CT and DXA Bone Fragility Evaluation after Bariatric Surgery
Marine Fauny1,2*, Marion Halin1, Edem Allado3,4, Laurent Brunaud5,6, Claire Nomine-Criqui5,6, Eliane Albuisson7,8, Isabelle Chary-Valckenaere1,9, Didier Quilliot5,10, Damien Loeuille1,9
1Department of Rheumatology, University Hospital, Nancy, France
2Department of Rheumatology, Saint Charles Hospital, Toul, France
3CHRU-Nancy, University Center of Sports Medicine and Adapted Physical Activity, F-54000, Nancy, France
4Université de Lorraine, DevAH, F-54000 Nancy, France
5Inserm UMRS 1256 N-GERE (Nutrition-Genetics-Environmental Risks) - University de Lorraine, Faculty of Medicine, Nancy, France
6Department of Gastrointestinal, Visceral, Metabolic, and Cancer Surgery (CVMC), University Hospital, Nancy, France
7University of Lorraine, CNRS, IECL, F-54000 Nancy, France
8DRCI, MPI Department, Methodology Unit, Data Management and Statistics UMDS, University Hospital Nancy, Nancy, France
9IMoPA. UMR 7365 CNRS –University of Lorraine
10Department of Endocrinology Diabetology and Nutrition, University Hospital, Nancy, France
*Corresponding Author: Marine Fauny, Department of Rheumatology, University Hospital Nancy, 5 rue du Morvan 54500 VANDOEUVRE les Nancy, France.
Received: 22 August 2025; Accepted: 28 August 2025; Published: 05 September 2025.
Article Information
Citation: Marine Fauny, Marion Halin, Edem Allado, Laurent Brunaud, Claire Nomine-Criqui, Eliane Albuisson, Isabelle Chary-Valckenaere, Didier Quilliot, Damien Loeuille. CT and DXA Bone Fragility Evaluation after Bariatric Surgery. Fortune Journal of Rheumatology 7 (2025): 32-40.
View / Download Pdf Share at FacebookAbstract
Objectives: The objectives were to determine the prevalence of bone fragility 3 years (±6 months) after bariatric surgery via DXA and computed tomography (CT) in patients with obesity; to identify the risk factors for the bone fragility development (bone mineral density [BMD] ≤-2SD or a scanographic bone attenuation coefficient of the first lumbar vertebra (SBAC-L1) ≤145 Hounsfield units (HU)) and to compare the results obtained via CT and DXA. Methods: This descriptive study included patients with obesity who underwent bariatric surgery and DXA and CT before and 3 years (±6 months) after bariatric surgery. Results: Among the 44 included patients, 84.1% were women, with a mean age of 53.9 years (±10.7). After 3 years, there was a greater prevalence of osteoporosis (p=0.002), the postsurgery T-scores were significantly lower (p<0.001) than those at baseline. The SBAC-L1 was significantly lower after surgery than before surgery (p=0.008). According to multivariate analysis, no risk factor was significantly associated with the development of bone fragility at 3 years. The correlation between CT and DXA results was positive and moderate to strong (0.53 to 0.63). Conclusion: There was a significantly greater prevalence of osteoporosis and a lower SBAC-L1 3 years after bariatric surgery than before.
Keywords
<p>Obesity; Bariatric surgery; Bone mineral density (BMD); DXA; Computed tomography (CT); Osteoporosis.</p>
Article Details
1. Introduction
Obesity has become a global public health priority due to its increasing prevalence and risk of comorbid conditions, including diabetes, cardiovascular disease and several types of cancers. In addition, it affects quality of life and life expectancy [1]. Bariatric surgery has become the most successful treatment for patients who have failed to experience supervised medical weight loss [2]. In patients with morbid obesity (body mass index [BMI] ≥ 40) or BMI ≥ 35 kg/m2 and comorbidities (diabetes, hypertension, osteoarthritis, obstructive sleep apnea), bariatric surgery is presently considered to be an effective therapy [3]. Despite multiple clinical benefits, a number of surgical and gastrointestinal complications can occur following bariatric procedures [4]. Among the complications, nutritional deficiencies, a consequence of reduced intake and/or malabsorption of nutrients and metabolic bone disease that leads to osteoporosis and osteoporotic fracture, warrant careful consideration [5]. The pathophysiology of bone disease in patients with obesity is multifactorial, ranging from inadequate nutrition due to chronic dieting practices to a lack of physical activity and increased sequestration of vitamin D within adipocytes. Bariatric surgery and the associated major weight loss can impact bone metabolism and induce significant changes, such as decreased mechanical loading, calcium/vitamin D malabsorption with secondary hyperparathyroidism, nutritional deprivation, changes in fat mass and alterations in fat-, bone- and gut-derived hormones, and can lead to increased bone resorption with a pronounced reduction in the degree of bone mineralization [4-9 ]. Bariatric surgery is also associated with an increased risk of fractures [10, 11]. An assessment of fracture risk is recommended before the first bariatric surgery procedure (for RYGB and biliopancreatic diversion and in patients at high risk of fracture, regardless of age, and in all menopausal women and all men ≥ 50 years old, regardless of the type of bariatric surgical procedure) [12]. Anti-osteoporosis treatment is indicated for menopausal women and men ≥ 50 years old with no history of fracture and a T-score ≤ -2 [12]. The objective of this study was to determine the prevalence of bone fragility 3 years (±6 months) after bariatric surgery via dual X-ray absorptiometry (DXA) and computed tomography (CT) in patients with obesity. The secondary objectives were to identify the risk factors for the development of bone fragility (bone mineral density [BMD] ≤ -2 SD at any site or a scanographic bone attenuation coefficient of the first lumbar vertebra (SBAC-L1) ≤145 Hounsfield units (HU)) and to compare the results derived from computed tomography (CT) to those derived from dual X-ray absorptiometry (DXA).
