Different Dosing Regimens of Rituximab Biosimilar in Rheumatoid Arthritis: A Comparative Analysis of Disease Activity Control, B-cell Depletion and Adverse Effect Profile
Syamasis Bandyopadhyay, Aheli Ghosh Dastidar*, Sandip Kumar Chandra
Department of Internal Medicine, Apollo Multispeciality Hospitals, Kolkata, India
*Corresponding Author: Dr Aheli Ghosh Dastidar, Post-graduate Trainee, Department of Internal Medicine, Apollo Multispecialty Hospitals, Kolkata, India
Received: 07 October 2025; Accepted: 15 October 2025; Published: 27 October 2025
Article Information
Citation: Syamasis Bandyopadhyay, Aheli Ghosh Dastidar, Sandip Kumar Chandra. Different Aosing Regimens of Rituximab Biosimilar in Rheumatoid Arthritis: A Comparative Analysis of Disease Activity Control, B-cell Depletion and Adverse Effect Profile. Fortune Journal of Rheumatology 7 (2025): 41-50.
View / Download Pdf Share at FacebookAbstract
Introduction: Rituximab is an established biologic DMARD for refractory rheumatoid arthritis (RA), but its high cost restricts access in low- and middle-income countries. Biosimilar rituximab (bRTX) provides a more affordable option with proven efficacy and safety. While reduced-dose regimens have shown non-inferiority to the standard regimen in originator RTX, real-world evidence on bRTX in India is limited. This study compares clinical outcomes and B-cell depletion with two bRTX dosing strategies in RA. Methods: We retrospectively analysed bDMARD-naïve RA patients with inadequate response to conventional DMARDs who chose to receive either 1000 mg × 2 or 500 mg × 2 doses of bRTX. Disease activity (DAS28-ESR/CRP), ACR50 responses, and CD19+ B-cell counts were assessed at baseline and 12 months. Between-group comparisons were performed using Student’s t- test. Results: The mean DAS28-CRP decreased from 5.23 ± 0.10 to 3.62 ± 0.44 in the 1000 mg group and from 5.24 ± 0.09 to 3.65 ± 0.59 in the 500 mg group. ACR50 response rates were 84.6% and 78.6% in the standard- and reduced-dose groups, respectively, with no significant difference in disease activity reduction (p > 0.05). CD19+ counts (/uL) fell from 1222 to 127 in the 1000 mg group and from 1223to 134 in the 500 mg group. Conclusion: Both standard and reduced bRTX regimens achieved comparable clinical efficacy and B-cell depletion over 12 months. The 500 mg regimen may represent an economically-viable alternative for RA management in resource-constrained settings.
Keywords
Rituximab, Biosimilar, Rheumatoid Arthritis, CD19, DAS28, ACR response.
Rituximab articles; Biosimilar articles; Rheumatoid Arthritis articles; CD19 articles; DAS28 articles; ACR response articles
Article Details
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune condition that remains challenging to manage universally, more so in resource limited countries. Both prevalence and incidence of RA are on the rise globally [1]. Effective management of RA demands early diagnosis and initiation of disease-modifying antirheumatic drugs (DMARDs) with a treat-to-target approach to achieve remission or low disease activity, thereby optimizing long-term clinical outcomes [2-5].
