ELISA Kit (Cayman Chemicals, #501620), Mouse MPO Quantikine ELISA Kit (ThermoFisher, #EMMPO), and Mouse Elastase Platinum ELISA (Abcam, #ab252356) according to the manufacturer’s instructions, respectively.
2.5 Statistical analysis
One-way ANOVA with post-hoc Tukey’s test was applied in GraphPad Prism version 9.1 for the data from multiple groups. Pearson’s correlation analysis was used for the relationship between NETosis biomarkers and all parameters of whole blood cell count and the ratios of neutrophils, lymphocytes, and platelets. All data are presented as mean ± SEM. Statistical significance was defined as p < 0.05 for all analyses.
3. Results
3.1 Brain IR affects the profiles of peripheral blood cells
Brain IR significantly affected the profiles of peripheral immune cells. Compared to the control (Ctrl) mice group, total leukocytes [Leu, Ctrl: (6.70±0.62)×103/µl; IR-day1: (3.03±0.32) ×103/ µl, p<0.01; IR-day2: (3.49±0.71) ×103/µl, p<0.01], Lymphocytes [Lym, Ctrl: (5.01±0.44)×103/µl, IR-day1: 1.72±0.30 ×103/µl, p<0.01; IR-day2: 2.41±0.59 ×103/µl, p<0.01], and monocytes [Mon, Ctrl: (0.210±0.033)×103/µl, IR-day1: (0.073±0.015)×103/µl, p<0.05; IR-day2: 0.060±0.017)×103/µl, p<0.05], decreased significantly, while neutrophils [Neu, Ctrl: (1.24±0.13) ×103/µl, IR-day1: (1.56±0.22) ×103/µl, p<0.05; IR-day2: (0.95± 0.13) ×103/µl] dramatically increased on day 1 after IR. From day 3 to day 7, these changes slowly returned to baseline levels (Figure 1). There were no significant changes to the profiles of red blood cells (RBC), hemoglobin (HB, data not shown), or platelets (PLT) after IR.
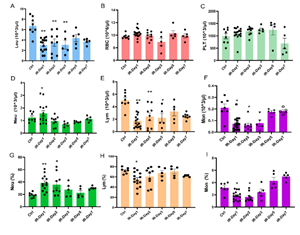
Figure 1: Brain IR affects the profiles of peripheral blood cells. Whole blood was collected from control mice (Ctrl, n=8) and brain IR mice on day 1 (IR-day1, n=16), day 2 (IR-day2, n=9), day 3 (IR-day3, n=5), day 5 (IR-day5, n=5), and day 7 (IR-day7, n=5) after IR, and peripheral blood cells were counted. A-F) represent the absolute values of cells: A)Leukocyte (Leu), B)Red blood cell (RBC), C)Platelet (PLT), D)Neutrophil (Neu), E) Lymphocyte (Lym), F) Monocyte (Mon). G-I) present the relative percentage values within whole blood cells: G) Neutrophil (Neu), H) Lymphocyte (Lym), I) Monocyte (Mon). * p<0.05, ** p<0.01, compared to Ctrl group.
3.2 Brain IR alters the ratios of peripheral blood cells with time
Stroke-associated immunosuppression and inflammation are increasingly recognized as factors that facilitate infections and potentially influence outcomes after stroke. Recent studies demonstrated that elevated neutrophil to lymphocyte ratio is a significant predictor of worse outcomes in patients with ischemic stroke [19]. In the present study, the data show that the ratios of neutrophil to lymphocyte [Neu/Lym, Ctrl: 0.25±0.02, IR-day1: 0.74±0.13 (p<0.01 vs Ctrl); IR-day2: 0.75 ±0.21], neutrophil to leukocyte [Neu/Leu, Ctrl: 0.19±0.01, IR-day1: 0.46±0.05 (p<0.01 vs Ctrl); IR-day2: 0.36±0.06], lymphocyte to monocyte [Lym/Mon, Ctrl: 22.8±1.3, IR-day1: 34.4±5.4 (p<0.05 vs Ctrl), IR-day2: 39.6±7.5], and platelet to lymphocyte [PLT/Lym, Ctrl: 13.1±1.2, IR-day1: 20.9±1.9 (p<0.01 vs Ctrl); IR-day2: 21.9±3.0] dramatically increased, while the ratio of platelet to neutrophil [PLT/Neu, Ctrl: 56.6±9.4, IR-day1: 33.2±4.0 (p<0.05 vs Ctrl), IR-day2: 40.4±6.4] significantly decreased at day 1 and day 2 after IR (Figure 2.). These ratios trended towards baseline on day 3.