2. Materials and methods
2.1 Population
This descriptive study included patients with obesity who underwent bariatric surgery at our specialized obesity center between January 2014 and December 2019 [13]. Patients who underwent DXA and CT, performed at the same center during routine follow-up, before and at 3 years (±6 months) after bariatric surgery were included. The exclusion criteria were surgery for gastric banding and ring ablation [13]. Demographic and anthropometric data (age, sex, smoking and alcohol consumption habits, body height and weight to calculate BMI), vitamin D status (vitamin D deficiency was defined as a level less than 30 ng/mL), and comorbidities (diabetes and cardiovascular risk factors) were collected from complete medical records [13].
2.2 DXA evaluation
Patients were included if they underwent preoperative DXA and one postoperative DXA 3 years (±6 months) after bariatric surgery. The preoperative DXA closest to the date of surgery was retained. All DXA measurements were performed on a Lunar Prodigy densitometer (Advance PA +301010, Encore, version 14.10.022; Madison, WI, 53718, USA). BMD and T-score at the lumbar spine, femoral neck and total hip; data on lean and fat mass and their distributions were assessed for each patient [13]. A diagnosis of osteoporosis was defined as a T-score ≤ − 2.5 SD at any measured location. Osteopenia was defined as -2.5 SD < T-score ≤ -1 SD [14].
2.3 CT evaluation
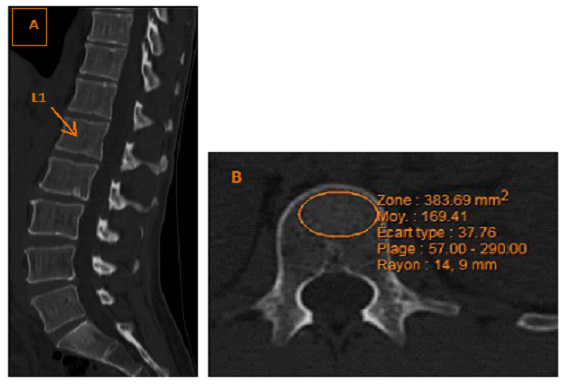
Patients were included if they underwent preoperative CT and one postoperative CT 3 years (±6 months) after bariatric surgery. CT was retained if it included the first lumbar vertebra (L1). The preoperative CT closest to the date of surgery was retained. The CT images were analyzed on a Synapse Mobility Web, V.6.0, 2016, FUJIFILM Medical Systems U.S.A., Inc. We also collected the technical CT details. On axial sections in the bone window, the scanographic bone attenuation coefficient of L1 (SBAC-L1) was measured in Hounsfield units (HU) (Figure 1), blinded to the clinical data, in trabecular bone thanks to a region of interest (ROI). The intra- and interreader reliability of the SBAC-L1measure was previously evaluated (kappa > 0.9) [15]. In the case of fracture or discovertebral damage of L1 with osteosclerosis of the vertebral endplate, SBAC was measured on the adjacent vertebrae using CT data acquired similarly from T12 to L5 [16]. A threshold of 145 HU was used [16].
2.4 Ethics approval
All of the data used were obtained from medical records, collected during routine follow-up. This study is registered with the Information Technology and Freedoms Commission for our University Hospital and on Clinicaltrials.gov (file number: 2019PI216) and was designed in accordance with the general ethical principles outlined in the Declaration of Helsinki. The protocol of this study was approved by the Information Technology and Freedoms Commission for our University Hospital. All patients provided their consent for the use of their medical data from the time they received medical care at the University Hospital.