Treatment for RA has evolved from salicylates through non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, conventional disease-modifying antirheumatic drugs (cDMARDs), to latest biologicals and targeted synthetic DMARDs. The American College of Rheumatology (ACR) guideline recommends biological or targeted synthetic DMARDs (tsDMARD) when patients show suboptimal response to cDMARDs like methotrexate [4]. A range of biological DMARDs have been approved for treating RA, and they include tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, golimumab, certolizumab pegol), T-cell costimulatory inhibitor (abatacept), interleukin (IL)-6 receptor inhibitors (tocilizumab, sarilumab), and anti-CD20 antibody (rituximab) [4]. With the introduction of biologics, the cumulative cost of RA therapy worldwide has gone up substantially [6]. In emerging economies like India, high cost of such therapies restricts their early and effective usage thereby hindering optimum disease control. The requirement of long-term treatment with biologicals in RA imposes a significant financial burden. In this context biosimilars offer a cost-effective option for managing RA, not only in India but also in countries with similar demography [7]. The expiration of RTX patents and the advent of biosimilars have improved access to this therapy, offering cost-effective alternatives with comparable efficacy and safety [8,9]. Biosimilars to biologicals are approved for use provided their safety, purity, and potency are similar to original or reference drug [10,11]. Rituximab (RTX) is a genetically engineered chimeric anti-CD20 monoclonal antibody, approved for treatment of RA [12]. The European Medicines Agency (EMA) and the United States of America Food and Drug Administration (US-FDA) have approved a few rituximab biosimilars for treating RA [8]. A systematic review and meta-analysis of biosimilar Rituximab (bRTX) in RA and non-Hodgkin’s lymphoma patients established the clinical response and safety [13]. In India, the central drug control organisation headed by drugs controller general of India has approved one such biosimilar developed by Hetero and subsequently marketed by Zydus Life sciences ltd as VortuxiTM for treatment of RA [14]. Rituximab acts by targeting CD20, a surface transmembrane protein marker expressed on B cells throughout their differentiation from pre-B cell till plasma cell stage [16] RTX depletes pre-B cells and mature B cells sparing the stem cells, pro B cells, plasma cells and plasmablasts.[10] RTX therapy has been shown to reduce B cells in peripheral blood and brings a variable decline in bone marrow and synovial B cell population [11,12]. Nakou et al demonstrated a substantial reduction of CD19+B cells, along with a significant reduction in the activated CD19+HLA-DR+subset in both peripheral blood and bone marrow with RTX therapy [17] This decrease in CD 19 B cells was followed by a clinical response in RA patients [17] Failure of peripheral blood CD19 B-cell depletion has been observed in non-responders to RTX therapy [15] B cell depletion following Rituximab therapy has been shown to be predictive of clinical response in RA [17] Some reports have also explored role of B cell repletion in predicting relapse in RA [16] B cell repopulation was found to precede clinical relapse by around 4 months in the study reported by Trouvin et al [15] In our centre, CD19 cell counts are routinely assessed at baseline and at specific intervals following initiation of bRTX therapy, as an aid to assess B-cell depletion which helps to estimate the optimal timing of subsequent dosing. RTX is conventionally administered as a 1000 mg intravenous infusion on days 1 and 15. (Rituxan_prescribing.Pdf, n.d.) [18] But several trials have shown comparable disease control with two doses of 500mg RTX given 14 days apart [19-22] The CERRERA collaboration combining data from 12 European registries demonstrated a comparable efficacy of 500mg x 2 dosing regimens with 1000mg x2 regimen of Rituximab [23]. However, evidence supporting lower dosing regimen of bRTX in RA is currently lacking. Utilizing a lower, yet efficacious dose may potentially confer a more favourable adverse effect profile while also improving economic sustainability at both the individual and national levels. This retrospective real-world comparative study examines the impact of conventional and low doses of bRTX on RA disease severity in Indian clinical practice and compares B cell depletion levels (measured by absolute CD19 cell count) between both regimes as a molecular marker of effectivity.