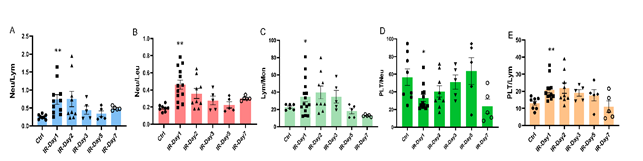
Figure 2: Brain IR alters the ratios of peripheral blood cells with time. The ratios of neutrophil to lymphocyte (Neu/Lym), neutrophil to leukocyte (Neu/Leu), lymphocyte to monocyte (Lym/Mon), platelet to neutrophil (PLT/Neu), and platelet to lymphocyte (PLT/Lym) were analyzed based on the values of whole blood cell count and their relative percentage. A) The ratio of Neu/Lym, B) The ratio of Neu/Leu, C) The ratio of Lym/Mon, D) The ratio of PLT/Neu, E) The ratio of PLT/Lym. * p<0.05, ** p<0.01 compared to Ctrl group.
3.3 Brain IR increases plasma NETosis biomarkers
Four NETosis biomarkers (CitH3, NE, dsDNA, and MPO) in plasma were tested using commercial ELISA kits according to manufacturer protocols. Interestingly, the biomarkers exhibited their peaks at different time points after IR. The concentrations of plasma CitH3 (IR-day1: 0.437±0.035 ng/ml, Ctrl: 0.298±0.022 ng/ml, p<0.05) and plasma NE (IR-day1: 17.02±0.86 ng/ml, Ctrl: 11.37±0.85 ng/ml, p<0.05) significantly increased on day 1, while the concentration of plasma MPO (IR-day2: 4.86±0.14 ng/ml, Ctrl: 4.45±0.13 ng/ml) reached its peak on day 2 and the concentration of plasma dsDNA (IR-day3: 1077.0±21.1 ng/ml, Ctrl: 953.7±25.8 ng/ml) reached its peak on day 3. These elevations returned close to Ctrl levels by day 5 (CitH3: 0.323±0.021 ng/ml, NE: 15.36±1.53 ng/ml, dsDNA: 942.3±60.9 ng/ml, and MPO: 4.26±0.23 ng/ml) (Figure 3.).
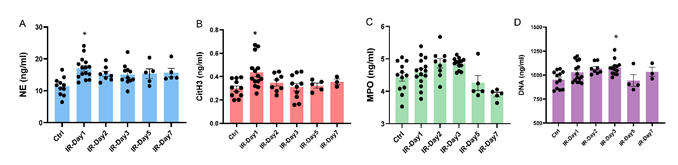
Figure 3: Brain IR increases plasma NETosis biomarkers. Plasma was collected from Ctrl and IR mice, plasma levels of Neutrophil Elastase (NE), Citrullinated Histone H3 (CitH3), Myeloperoxidase (MPO), and double stranded deoxyribonucleic acid (dsDNA) were measured using commercial kits following the manufacturer’s instructions. A) Plasma NE concentration, B) Plasma CitH3 concentration, C) Plasma dsDNA concentration, and D) Plasma MPO concentration. * p<0.05 compared to Ctrl group.
3.4 Brain IR affects correlation between NETosis biomarkers and peripheral immune cells
Pearson’s correlation analysis was used for the relationship between NETosis biomarkers and peripheral immune cell counts. The concentration of plasma NE was positively related with neutrophil percentage (r=0.356, p<0.01), and inversely correlated with lymphocyte percentage (r=-0.325, p<0.05, Figure 4. A, B). The concentration of plasma CitH3 was positively correlated with neutrophil percentage (r=0.310, p<0.05), and inversely correlated with leukocytes count (r=-0.311, p<0.05, Figure 4. C, D). The concentration of plasma MPO was positively correlated with neutrophils percentage (r=0.325, p<0.05), and inversely related with lymphocyte percentage (r=-0.304, p<0.05, Figure 4. E, F).
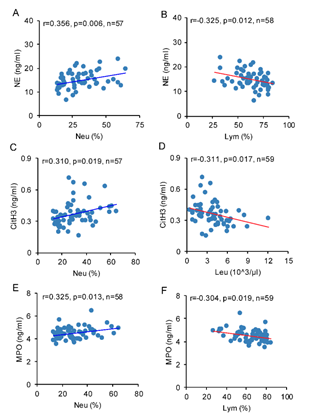
Figure 4: Brain IR affects correlation between NETosis biomarkers and peripheral immune cells. Pearson correlation analysis was used to identify the relationship between NETosis biomarkers and peripheral immune cell counts. The concentration of plasma NE was correlated with A) Neu (%) and B) Lym (%). The concentration of plasma CitH3 was correlated with C) Neu (%), D) Leu. The concentration of plasma MPO was correlated with E) Neu (%) and F) Lym (%).