2.5 Statistical analysis
Both descriptive and comparative analyses were conducted by accounting for the nature and distribution of the variables. Qualitative variables are described as frequencies and percentages; quantitative variables are reported as the mean and standard deviation (SD) or as the median and interquartile range (IQR). The Kolmogorov–Smirnov test showed that among the continuous demographic and clinical variables, only age, height, and weight followed a normal distribution. Comparative analyses were conducted in accordance with the nature of the variable and its distribution. The test is specified in the legend of each table. Logistic regression was performed to identify the variables significantly associated with the appearance of a DXA ≤ -2 SD for at least one measurement site at 3 years (Table 5) or an SBAC-L1 ≤ 145 HU on CT at 3 years. Significant results (from univariate and multivariate analyses) are presented as odds ratios (ORs) and 95% confidence intervals (CIs). All the analyses were adjusted based on the duration between the two DXA or CT exams. To study the correlation between CT and DXA results, the Pearson coefficients were calculated. The significance level was set at 0.05 for all statistical analyses. IBM SPSS Statistics v23 was used for the data analysis.
3. Results
3.1 Population
The characteristics of the 44 patients, who underwent DXA and CT before and at 3 years (±6 months) after bariatric surgery, are described in Table 1. The mean age was 53.9 (±10.7) years, and the majority of the patients were women (84.1%). The mean BMI before the surgery was 46.9 (±8.8). Eight patients (18.2%) underwent sleeve gastrectomy, and 36 (81.8%) underwent gastric bypass (GBP). On DXA, before surgery, 5 patients (11.4%) had osteopenia for at least one measurement site, and one (2.3%) had osteoporosis for at least one measurement site. Two patients (4.5%) had a T-score ≤ -2 SD at baseline. On CT, the mean SBAC-L1 was 183.2 (±60.1) HU, and 11 patients (4.8%) had an SBAC-L1 under the fracture threshold of 145 HU.
3.2 Comparison of the included patients’ characteristics before and 3 years after bariatric surgery (Table 2)
At 3 years after bariatric surgery, patients had significant decreases in weight and BMI (p<0.001), lean mass and fat mass, the android/gynoid fat-mass ratio, and visceral adipose tissue (VAT) (p<0.001 for each variable). Regarding DXA results, there was a significant increase in the prevalence of osteoporosis for at least one measurement site: 2.3% before surgery and 9.1% after surgery (p=0.002). BMD and T-scores were significantly lower for the femoral neck and hip (p<0.001), and more patients had osteopenia at the femoral neck or hip (p=0.002 and 0.004, respectively). Regarding measurements taken at the spine, only the T-score was significantly lower 3 years after bariatric surgery (p=0.0001). On CT, the SBAC-L1 was significantly lower at 3 years (p=0.008): 183.2 HU before surgery and 164.8 HU after surgery.
3.3 DXA evaluation (Tables 3 and 5)
At baseline, only 2 patients (4.5%) had a T-score ≤ -2 SD. Among the 42 patients with a T-score > -2 SD at baseline, 37 (88.1%) maintained a T-score > -2 SD 3 years after bariatric surgery, and 5 (11.9%) had a T-score ≤ -2 SD. Patients who had a T-score ≤ -2 SD at 3 years after bariatric surgery had a significantly lower weight (p=0.004), lower fat mass (p=0.005) and a lower SBAC-L1 (p=0.015) at baseline, before surgery. According to the multivariate analysis (Table 5), there was no statistically significant difference between the 2 groups.
3.4 CT evaluation (Table 4)
At baseline, 11 patients (25%) had an SBAC-L1 ≤ 145 HU. Among the 33 patients with an SBAC-L1 > 145 HU at baseline, 27 (81.8) maintained an SBAC-L1 > 145 HU, and 6 (18.2) had an SBAC-L1 ≤ 145 HU 3 years after bariatric surgery. None demographic variable or DXA measurement was significantly associated with an SBAC-L1 ≤ 145 HU 3 years after bariatric surgery. According to these results, no multivariate analysis was performed.
3.5 Comparison of the 2 methods of bone fragility evaluation
The 2 patients with a DXA ≤ -2 SD at baseline also had an SBAC-L1 ≤ 145 HU at baseline. Among the 11 patients with an SBAC-L1 ≤ 145 HU at baseline, 2 also had a DXA ≤ -2 SD after bariatric surgery. Only one patient with a DXA > -2 SD and SBAC-L1 > 145 HU at baseline had a DXA ≤ -2 SD and SBAC-L1 ≤ 145 HU 3 years after bariatric surgery. The correlation coefficient was 0.53 between the femoral neck T-score and SBAC-L1 before surgery and 0.63 after surgery. The correlation was positive, significant and moderate to strong. For the spine T-score, the correlation with the SBAC-L1 was positive and poor to moderate (0.42 before surgery and 0.51 after surgery).