Methods
This retrospective work was done on a cohort of RA patients attending rheumatology outpatient of a tertiary care hospital in eastern India. Prior ethical approval was obtained from the institutional ethics committee (IEC/BR/2025/01/06). Adult biological-naive RA patients meeting EULAR 2010 criteria, with inadequate response to conventional DMARDs who had voluntarily opted for either 1000 mg x 2 or 500mg x 2 of bRTX, after considering all potential therapeutic options were eligible for this analysis. In situations where disease control remains suboptimal despite ongoing therapy, it is standard practice in our institute to engage in a comprehensive discussion with the patient and their family regarding all available alternative or additional therapeutic options. This includes a detailed review of each agent—whether conventional, biological, or targeted synthetic DMARDs—addressing factors such as cost, established efficacy, dosing regimens, and potential adverse effects, therapy duration. Such discussions are integral to a shared decision-making process prior to the initiation of any new therapeutic intervention. The same was followed in these patients before initiation of Rituximb therapy. After consideration of stated factors those patients who chose to receive either 500mg or 1000mg were included for the analysis. Consistent with usual care, before Rituximab therapy initiation, patients were screened for Hepatitis B, hepatitis C, HIV positivity, low IgG, IgM serum levels and cytopenias (TLC <4000 /cumm, HB <8 g/dL, platelet < 1lakh/cumm). Patients with history of recent hospitalisation within 3 months, pregnant women, female patients unwilling to be on appropriate contraception, patients with history of malignancy and known ischaemic heart disease especially with heart failure (EF <40%) were excluded as per routine extant clinical practice. Patients were administered intravenous infusions of two doses of either 1000 mg or 500 mg of bRTX with standard premedication and monitoring 15 days apart. Any infusion related side effects were recorded and appropriately dealt with. All patients continued cDMARD with their biological treatment and were followed up in OPD as per schedule. Data of 27 RA patients on regular OPD follow-up from October 2023 to October 2024, receiving two doses of either 500mg or 1000mg of bRTX, was retrieved from patient records system. Patient data were compiled in Microsoft Excel, encompassing sociodemographic variables, clinical disease activity parameters (tender joint counts, swollen joint counts, physician and patient global assessment scores, visual analogue score for pain), and laboratory investigations (ESR, CRP, Complete Blood Count and CD19 cell count at baseline and after 12 months of treatment). Any adverse events reported by the patients while on bRTX therapy or the clinician were also recorded.
Statistical Methods
Baseline comparability of both groups of patients (receiving either 1000mg or 500mg) was confirmed. To account for residual imbalances, linear regression/ANOVA was performed adjusted for age, baseline DAS28, etc. No statistically significant differences in baseline characteristics between two groups were found. For accommodation of non-normal data non-parametric tests like Mann-Whitney U tests and median difference were applied. Bonferroni or false discovery rate (FDR) corrections were applied for multiple comparisons. Data of changes in CD19 count, DAS28-ESR (Disease Activity Score, Erythrocyte sedimentation rate) and DAS28-CRP scores CRP scores (C-reactive protein), ACR 50 responses at baseline and after 12 months of therapy across both groups of patients were compared using Student’s t test. Microsoft Excel statistical package and Python software was used for data analysis. Correlation between DAS28 ESR and Absolute CD 19 cell count was calculated using Pearson’s correlation method.