3.5 Brain IR affects correlation between NETosis biomarkers and peripheral immune cell ratios
Pearson’s correlation analysis was used for the relationship between NETosis biomarkers and peripheral immune cell ratios. The concentration of plasma NE was positively related with the ratios of neutrophil to lymphocyte (r=0.332, p <0.05), and neutrophil to leukocyte (r=0.377, p<0.01, Figure 5. A, B). The concentration of plasma CitH3 was positively correlated with the ratio of neutrophil to lymphocyte (r=0.311, p <0.05), and inversely correlated with the ratio of platelet to neutrophil (r=-0.270, p<0.05, Figure 5. C, D). The concentration of plasma MPO was positively correlated with the ratios of neutrophil to leukocyte (r=0.365, p<0.01) and platelet to lymphocyte (r=0.366, p<0.01, Figure 5. E, F).
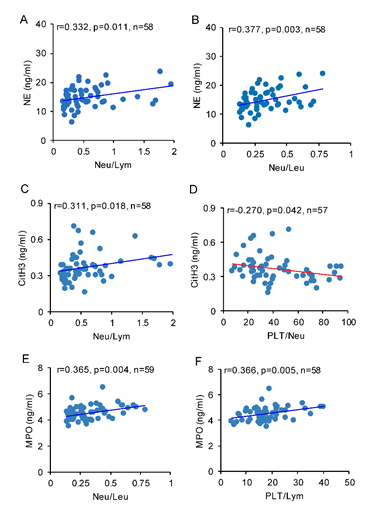
Figure 5: Brain IR affects correlation between NETosis biomarkers and peripheral immune cell ratios. Pearson correlation analysis was used to identify the relationship between NETosis biomarkers and peripheral immune cell ratios. The concentration of plasma NE was correlated with A) Neu/Lym and B) Neu/Leu. The concentration of plasma CitH3 was correlated with C) Neu/Lym and D) PLT/Neu. The concentration of plasma MPO was correlated with E) Neu/Leu and F) PLT/Lym.
4. Discussion
Ischemic stroke is a major public health disease with high cost, social burden, and incidence worldwide. Intravenous tissue plasminogen activator and endovascular mechanical thrombectomy are the only FDA approved treatments for AIS, and there remains a large subset of stroke survivors suffering from profound long-term impairment and poor prognosis. Reperfusion resistance exacerbated by impaired microvascular perfusion continues to be a major challenge encountered with thrombolytic treatment of AIS [7, 8]. Recent evidence suggests that NETosis facilitates thrombosis and increases reperfusion resistance [7]. Moreover, prevention of NETosis is reported to have pro-thrombolytic potential in the treatment of AIS [12-18]. Details regarding the precise evolution of various post stroke NETosis biomarkers and their implication and potential effect on cerebral infarct expansion and aggravation of functional deficits are still not fully understood. In cerebral ischemia, neutrophils are the first group of cells to infiltrate the damaged tissue and undergo NETosis in the brain parenchyma and microvasculature [3-6]. In this study, the time course of NETosis biomarkers in mouse plasma is reported. The data show that four NETosis plasma biomarkers (CitH3, NE, dsDNA, and MPO) increased after stroke. CitH3 and NE reached their respective peaks at 24 hours while dsDNA and MPO peaked at 3 days. All plasma NETosis biomarkers levels exhibited resolution from peak during our seven day timeline reaching their respective baselines by day 5 post stroke. A previous mouse study reported that the level of CitH3 protein in brain tissue reached its peak around 3 to 5 days after stroke [21]. The delayed peak of CitH3 observed in the previous study could be due to the fact that it employed a distal MCAO model that results in smaller infarction and lower severity of BBB impairment. Moreover, there may be an interval between neutrophils infarct infiltration and their ongoing NETosis and CitH3 release in brain. However, such a relationship between NETosis biomarker peaks in peripheral blood and brain parenchyma remains to be tested. Previous human data showed that the maximum lesion volume was reached at day 3 after stroke onset, plateaued at day 4, and then slowly decreased starting on day 5 [22]. This human data along with our and others’ preclinical data suggests that the detection and tracking of plasma NETosis biomarkers may be a promising strategy for optimizing NETosis –based therapies aimed at mitigating cerebral infarction progression.