Table 1: Characteristics of the patients included at baseline (n=44)
|
Demographical data |
|
|
Age |
53.9 (10.7) |
|
Sex (women) |
37 (84.1) |
|
Weight (kg) |
126.5 (26.1) |
|
Height (cm) |
164.2 (7.2) |
|
BMI (g/cm2) |
46.9 (8.8) |
|
Diabetes |
24 (54.5) |
|
Cardiovascular risk factors |
35 (79.5) |
|
Tobacco use |
21 (47.7) |
|
Alcohol consumption |
1 (2.3) |
|
Vitamin D deficiency |
42 (95.5) |
|
Type of surgery |
|
|
GBP |
36 (81.8) |
|
Sleeve |
8 (18.2) |
|
CT |
|
|
SBAC-L1 (HU) |
183.2 (60.1) |
|
SBAC-L1 ≤ 145 HU |
11 (4.8) |
|
DXA |
|
|
Osteoporosis for at least one measurement site |
1 (2.3) |
|
Osteopenia for at least one measurement site |
5 (11.4) |
|
Femoral neck |
|
|
BMD (g/cm2) |
1.095 (0.166) |
|
T-score |
0.9 (1.4) |
|
Osteoporosis |
0 (0) |
|
Osteopenia |
3 (6.8) |
|
Hip |
|
|
BMD (g/cm2) |
1.160 (0.162) |
|
T-score |
1.3 (1.4) |
|
Osteoporosis |
0 (0) |
|
Osteopenia |
2 (4.5) |
|
Spine |
|
|
BMD (g/cm2) |
1.286 (0.188) |
|
T-score |
1.0 (1.6) |
|
Osteoporosis |
1 (2.3) |
|
Osteopenia |
4 (9.1) |
|
Body composition |
|
|
Lean mass (kg) |
57.1 (9.9) |
|
Fat mass (kg) |
65.5 (17.4) |
|
Android fat mass/Gynoid fat mass |
1.6 (0.5) |
|
VAT (cm3) |
3101.0 (1307.5) |
The data are presented as the n (%) for dichotomous variables, the mean (SD) for continuous demographic variables with a normal distribution and the median (interquartile range) for variables with a nonnormal distribution. ALM/H2: appendicular lean mass adjusted to height; ALM/W: appendicular lean mass adjusted to body weight; BMD: bone mineral density; BMI: body mass index; DXA: dual-energy X-ray absorptiometry; VAT: visceral adipose tissue. The percentage was calculated based on the available data for each variable. Osteoporosis was defined by a T-score ≤ −2.5 SD at any measured location, and osteopenia was defined by −2.5 SD < T-score ≤ −1 SD. Vitamin D deficiency was defined as a concentration less than 30 ng/mL.
Table 2: Comparison of the included patients’ characteristics before and at 3 years (±6 months) after bariatric surgery (n=44)
|
BEFORE |
3 years AFTER |
p value |
|
|
Demographical data |
|||
|
Weight (kg) |
126.5 (26.1) |
84.8 (26.3) |
<0.001 |
|
BMI (g/cm2) |
46.9 (8.8) |
31.4 (9.2) |
<0.001 |
|
DXA |
|||
|
Osteoporosis for at least one measurement site |
1 (2.3) |
4 (9.1) |
0.002 |
|
Osteopenia for at least one measurement site |
5 (11.4) |
13 (29.5) |
0.25 |
|
Femoral neck |
|||
|
BMD (g/cm2) |
1.095 (0.166) |
0.943 (0.165) |
<0.001 |
|
T-score |
0.9 (1.4) |
-0.4 (1.4) |
0.0001 |
|
Osteoporosis |
0 (0) |
3 (6.8) |
/ |
|
Osteopenia |
3 (6.8) |
12 (27.3) |
0.002 |
|
Hip |
|||
|
BMD (g/cm2) |
1.160 (0.162) |
0.973 (0.190) |
0.0001 |
|
T-score |
1.3 (1.4) |
-0.3 (1.6) |
0.0001 |
|
Osteoporosis |
1 (2.3) |
3 (6.8) |
0.500 |
|
Osteopenia |
4 (9.1) |
12 (27.3) |
0.004 |
|
Spine |
|||
|
BMD (g/cm2) |
1.286 (0.188) |
1.185 (0.209) |
0.259 |
|
T-score |
1.0 (1.6) |
0 (1.8) |
0.0001 |
|
Osteoporosis |
1 (2.3) |
4 (9.1) |
0.500 |
|
Osteopenia |
4 (9.1) |
8 (18.2) |
0.063 |
|
Body composition |
|||
|
Lean mass (kg) |
57.1 (9.9) |
47.6 (9.8) |
<0.001 |
|
Fat mass (kg) |
65.5 (17.4) |
34.8 (17.2) |
<0.001 |
|
Android fat mass/Gynoid fat mass |
1.6 (0.5) |
1.4 (0.5) |
<0.001 |
|
VAT (cm3) |
3101.0 (1307.5) |
1130.9 (906.7) |
0.0001 |
|
CT |
|||
|
SBAC-L1 (HU) |
183.2 (60.1) |
164.8 (44.4) |
0.008 |
|
SBAC-L1 ≤ 145 HU |
11 (4.8) |
14 (6.2) |
0.508 |
The data are presented as the n (%) for dichotomous variables, the mean (SD) for continuous demographic variables with a normal distribution and the median (interquartile range) for variables with a nonnormal distribution. ALM/H2: appendicular lean mass adjusted to height; ALM/W: appendicular lean mass adjusted to body weight; BMD: bone mineral density; BMI: body mass index; DXA: dual-energy X-ray absorptiometry; VAT: visceral adipose tissue. The percentage was calculated based on the available data for each variable. Osteoporosis was defined by a T-score ≤ −2.5 SD at any measured location, and osteopenia was defined by −2.5 SD < T-score ≤ −1 SD. Vitamin D deficiency was defined as a concentration less than 30 ng/mL. The results in bold are statistically significant (p<0.05).