Results
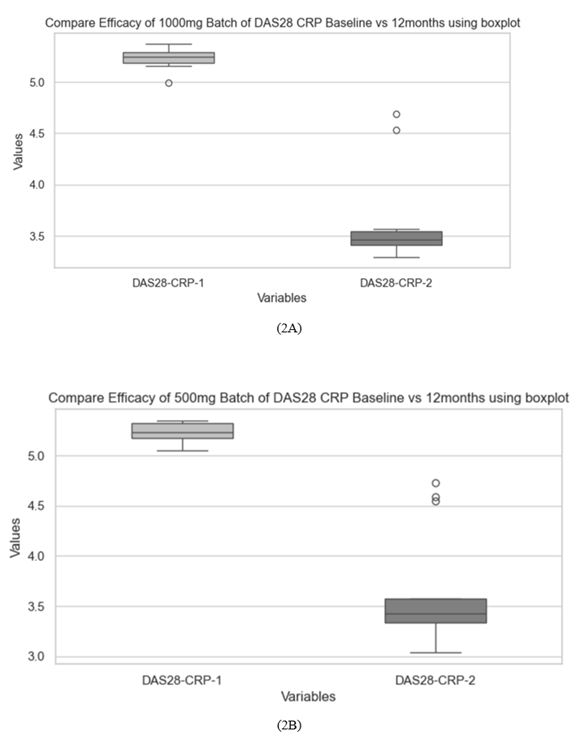
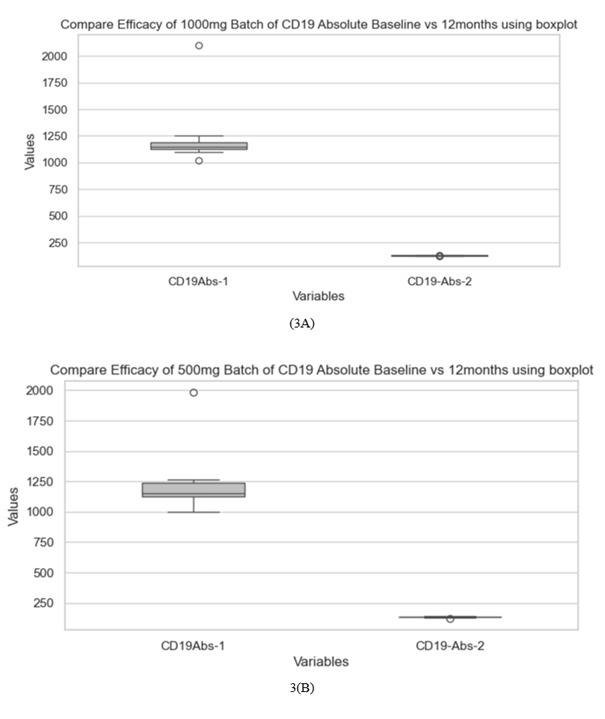
Twenty-seven RA patients with inadequate response to conventional DMARDs who opted for bRTX were included. 13 of them opted for 2 doses of 1000mg and 14 chose to receive 2 doses of 500mg of the drug, two weeks apart. These patients remained on varying combinations of methotrexate (MTX), hydroxychloroquine (HCQS) and sulfasalazine (SSZ)), as tolerated throughout bRTX therapy period. Their mean age was 40.9 ±4.83 years, 17 (62.96%) of them were females. The mean disease duration was 6.44 ± 1.44 years. Demographic characteristics were similar across both groups. (table 1) Baseline laboratory parameters and disease severity indices were comparable in both groups of patients receiving either 1000mg or 500mg bRTX. (table 2, table 3) (figure 1). Treatment with both doses of bRTX resulted in satisfactory clinical response as seen by substantial reduction in DAS28-ESR and DAS28-CRP scores at 12 months of therapy compared to baseline (table 2) (figure 2, figure 1). 84.61% patients in the 1000mg bRTX group achieved an ACR 50 response as compared to 78.57% patients in the 500mg bRTX group. (table 2) Therapy with both doses of bRTX resulted in a sustained B cell depletion as evidenced by significantly lower CD19 cell count in both 1000mg and 500mg doses of bRTX therapy group at 12 months. (table3) (figure 3). However mean CD 19 cell count at 12 months of therapy was significantly lower in the 1000mg bRTX group as compared to the 500mg group. (table 3). Both biochemical and haematological parameters were also comparable. The degree of correlation between DAS28-ESR and CD 19 count was detected as 0.4.
Table 1: Demographic parameters of patients receiving 500mg or 1000mg of bRTX.
|
Parameter |
bRTX1000mg (n=13) (Mean±SD) |
bRTX 500mg (n=14) (Mean±SD) |
||
|
Gender |
||||
|
Female |
8(61%) |
9(64%) |
||
|
Male |
5 (39%) |
5(36%) |
||
|
Age (Average) |
40.96±4.83 |
38.69±2.86 |
||
|
Comorbidities |
||||
|
Hypertension |
5 (38.5%) |
4 (28.6%) |
||
|
Hyperlipidaemia |
2 (15.4%) |
2 (14.28%) |
||
|
Diabetes Mellitus |
3(23.08%) |
0 |
||
|
Educational Qualification |
||||
|
Graduation |
6 (46.15%) |
5 (35.71%) |
||
|
Post Graduation |
3 (23.07%) |
4 (28.57%) |
||
|
Higher secondary |
4 (30.77%) |
5 (35.71%) |
||
|
Time from diagnosis |
6.23±1.59 years |
6.77±1.42 years |
||
|
DMARD history |
||||
|
Methotrexate |
12 (92.30%) |
13 (92.85%) |
||
|
HCQ |
12 (92.30%) |
11 (78.57%) |
||
|
SSZ |
9 (69.23%) |
9 (64.28%) |
||
Table 2: Disease activity at baseline and after 12 months of therapy with bRTX
|
Parameter |
B RTX 1000mg |
B RTX 500mg |
||
|
At Baseline |
After 12 months |
At Baseline |
After 12 months |
|
|
DAS 28 ESR |
6.36 ± 0.10 |
4.48 ± 0.52 |
6.35 ± 0.09 |
4.53 ± 0.70 |
|
DAS 28 CRP |
5.23 ± 0.10 |
3.62 ± 0.44 |
5.24 ± 0.09 |
3.65 ± 0.59 |
|
VAS for pain |
8.30 ± 0.85 |
4.0 ± 1.15 |
8.35 ± 0.77 |
3.92 ± 1.47 |
|
% of ACR 50 response |
84.61% |
78.57% |
||
Table 3: Cumulative table of Disease parameters, Laboratory parameters and CD 19 cell counts at baseline and at 12 months of bRTX therapy
|
Parameter |
bRTX 500mg (Mean ± SD) |
bRTX 1000mg (Mean ± SD) |
Mean Difference (95% CI) |
p-value |
|
Disease Activity |
||||
|
DAS28 CRP Baseline |