NE and MPO are both released from neutrophils and can facilitate histone decondensation and exacerbate NETosis. A recent study reported that a neutrophil elastase inhibitor (Agaphelin) exhibited a neuroprotective effect by reducing infarct volume, improving neurological function and decreasing mortality following ischemic stroke in mice [23]. The antithrombotic effects, promotion of brain tissue survival, and inhibition of thromboinflammation identifies neutrophil elastase inhibitors as another potential treatment option for ischemic stroke. MPO plasma concentration in patients suffering from acute cerebral ischemia has also shown positive correlation with stroke severity and worse outcome [24]. Our study, along with this additional data suggests potential strategies for early intervention of NETosis with efficacy monitoring via plasma NET biomarkers consequently resulting in better outcomes after AIS. Evidence shows that neutrophil counts increase after AIS and higher circulating neutrophil counts are independently associated with higher mortality rates and worse outcomes in patients with AIS [20, 21, 24]. However, it is not fully understood how the profiles of peripheral blood cells change with time after stroke. To address these key questions, whole blood cell counts were assessed at multiple time points after ischemia-reperfusion in this study. It was found that neutrophils dramatically increased while leukocytes, lymphocytes, and monocytes significantly decreased on day 1 and day 2 after AIS. Our finding is further supported by another MCAO study in which an increase in leukocyte levels was observed within 15 minutes of reperfusion and the levels decreased significantly at 24-hours post-reperfusion[25]. Interestingly, human patients are presented with higher leukocytes at the time of admission and these levels remain elevated for at least the first 24-hours after AIS [20]. This discrepancy in leukocytes level between the preclinical models and clinical observation at 24-hours is possible because of the percentage of neutrophils in peripheral WBCs, generally 20-30% in mice [26] and 50-70% in humans. Therapeutic interventions, such as tPA or endovascular thrombectomy, may also modify leukocyte profiles in the first 24-hours post-stroke [20, 27]. However, a comprehensive study is required to fully understand the discrepancies found in human and preclinical stroke studies. In our study, the data show that peripheral blood cell profiles gradually returned to their basal level by day 7 post stroke. Interestingly, the correlation analysis showed a significant, positive relationship between the concentrations of CitH3, NE, and MPO in mice plasma and neutrophil counts, while a significant inverse correlation was observed between the concentrations of plasma NETosis biomarkers (NE, CitH3, and MPO) with leukocyte and lymphocyte counts. This data suggest that higher plasma CitH3, NE, and MPO may be positively associated with worse outcomes in mice; however, further investigation is needed to confirm this hypothesis and its clinical implication.
Recently, the changes in neutrophil, lymphocyte, and platelet ratios as well as their relationship with the stroke neurologic score, mortality rate, and overall post-stroke functional outcomes has attracted much attention [20, 24, 28, 29]. A higher neutrophil to lymphocyte ratio was associated with increased stroke severity and worse outcomes [24]. We observed a significant increase in neutrophil to lymphocyte ratios from day 1 to day 3, and an increase in platelet to lymphocyte ratios at day 1. Correlations between neurological function scores and changes in the ratios of neutrophil to lymphocyte and platelet to lymphocyte were also reported [20]. Several studies have demonstrated that an elevated neutrophil to lymphocyte ratio may be an independent predictor for stroke outcomes [19, 20, 24, 28, 29]. As expected, the data from this study shows that the ratios of neutrophil to lymphocyte, neutrophil to leukocyte, lymphocyte to monocyte, and platelet to lymphocyte dramatically increased, while the ratio of platelet to lymphocyte significantly decreased on day 1 and day 2 in mice after AIS. Importantly, there was a significant positive correlation between the concentration of plasma NETosis biomarkers (CitH3, NE, and MPO) and neutrophil to lymphocyte ratio. Conversely, there was an inverse correlation between the concentration of plasma NETosis biomarkers (CitH3 and NE) and platelet to neutrophil ratio. Whether there are similar relationships between the concentrations of NETosis biomarkers and the ratios of these cells in human patients with AIS remains to be investigated.
5. Conclusions
Taken together, there were significant changes in NETosis biomarkers in mice plasma after stroke, and there were relationships between the concentrations of NETosis biomarkers (CitH3, NE, and MPO) in mice plasma and peripheral immune cell counts (leukocyte, neutrophil, and lymphocyte), as well as between NETosis biomarkers concentrations and neutrophil to lymphocyte ratio. These data suggest that NETosis biomarkers (CitH3, NE, and MPO) could be potential therapeutic targets for AIS treatment. Further investigation is needed to better understand whether the evolution of plasma NETosis biomarkers correlate with NETosis-based therapies to mitigate brain infarction and improve functional outcomes.