For comparisons of the data before and 3 years after surgery, paired Student’s t tests were used for variables with a normal distribution, and Wilcoxon signed rank tests were used for other continuous variables. For qualitative variables, the McNemar test was used.
Table 3: 3-years postsurgery (± 6 months) characteristics of the patients with a T-score > -2 SD on DXA at baseline (before bariatric surgery) (n=42)
|
T-score > -2 SD at 3 years |
T-score £ -2 SD at 3 years |
p value |
|
|
Demographical data |
N=37 |
N=5 |
|
|
Age |
53.7 (10.4) |
57.6 (7.3) |
0.071 |
|
Sex (women) |
31 (83.8) |
4 (80) |
0.618 |
|
Weight before surgery (kg) |
130.4 (26.3) |
110.8 (10.5) |
0.004 |
|
Weight after surgery (kg) |
88.0 (24.0) |
69.5 (17.6) |
0.204 |
|
BMI before surgery (g/cm2) |
46.7 [11.6] |
40.8 [3.8] |
0.055 |
|
BMI after surgery (g/cm2) |
29.4 [11.1] |
24.9 [11.5] |
0.253 |
|
D BMI |
15.5 (7.2) |
15.8 (7.4) |
0.938 |
|
Diabetes |
22 (59.5) |
2 (40) |
0.361 |
|
Cardiovascular risk factors |
31 (83.8) |
3 (60) |
0.673 |
|
Tobacco use |
18 (48.6) |
2 (40) |
0.547 |
|
Alcohol consumption |
0 (0) |
0 (0) |
/ |
|
Vitamin D deficiency |
35 (94.6) |
5 (100) |
0.774 |
|
Type of surgery |
|||
|
GBP |
29 (78.4) |
5 (100) |
0.327 |
|
Sleeve |
8 (21.6) |
0 (0) |
/ |
|
DXA (before surgery) |
|||
|
Lean mass (kg) |
58.4 (9.7) |
52.5 (9.1) |
0.239 |
|
Fat mass (kg) |
67.6 (18.2) |
55.8 (5.5) |
0.005 |
|
Android fat mass/Gynoid fat mass |
1.7 (0.5) |
1.5 (0.8) |
0.649 |
|
VAT (cm3) |
3190.1 (1372.8) |
2970.8 (1221.0) |
0.992 |
|
CT (before surgery) |
|||
|
SBAC-L1 (HU) |
191.2 (59.4) |
147.8 (54.0) |
0.015 |
|
SBAC-L1 ≤ 145 HU |
7 (18.9) |
2 (40) |
0.057 |
The data are presented as the n (%) for dichotomous variables, the mean (SD) for continuous demographic variables with a normal distribution and the median (interquartile range) for variables with a nonnormal distribution. ALM/H2: appendicular lean mass adjusted to height; ALM/W: appendicular lean mass adjusted to body weight; BMD: bone mineral density; BMI: body mass index; DXA: dual-energy X-ray absorptiometry; VAT: visceral adipose tissue. The percentage was calculated based on the available data for each variable. Osteoporosis was defined by a T-score ≤ −2.5 SD at any measured location, and osteopenia was defined by −2.5 SD < T-score ≤ −1 SD. Vitamin D deficiency was defined as a concentration less than 30 ng/mL. p value: Logistic regression was performed to identify the variables significantly associated with the binary outcome of a T-score ≤ -2 SD. The results in bold are statistically significant (p<0.05). Paired Student’s t tests were used for variables with a normal distribution, and Mann-Whitney tests were used for other continuous variables. For qualitative variables, Fisher’s exact test was used.