5.23 ± 0.09 |
5.23 ± 0.10 |
0.00 (-0.08, 0.08) |
0.999 |
|
DAS28 CRP 12 months |
3.65 ± 0.55 |
3.62 ± 0.45 |
0.02 (-0.39, 0.44) |
0.903 |
|
DAS28 ESR Baseline |
6.35 ± 0.09 |
6.36 ± 0.10 |
-0.01 (-0.09, 0.07) |
0.821 |
|
DAS28 ESR 12 months |
4.53 ± 0.65 |
4.48 ± 0.52 |
0.04 (-0.44, 0.53) |
0.846 |
|
VAS Baseline |
8.36 ± 0.74 |
8.31 ± 0.85 |
0.05 (-0.63, 0.72) |
0.874 |
|
VAS 12 months |
3.93 ± 1.38 |
4.00 ± 1.15 |
-0.07 (-1.14, 0.99) |
0.885 |
|
Laboratory Markers |
||||
|
Platelet Baseline (Lakh/cu mm) |
1.74 ± 0.09 |
1.85 ± 0.32 |
-0.12 (-0.32, 0.08) |
0.225 |
|
Platelet 12 months (Lak/cu mm) |
1.75 ± 0.09 |
1.85 ± 0.48 |
-0.11 (-0.40, 0.19) |
0.443 |
|
WBC Baseline (cells/uL) |
6137.86 ± 977.45 |
5992.31 ± 1426.80 |
145.55 (-887.59, 1178.69) |
0.762 |
|
WBC 12 months (cells/uL) |
6157.14 ± 1904.13 |
6653.85 ± 1349.45 |
-496.70 (-1873.08, 879.68) |
0.44 |
|
WBC Baseline (cells/uL) |
6137.86 ± 977.45 |
5992.31 ± 1426.80 |
145.55 (-887.59, 1178.69) |
0.762 |
|
WBC 12 months (cells/uL) |
6157.14 ± 1904.13 |
6653.85 ± 1349.45 |
-496.70 (-1873.08, 879.68) |
0.44 |
|
ESR Baseline (mm/hr) |
56.93 ± 3.38 |
55.77 ± 4.59 |
1.16 (-2.24, 4.56) |
0.465 |
|
ESR 12 months (mm/hr) |
28.57 ± 9.23 |
26.69 ± 7.66 |
1.88 (-5.22, 8.97) |
0.569 |
|
CRP Baseline(mg/L) |
7.04 ± 0.78 |
6.55 ± 0.94 |
0.50 (-0.23, 1.23) |
0.151 |
|
CRP 12 months (mg/L) |
2.91 ± 1.11 |
2.71 ± 1.14 |
0.20 (-0.74, 1.14) |
0.649 |
|
B-Cell Depletion |
||||
|
CD19% Baseline |
13.36 ± 1.08 |
12.92 ± 1.50 |
0.43 (-0.67, 1.54) |
0.4 |
|
CD19% 12 months |
2.34 ± 0.59 |
1.98 ± 0.43 |
0.36 (-0.07, 0.79) |
0.078 |
|
CD19 Absolute Baseline (cells/uL) |
1217.29 ± 231.42 |
1221.54 ± 270.14 |
-4.25 (-215.93, 207.43) |
0.965 |
|
CD19 Absolute 12 months (cells/uL) |
134.21 ± 4.95 |
126.77 ± 3.42 |
7.45 (3.90, 10.99) |
<0.001 |
Table 4: Lab parameters at baseline and after 12 months of therapy
|
Parameter |
bRTX 1000mg |
bRTX 500mg |
||
|
At Baseline |
After 12 months |
At Baseline |
After 12 months |
|
|
Haemoglobin (g/dL |
10.88 ± 0.94 |
11.16 ± 0.55 |
10.00 ± 0.66 |
10.05 ± 0.64 |
|
WBC Count (/cu.mm |
5992.31 ± 1426.80 |
6653.84 ± 1349.45 |
6179.23 ± 1044.34 |
6284.61 ± 1949.10 |
|
Platelet Count(lakh/cumm) |
1.85 ± 0.32 |
1.85 ± 0.47 |
1.73 ± 0.09 |
1.74 ± 0.10 |
|
ESR (mm/hr) |
55.77 ± 4.58 |
26.69 ± 7.66 |
56.92 ± 3.67 |
28.92 ± 9.69 |
|
CRP (mg/dL) |
6.54 ± 0.94 |
2.71 ± 1.13 |
7.04 ± 0.85 |
2.95 ± 1.16 |
|
CD19 Absolute Count(/uL) |
1221.53 ± 270.14 |
126.77 ± 3.42 |
1222.07 ± 249.65 |
133.84 ± 5.15 |
Adverse Events
Two patients receiving 1000mg bRTX and one patient receiving 500mg bRTX developed infusion reaction in form of chills during the first dose infusion, all of which was managed conservatively and subsequently they received full planned dose as per schedule. No major side effects requiring drug discontinuation or hospitalisation were observed. The patients did not report any infection requiring hospitalisation or adverse health event possibly linked to bRTX therapy in the year following initial bRTX infusion.
Discussion
This real-world comparative analysis demonstrates that administration of two doses of both 1000mg and 500mg bRTX showed a significant reduction in disease activity among biologic-naïve RA patients. DAS28-ESR and DAS28-CRP showed significant improvement at 12 months in both groups, reinforcing bRTX’s role in reducing inflammation and joint involvement. Impressive ACR50 responses in both groups were indicative of the same. These findings align with prior studies of RTX in RA, which highlight its ability to modulate B-cell-driven pathogenesis, bringing about