Author Contributions
Conceptualization, Y.J., A.S.A, and W.F.M; methodology, Y.J., A.S.A, and W.M.; validation, Y.J., A.S.A, and W.M.; formal analysis, Y.J., and W.M; investigation, Y.J., A.S.A, and W.M.; resources, W.F.M.; data curation, Y.J., A.S.A, and W.M.; writing—original draft preparation, Y.J.; writing—review and editing, A.S.A, W.M. A.S, and D.A; visualization, Y.J., and A.S.A.; supervision. W.F.M.; funding acquisition, A.S.A. and W.F.M. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This research was funded by a grant from the Barrow Neurological Foundation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 141 (2020): e139-e596.
- Li Y, Wang Y, Yao Y, et al. Systematic Study of the Immune Components after Ischemic Stroke Using CyTOF Techniques. J Immunol Res 2020 (2020): 9132410.
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 303 (2004): 1532-1535.
- Yuen J, Pluthero FG, Douda DN, et al. NETosing Neutrophils Activate Complement both on Their Own NETs and Bacteria via Alternative and Non-alternative Pathways. Front Immunol 7 (2016): 137.
- Sollberger G, Tilley DO, Zychlinsky A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Dev Cell 44 (2018): 542-553.
- Yousefi S, Stojkov D, Germic N, et al. Untangling "NETosis" from NETs. Eur J Immunol 49 (2019): 221-227.
- Kim S W, Lee JK. Role of HMGB1 in the Interplay between NETosis and Thrombosis in Ischemic Stroke: A Review. Cells 9 (2020).
- Novotny J, Oberdieck P, Titova A, et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 94 (2020): e2346-e2360.
- Rainer TH, Wong LK, Lam W, et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem 49 (2003): 562-569.
- Bang OY, Chung JW, Cho YH, et al. Circulating DNAs, a Marker of Neutrophil Extracellular Traposis and Cancer-Related Stroke: The OASIS-Cancer Study. Stroke 50 (2019): 2944-2947.
- Tsai NW, Lin TK, Chen SD, et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta 412 (2011): 476-479.
- Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 82 (2017): 223-232.
- Hamam HJ, Khan MA, Palaniyar N. Histone Acetylation Promotes Neutrophil Extracellular Trap Formation. Biomolecules 9 (2019).
- Neeli I, Radic M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol 4 (2013): 38.
- Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207 (2010): 1853-1862.
- Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184 (2009): 205-213.
- Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180 (2008): 1895-1902.
- Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA 110 (2013): 8674-8679.
- Giede-Jeppe A, Bobinger T, Gerner ST, et al. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor for In-Hospital Mortality in Spontaneous Intracerebral Hemorrhage. Cerebrovasc Dis 44 (2017): 26-34.
- Komurcu HF, Gozke E, Dogan Ak P, et al. Changes in neutrophil, lymphocyte, platelet ratios and their relationship with NIHSS after rtPA and/or thrombectomy in ischemic stroke. J Stroke Cerebrovasc Dis 29 (2020): 105004.
- Kang L, Yu H, Yang X, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 11 (2020): 2488.
- Lansberg MG, O'Brien MW, Tong DC, et al. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol 58 (2001): 613-617.
- Leinweber J, Mizurini DM, Francischetti IMB, et al. Elastase inhibitor agaphelin protects from acute ischemic stroke in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Brain Behav Immun 93 (2021): 288-298.
- Maestrini I, Tagzirt M, Gautier S, et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: Cohort study. Neurology 95 (2020): e97-e108.
- Morrison H, McKee D, Ritter L. Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol Res Nurs 13 (2011): 154-163.
- O'Connell KE, Mikkola AM, Stepanek AM, et al. Practical murine hematopathology: a comparative review and implications for research. Comp Med 65 (2015): 96-113.
- Semerano A, Laredo C, Zhao Y, et al. Leukocytes, Collateral Circulation, and Reperfusion in Ischemic Stroke Patients Treated With Mechanical Thrombectomy. Stroke 50 (2019): 3456-3464.
- Bi Y, Shen J, Chen SC, et al. Prognostic value of neutrophil to lymphocyte ratio in acute ischemic stroke after reperfusion therapy. Sci Rep 11 (2021): 6177.
- Ferro D, Matias M, Neto J, et al. Neutrophil-to-Lymphocyte Ratio Predicts Cerebral Edema and Clinical Worsening Early After Reperfusion Therapy in Stroke. Stroke 52 (2021): 859-867.
- Yin J, Han P, Tang Z, et al. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab 35 (2015): 1783-1789.

 Impact Factor: * 1.7
Impact Factor: * 1.7 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 