Table 4: 3-years-postsurgery (± 6 months) characteristics of patients with an SBAC-L1 > 145 HU at baseline (before bariatric surgery) (n=33)
|
SBAC-L1 > 145 HU |
SBAC-L1 £ 145 HU |
p value |
|
|
Demographical data |
N = 27 |
N=6 |
|
|
Age |
52.4 (10.6) |
55.3 (8.7) |
0.498 |
|
Sex (women) |
24 (88.9) |
5 (83.3) |
0.798 |
|
Weight before surgery (kg) |
126.9 (23.5) |
122.6 (25.2) |
0.855 |
|
Weight after surgery (kg) |
82.8 (23.9) |
80.8 (26.2) |
0.978 |
|
BMI before surgery (g/cm2) |
46.7 [9.0] |
41.7 [9.5] |
0.803 |
|
BMI after surgery (g/cm2) |
28.9 [10.9] |
27.7 [6.9] |
0.91 |
|
D BMI |
16.5 (7.5) |
15.4 (3.9) |
0.757 |
|
Diabetes |
13 (48.1) |
4 (66.7) |
0.358 |
|
Cardiovascular risk factors |
20 (74.1) |
6 (100) |
0.208 |
|
Tobacco use |
12 (44.4) |
4 (66.7) |
0.298 |
|
Alcohol consumption |
0 (0) |
0 (0) |
/ |
|
Vitamin D deficiency |
26 (96.3) |
6 (100) |
0.818 |
|
Type of surgery |
|||
|
GBP |
22 (81.5) |
5 (83.3) |
0.705 |
|
Sleeve |
5 (18.5) |
1 (16.7) |
|
|
DXA (before surgery) |
|||
|
Osteoporosis for at least one measurement site |
0 (0) |
0 (0) |
NA |
|
Osteopenia for at least one measurement site |
1 (3.0) |
0 (0) |
0.818 |
|
T-score ≤ -2 SD for at least one measurement site |
0 (0) |
0 (0) |
0.335 |
|
Lean mass (kg) |
56.4 (7.5) |
55.6 (12.4) |
0.891 |
|
Fat mass (kg) |
65.0 (16.9) |
63.1 (16.5) |
0.805 |
|
Android fat mass/Gynoid fat mass |
1.7 (0.5) |
1.8 (0.4) |
0.518 |
|
VAT (cm3) |
3049.3 (1348.8) |
2646.6 (1387.3) |
0.582 |
The data are presented as the n (%) for dichotomous variables, the mean (SD) for continuous demographic variables with a normal distribution and the median (interquartile range) for variables with a nonnormal distribution. ALM/H2: appendicular lean mass adjusted to height; ALM/W: appendicular lean mass adjusted to body weight; BMD: bone mineral density; BMI: body mass index; DXA: dual-energy X-ray absorptiometry; VAT: visceral adipose tissue. The percentage was calculated based on the available data for each variable. Osteoporosis was defined by a T-score ≤ −2.5 SD at any measured location, and osteopenia was defined by −2.5 SD < T-score ≤ −1 SD. Vitamin D deficiency was defined as a concentration less than 30 ng/mL. p value: Logistic regression was performed to identify the variables significantly associated with the binary outcome of SBAC-L1 ≤ 145 HU. The results in bold are statistically significant (p<0.05). Paired Student’s t tests were used for variables with a normal distribution, and Mann-Whitney tests were used for other continuous variables. For qualitative variables, Fisher’s exact test was used.
Table 5: Multivariate analysis for DXA (threshold: T-score ≤ -2 SD)
|
T-score > -2 SD at 3 years |
T-score £ -2 SD at 3 years |
Multivariate analysis |
|
|
N=37 |
N=5 |
||
|
Demographical data |
OR (95%CI) |
||
|
Age |
53.7 (10.4) |
57.6 (7.3) |
0.969 [0.81-1.16] |
|
BMI before surgery (g/cm2) |
46.7 [11.6] |
40.8 [3.8] |
0.751 [0.45-1.27] |
|
DXA (before surgery) |
|||
|
Fat mass (kg) |
67.6 (18.2) |
55.8 (5.5) |
0.97 [0.74-1.29] |
|
CT (before surgery) |
|||
|
SBAC-L1 (HU) |
191.2 (59.4) |
147.8 (54.0) |
0.96 [0.91-1.01] |
The data are presented as the n (%) for dichotomous variables, the mean (SD) for continuous demographic variables with a normal distribution and the median (interquartile range) for variables with a nonnormal distribution. BMD: bone mineral density; BMI: body mass index; DXA: dual-energy X-ray absorptiometry. The percentage was calculated based on the available data for each variable. Osteoporosis was defined by a T-score ≤ −2.5 SD at any measured location, and osteopenia was defined by −2.5 SD < T-score ≤ −1 SD. p value: Logistic regression was performed to identify the variables significantly associated with the binary outcome of a T-score ≤ -2 SD. The results in bold are statistically significant (p<0.05). The results are adjusted for time between DXA and surgery.