sustained disease control [3,24]. The comparable clinical response brought about by the two dosing regimens of bRTX is consistent with some studies on originator RTX [13]. Clinical effectiveness of RTX is well established across multiple rheumatological disorders and also beyond domains of rheumatology. There exists variation of recommended dosing of Rituximab among individual conditions. In ANCA vasculitis 500mg 6 monthly Rituximab proved a better agent than Azathioprine as maintenance therapy [25]. In lupus nephritis, multiple dosing strategies of Rituximab have been used ranging from 2 infusions of either 500mg or 1000mg to 375mg/m2 every week for 4 weeks. Notably, regimen comprising 2 doses of 500mg showed comparability with other dosing regimens [26-28]. An observational study evaluating the impact of off-label low dose rituximab in a variety of autoimmune conditions reported decent clinical response in majority patients [29]. Few reports comparing the effectiveness of 500mg and 1000mg doses of RTX in RA patients with an inadequate response to MTX found significant clinical improvement with both regimens [19] The SERENE trial concluded that two infusions of either dose, combined with MTX, led to substantial improvement at 24 weeks, with sustained benefits at 48 weeks. Similar proportions of patients in the RTX 500 mg and RTX 1000 mg groups achieved ACR20 (54.5% vs. 50.6%) and ACR50 (26.3% vs. 25.9%) responses. Additionally, mean DAS28-ESR scores over 48 weeks were comparable between the two groups [19]. The MIRROR trial showed that escalating the dose from 500mg to 1000mg did not improve the clinical outcomes [20]. IMAGE trial demonstrated that MTX combined with 1000mg of RTX significantly reduced joint damage progression and improved clinical outcomes, while the MTX + 500 mg RTX group showed significant clinical improvement, but joint damage progression remained unchanged [22]. The DANCER trial further confirmed improvement of health-related quality of life with both doses [30]. Findings from CERERRA and a meta-analysis by Bredemeier et al. also demonstrated comparable clinical outcomes between 500 mg and 1000 mg doses [23,24]. Furthermore, lower-dose regimens (e.g,500 mg every six months) have been explored as cost-saving alternatives without compromising efficacy in select patient populations [31]. Our study data mirrors similar clinical outcomes with both low and high doses of bRTX, as seen in clinical trials of originator molecule. Parallel evaluation of CD 19 levels alongside disease activity indices, helps in depicting a clearer picture of disease control and therapeutic response. This sustained B cell depletion with clinical effectiveness was seen even at 1 year of bRTX dosing. The cost implications of such changes will be substantial, improving affordability of the molecule in Indian background and possibly beyond. Interestingly, the significantly lower CD19 counts at 12 months in the 1000 mg group suggests a dose-dependent impact on B-cell depletion, a finding consistent with previous studies [18,32]. In our study significantly higher CD19 depletion at 12 months in 1000mg bRTX group was not associated with significantly superior clinical disease control. A plausible explanation of this disparity might be that the level of CD19 depletion brought about by 500mg bRTX was sufficient for therapeutic response with no additional benefit being conferred by a greater CD19 depletion in 1000mg group in this group of RA patients. However, a more pronounced depletion may have implications for long-term disease control and relapse rates. Additional longitudinal studies are required to establish whether this translates into superior clinical outcomes over time. The high degree of correlation between CD 19 cell depletion and clinical disease remission brought about by both doses further helps to reinforce clinical confidence in utilizing lower dose of bRTX in regular practice. Notably, as most patients were on MTX while on bRTX treatment with a few continuing other DMARDs as well, the ACR 50 response was impressive. All the patients were educated individuals and thereby showed compliance and motivation towards treatment. The near complete CD 19 positive B cell depletion in all patients laid the molecular foundation of the remarkable clinical response observed.
Limitation
However, the retrospective design, small sample size, observational nature, lack of head-to-head comparison, and absence of imaging evaluation preclude causal inference. Additionally, the 12-month follow-up may be insufficient to capture long-term differences in effectiveness and safety. Future studies with extended follow-up and larger cohorts are needed to confirm these findings and refine optimal dosing strategies for bRTX in different RA patient subgroups.
Conclusion
The findings of this study suggest that a lower dose of bRTX may be sufficient for satisfactory disease control in real-world setting, with significant economic benefit that may transgress to better biologic penetration. Given the comparable effectiveness in disease activity control and sustained B-cell depletion for 12 months, the 500 mg bRTX regimen may offer a cost-effective alternative without compromising therapeutic benefit. The moderate-to-high degree of correlation between CD19 cell depletion and disease activity reduction establishes potential prospect of absolute CD 19 cell count assessment as a molecular marker of disease remission, guiding subsequent dosing of bRTX. Future research focusing on long-term outcomes including remission and response rates, potential differences in immunogenicity between the two-dosing regimen and ethnic difference is essential for universal recommendation of low dose bRTX in RA.
Acknowledgement
The authors acknowledge the immense contribution of Mr Subhendu Dutta Bhowmick, data scientist, in analysing and drafting a comprehensive interpretation of the dataset
References
- Chopra A, Ghorpade R, Sarmukkadam S, et al. 5 Million Patients and Not 0.34% Is Worrisome: Burden of Rheumatoid Arthritis in India Based On a Bone and Joint Decade India Community Oriented Program for Control of Rheumatic Disease. In Arthritis and Rheumatism 64 (2012); S23.
- Heidari B. Rheumatoid Arthritis: Early diagnosis and treatment outcomes. Caspian J Intern Med. Winter 2 (2011): 161-170.
- Smolen JS, Landewé RB, Bijlsma JW, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the rheumatic diseases 79 (2020): 685-699.
- Fraenkel L, Bathon JM, England BR St, et al. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis & Rheumatology 73 (2021): 1108-1123.
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. Jama 320 (2018): 1360-1372.
- Hresko A, Lin TC, Solomon DH. Medical care costs associated with rheumatoid arthritis in the US: a systematic literature review and meta-analysis. Arthritis care & research 70 (2018): 1431-1438.
- Scott DL. Developing new therapeutic approaches for rheumatoid arthritis: The continuing challenges of clinical assessments. F1000Research 5 (2016): F1000.
- Dörner T, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Annals of the rheumatic diseases 75 (2016): 974-982.