4. Discussion
This is the first study in which bone fragility was evaluated on both DXA and CT 3 years after bariatric surgery in patients with obesity. Before bariatric surgery, 4.8% of the patients had an SBAC-L1 ≤ 145 HU, 2.3% had osteoporosis on DXA, and 4.5% had low bone mass at least one measurement site. The prevalence of osteoporosis varies from 1.8% to 8% according to the literature [17, 18], but the prevalence of low bone mass observed in this study was lower than that reported in the literature: 29 to 51.6% [17, 19, 20]. Regarding the CT results, with SBAC-L1, the prevalence of bone fragility tended to be equivalent to that of low bone mass fragility, and these results suggest an underestimation of bone risk in patients with obesity through DXA. Three years after bariatric surgery, the BMI was significantly lower than that before surgery, the ratio of android/gynoid fat mass improved, and there were more patients with osteoporosis on DXA (9.1%; p=0.002). The T-scores at the 3 sites and SBAC-L1 were significantly lower than those before surgery, in accordance with previous studies [5, 21-26].
Current management of patients with obesity who performed bariatric surgery should be geared toward bone loss prevention and nutritional deficiency correction. Pharmacological treatments should be considered for high-risk patients or patients with fractures without traumatism [12]. Bone loss following bariatric surgery is multifactorial, with malabsorption, high-turnover bone loss and an increase of bone marrow adipose tissue [5, 23, 26, 27]. The risk factors associated with the development of a T-score ≤ -2 SD at least one measurement site at 3 years after bariatric surgery were lower weight, lower fat mass and a lower SBAC-L1 at baseline. However, there was no association in the multivariate analysis. None demographic or DXA data was significantly associated with an SBAC-L1 ≤ 145 HU at 3 years after bariatric surgery in patients with an SBAC-L1 >145 HU at baseline. According to these results, no multivariate analysis was performed. A limitation of this study is the lack of information about fractures and menopausal status. Indeed, the large percentage of female patients (84.1%) and the mean age of our population (53.9 years) could influence the results. The number of included patients was small, generating a lack of power, which could explain the lack of results in the univariate and multivariate analyses. Due to the small sample size, our study did not permit comparisons of bone evolution according to the type of bariatric surgery. Previous studies [28-30] have shown that malabsorptive and mixed procedures have an increased risk of fracture compared with their nonsurgical counterparts, while restrictive procedures alone do not increase the incidence of fracture.
The strength of this study was the evaluation of bone fragility with both DXA and CT for all patients. CT allows the avoidance of cortical bone and osteoarthritis, especially on the spine, in patients with obesity, and the SBAC-L1 measurement is less influenced by body fat. The threshold of 145 HU was used because it allowed the best compromise between sensitivity and specificity in a general population [16], but no study has been previously conducted in this specific population of patients with obesity. Moreover, one advantage of CT compared to DXA is its ability to accurately identify unsuspected osteoporotic vertebral fractures, which are clearly indicative of osteoporosis independent of the patient’s DXA T-score. DXA also has several limitations, such as reduced photon penetration through soft tissues [31]. Thus, BMD measured by DXA increases with BMI. Fat mass variation during follow-up could influence the DXA measurements, with an underestimation of bone fragility for patients with elevated fat mass. DXA remains the gold standard examination tool for osteoporosis screening, but it may not be the most reliable examination tool in patients with obesity [32, 33]. Another strength was the evaluation at 3 years post-surgery; because the amount of weight lost peaked after the 2-year follow-up and was relatively stable after this time point [34], we can consider weight stabilization to have occurred at 3 years. In previous studies, 2 years after bariatric surgery, we also found a significant increase in the prevalence of osteoporosis on DXA (0.9% before surgery vs. 3.6% after surgery, p=0.0001) and a significantly lower SBAC-L1 (196.2 HU before surgery vs. 189.2 HU after surgery) [35, Fauny M. Halin M, Allado E, et al. DXA evaluation of bone fragility 2 years after bariatric surgery in patients with obesity [preprint]]. Risk factor evaluation, especially through the use of a T-score threshold of -2 SD, allows to develop a post-surgery bone fragility prevention plan for patients at greater risk at baseline and to prevent fracture with possible treatment [12].
In conclusion, there was a significantly greater prevalence of osteoporosis and a lower SBAC-L1 3 years after bariatric surgery. According to multivariate analysis, nondemographic, CT or DXA data was significantly associated with an SBAC-L1 ≤ 145 HU or a T-score ≤ -2 SD 3 years after bariatric surgery. The correlation between CT and DXA was positive and moderate to strong.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interest statement
All authors have no conflicts of interest
Acknowledgments
None
Conflict of interest
All authors have no conflicts of interest
Funding
None
References
- Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet 387 (2016): 1947-1956.
- Borisenko O, Colpan Z, Dillemans B, et al. Clinical Indications, Utilization, and Funding of Bariatric Surgery in Europe. Obes Surg 25 (2015): 1408-1416.
- Stahl JM, Malhotra S. Obesity Surgery Indications And Contraindications. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2021).
- Lupoli R, Lembo E, Saldalamacchia G, et . Bariatric surgery and long-term nutritional issues. World J Diabetes 8 (2017): 464-474.
- Jammah AA. Endocrine and Metabolic Complications After Bariatric Surgery. Saudi J Gastroenterol 21 (2015): 269-277.
- Mieczkowska A, Irwin N, Flatt PR, et al. Glucose-dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone 56 (2013): 337-342.
- Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int 25 (2014): 423-439.
- Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 16 (2014): 1204-1213.
- Costa TM Da RL, Paganoto M, Radominski RB, et al. Impact of deficient nutrition in bone mass after bariatric surgery. Arq Bras Cir Dig 29 (2016): 38-42.
- Nakamura KM, Haglind EGC, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int 25 (2014): 151-158.
- Dix CF, Bauer JD, Wright ORL. A Systematic Review: Vitamin D Status and Sleeve Gastrectomy. Obes Surg 27 (2017): 215-225.
- Paccou J, Genser L, Lespessailles É, et al. French recommendations on the prevention and treatment of osteoporosis secondary to bariatric surgery. Joint Bone Spine 89 (2022): 105443.
- Fauny M, Halin M, Allado E, et al. DXA evaluation of bone fragility 2 years after bariatric surgery in patients with obesity. Bone Rep 22 (2024): 101782.
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843 (1994): 1-129.
- Fauny M, Bauer E, Albuisson E, et al. Vertebral fracture prevalence and measurement of the scanographic bone attenuation coefficient on CT-scan in patients with systemic sclerosis. Rheumatol Int 38 (2018): 1901-1910.
- Pickhardt PJ, Pooler BD, Lauder T, et al. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158 (2013): 588-595.
- Greco E A, Fornari R, Rossi F, et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int. J. Clin. Pract 64 (2010): 817-820.
- Hsu Y-H, A Venners S, A Terwedow H, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am. J. Clin. Nutr 83 (2006): 146-154.
- Premaor M O, Pilbrow L, Tonkin C, et al. Obesity and fractures in postmenopausal women. J. Bone Miner. Res 25 (2010): 292-297.
- Oldroyd A, Mitchell K, Bukhari M. The prevalence of osteoporosis in an older population with very high body mass index: Evidence for an association. Int. J. Clin. Pract 68 (2014): 771-774.
- Ko B-J, Myung SK, Cho K-H, et al. Relationship Between Bariatric Surgery and Bone Mineral Density: a Meta-analysis. Obes Surg 26 (2016): 1414-1421.
- Paccou J, Caiazzo R, Lespessailles E, et al. Bariatric Surgery and Osteoporosis. Calcif Tissue Int 110 (2022): 576-591.
- Krez AN, Stein EM. The Skeletal Consequences of Bariatric Surgery. Curr Osteoporos Rep 18 (2020): 262-272.
- von Mach MA, Stoeckli R, Bilz S, et al. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism 53 (2004): 918-921.
- Pugnale N, Giusti V, Suter M, et al. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric banding in obese pre-menopausal women. Int J Obes Relat Metab Disord 27 (2003): 110-116.
- Mele C, Caputo M, Ferrero A, et al. Bone Response to Weight Loss Following Bariatric Surgery. Front Endocrinol (Lausanne) 13 (2022): 921353.
- Ambrosi TH, Scialdone A, Graja A, et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 20 (2017): 771-784.
- Zhang Q, Dong J, Zhou D, et al. Comparative risk of fracture for bariatric procedures in patients with obesity: A systematic review and Bayesian network meta-analysis. Int J Surg 75 (2020): 13-23.
- Khalid SI, Omotosho PA, Spagnoli A, et al. Association of Bariatric Surgery With Risk of Fracture in Patients With Severe Obesity. JAMA Netw Open 3 (2020): 207419.
- Ahlin S, Peltonen M, Sjöholm K, et al. Fracture risk after three bariatric surgery procedures in Swedish obese subjects: up to 26 years follow-up of a controlled intervention study. J Intern Med 287 (2020): 546-557.
- Yu E W. Bone metabolism after bariatric surgery. J. Bone Miner. Res 29 (2014): 1507-1518.
- Oldroyd A, Mitchell K, Bukhari M. The prevalence of osteoporosis in an older population with very high body mass index: Evidence for an association. Int. J. Clin. Pract 68 (2014): 771-774.
- Lespessailles E, Paccou J, Javier R-M, et al. Obesity, Bariatric Surgery, and Fractures. J. Clin. Endocrinol. Metab 104 (2019): 4756-4768.
- O'Brien PE, Hindle A, Brennan L, et al. Long-Term Outcomes After Bariatric Surgery: a Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes Surg 29 (2019): 3-14.
- Fauny M, Halin M, Allado E, et al. CT evaluation of bone fragility 2 years after bariatric surgery: an observational study. J Bone Miner Metab 41 (2023): 105-112

 Impact Factor: * 1.7
Impact Factor: * 1.7 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 