- Greenwald M, Tesser J, Sewell KL. Biosimilars have arrived: rituximab. Arthritis 8 (2018): 3762-3864.
- Jung JY, Kim JW, Kim HA, et al. Rituximab biosimilar CT-P10 for the treatment of rheumatoid arthritis. Expert Opinion on Biological Therapy 19 (2019): 979-986.
- Subramanian R, Prasad S. Biologics: a tectonic shift in the management of rheumatoid arthritis. APIK Journal of Internal Medicine 7 (2019): 103-108.
- Mok CC. Rituximab for the treatment of rheumatoid arthritis: an update. Drug design, development and therapy 27 (2014): 87-100.
- Lee S, Lee H, Kim E. Comparative efficacy and safety of biosimilar rituximab and originator rituximab in rheumatoid arthritis and non-Hodgkin’s lymphoma: a systematic review and meta-analysis. Bio Drugs 33 (2019): 469-483.
- Suresh A, Ghanashyam B, Sinha S, et al. A Randomized, Multiple-Dose, Multicenter, Comparative Parallel Study to Evaluate the Safety and Efficacy of Intravenous Infusion of Rituximab (Hetero) and Reference Medicinal Product (Rituximab, Roche) in Indian Patients of Non-Hodgkin's Lymphoma (HERILY). Indian journal of medical and paediatric oncology 39 (2018): 316-320
- Trouvin AP, Jacquot S, Grigioni S, et al. Usefulness of monitoring of B cell depletion in rituximab-treated rheumatoid arthritis patients in order to predict clinical relapse: a prospective observational study. Clinical & Experimental Immunology 180 (2015): 11-18.
- Hanif N, Anwer F. Rituximab. In StatPearls (2025).
- Nakou M, Katsikas G, Sidiropoulos P, et al. Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis research & therapy 11 (2009): 1-8.
- Vital EM, Rawstron AC, Dass S, et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthritis & Rheumatism 63 (2011): 603-608.
- Emery P, Deodhar A, Rigby WF, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)). Annals of the rheumatic diseases 69 (2010): 1629-1635.
- Rubbert-Roth A, Tak PP, Zerbini C, et al. Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a Phase III randomized study (MIRROR). Rheumatology 49 (2010): 1683-1693.
- Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 54 (2006):1390-1400.
- Tak PP, Rigby WF, Rubbert-Roth A, et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Annals of the rheumatic diseases 70 (2011): 39-46.
- Chatzidionysiou K, Lie E, Nasonov E, et al. Effectiveness of two different doses of rituximab for the treatment of rheumatoid arthritis in an international cohort: data from the CERERRA collaboration. Arthritis research & therapy 18 (2016): 1-6.
- Bredemeier M, Campos GG, de Oliveira FK. Updated systematic review and meta-analysis of randomized controlled trials comparing low-versus high-dose rituximab for rheumatoid arthritis. Clinical rheumatology 34 (2015): 1801-1805.
- Guillevin L, Pagnoux C, Karras A, et al. French Vasculitis Study Group. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371 (2014): 1771-1780.
- Leandro MJ, Edwards JC, Cambridge G, et al. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 46 (2002): 2673-2677.
- Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, et al. Clinical and immunological effects of rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther 8 (2006): R83.
- Sutter JA, Kwan-Morley J, Dunham J, et al. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin Immunol 126 (2008): 282-290.
- Chay J, Donovan P, Cummins L, et al. Experience with low-dose rituximab in off-label indications at two tertiary hospitals. Intern Med J 43 (2013): 871-882.
- Mease PJ, Revicki DA, Szechinski J, et al. Improved health-related quality of life for patients with active rheumatoid arthritis receiving rituximab: Results of the Dose-Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) Trial. The Journal of rheumatology 35 (2008): 20-30.
- Van Vollenhoven RF, Emery P, Bingham III CO, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Annals of the rheumatic diseases 72 (2013): 1496-502.
- Edwards JC, Szczepański L, Szechiński J, et al. Efficacy of B-cell–targeted therapy with rituximab in patients with rheumatoid arthritis. New England Journal of Medicine 350 (2004): 2572-2581.


 Impact Factor: * 1.7
Impact Factor: * 1.7 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 