The Role of Repetitive Peripheral Magnetic Stimulation in Reducing Contractures and Enhancing Joint Function Among Elderly Residents of Skilled Nursing Facility: A Pilot Study
Raymond B. Esperida, PhD, MSPT¹ ², Shwe Sin Win Khin3, Hee Siew Chen3, Khin Hnin Khaing3, Ho Teh Chin3
¹Institute of Rehabilitative Sciences, Silliman University, Dumaguete, Negros Oriental, Philippines
²BTL Singapore Pte Ltd
³ECON Healthcare (ASIA) Ltd
*Corresponding Author: Raymond B Esperida, Institute of Rehabilitative Sciences, Silliman University, Dumaguete, Negros Oriental, Philippines.
Received: 16 December 2024; Accepted: 22 January 2025; Published: 12 February 2025
Article Information
Citation:
Raymond B Esperida, Shwe Sin Win Khin, Hee Siew Chen, Khin Hnin Khaing, Ho Teh Chin. The Role of Repetitive Peripheral Magnetic Stimulation in Reducing Contractures and Enhancing Joint Function Among Elderly Residents of Skilled Nursing Facility: A Pilot Study. Archives of Physiotherapy and Rehabilitation 8 (2025): 01-10.
View / Download Pdf Share at FacebookAbstract
Background: Contractures are a common issue in geriatric patients, particularly in nursing facilities, where prolonged immobility can reduce joint mobility and function. These conditions, characterized by tightness in muscles, tendons, or skin, impact quality of life and complicate caregiving. Repetitive Peripheral Magnetic Stimulation (RPMS) therapy is a promising non-invasive treatment for healing, reducing inflammation, and improving joint function, potentially aiding elderly patients in skilled nursing facilities.
Methods: This study employed an ABA design (baseline-treatment-baseline) to assess the effects of combined RPMS and Passive Range of Motion (PROM) therapy on knee contractures in elderly patients. Twelve participants (mean age 83.3 ± 5.2 years; 4 males, 8 females) were recruited into a study protocol consisting of 3 phases: Phase A1 (RPMS+PROM), Phase B (PROM), and Phase A2 (RPMS+PROM). The primary goal was to assess knee range of motion (ROM) improvements, with mobility changes as a secondary focus. ROM data were collected at baseline and after each phase using a goniometer.
Results: No participant experienced a decline in knee ROM after completing the treatment program, although some showed slight fluctuations during individual phases. The average improvement across both limbs was 20°, with statistically significant changes observed in the dominant limb after Phase B and in both limbs following Phase A2, compared to baseline.
Conclusions: RPMS therapy combined with PROM exercises showed promise for improving knee ROM. However, variability in outcomes highlights the need for personalized treatment plans. Future research should explore factors that contribute to sustained improvements and refine protocols for long-term benefits.
Keywords
<p>Pulsed electromagnetic field; Super inductive system; Contractures; Passive range of motion</p>
Article Details
1. Introduction
Contractures are a significant concern in the care of geriatric patients within nursing facilities. These conditions, characterized by the permanent tightening of muscles, tendons, or skin, lead to reduced joint mobility and function. In older adults, contractures often arise from a combination of prolonged immobility, muscle atrophy, and other age-related factors, posing challenges for both patients and caregivers [1].
Contractures in geriatric patients frequently develop as a consequence of extended periods of immobility, which can be common in nursing facilities due to underlying health conditions or injuries that necessitate reduced physical activity. These facilities cater to individuals who may have complex medical needs, including those recovering from surgery, managing chronic illnesses, or dealing with debilitating conditions such as stroke or severe arthritis [2].
The underlying pathophysiology of contractures involves a progressive shortening and tightening of soft tissues around a joint. This can result from several factors, including prolonged immobility. Extended bed rest or sedentary lifestyles reduce joint movement and flexibility, causing muscles and tendons to shorten and lose their elasticity [3]. Secondly, neurological impairments. Conditions such as stroke, Parkinson’s disease, or spinal cord injuries can impair motor function and lead to abnormal muscle tone and spasticity, contributing to contracture formation [4]. Thirdly, muscle atrophy. With age, muscle mass and strength can diminish, reducing the ability to maintain proper joint function and increasing the risk of contractures [5]. Fourth is inflammatory conditions. Chronic conditions like rheumatoid arthritis or systemic lupus erythematosus can cause joint inflammation and deformities, further promoting contracture development [6]. Lastly, reduced mobility and rehabilitation challenges. Limited movement options and challenges in performing rehabilitative exercises due to physical or cognitive impairments can exacerbate contracture development [7].
Contractures in geriatric patients can significantly impact their quality of life, leading to pain, decreased functional independence, and difficulty with activities of daily living. Additionally, they can complicate caregiving and increase the risk of pressure ulcers and other secondary complications [8].
Addressing contractures involves a multidisciplinary approach that includes regular physical therapy, occupational therapy, and, when necessary, surgical interventions. Preventive measures such as routine range-of-motion exercises, proper positioning, and patient education are crucial in managing and mitigating the effects of contractures in nursing facilities [9].
Repetitive Peripheral Magnetic Stimulation (RPMS) therapy is an emerging non-invasive treatment modality that utilizes electromagnetic fields to stimulate cellular processes and promote healing. This therapeutic approach involves the application of high-intensity, pulsed magnetic fields that enable stimulation of deep tissue structures. RPMS therapy is gaining attention for its potential benefits in addressing and preventing the development of contractures, particularly in geriatric patients [10,11].
Research into the efficacy of RPMS therapy for contractures is still evolving, but preliminary studies suggest promising results. Clinical trials have demonstrated that RPMS therapy can improve joint function and reduce pain and inflammation in various musculoskeletal conditions. Although specific studies on contractures are limited, the existing evidence supports its potential benefits.
In skilled nursing facilities, integrating RPMS therapy into patient care plans could offer a valuable tool for managing contractures. However, it is essential for healthcare providers to consider individual patient needs and integrate RPMS therapy as part of a comprehensive treatment approach.
RPMS therapy presents a promising intervention for addressing and preventing contractures in geriatric patients. By enhancing cellular function, reducing inflammation, improving circulation, and alleviating pain, RPMS therapy may offer significant benefits in maintaining joint health and mobility. As research continues to unfold, RPMS therapy could become an integral component of multidisciplinary strategies aimed at improving the quality of life for elderly individuals in skilled nursing facilities.
2. Materials and Methods
Research was conducted from August 13 to November 22, 2024, at ECON Healthcare Singapore, a private eldercare services provider. The study utilized an ABA design (baseline-treatment-baseline) to assess the effectiveness of RPMS therapy on contractures in elderly patients. The ABA design included three phases: RPMS and Passive Range of Motion exercises (PROM) (A1), PROM (B), and RPMS+PROM (A2). This design helped in understanding whether observed changes are due to the intervention or other factors.
The aim of this study was to determine the effect of RPMS therapy on reducing contractures in elderly patients, particularly those with limitations in knee joint mobility by assessing the degree of improvement in joint flexibility. Changes in Knee Passive Range of Motion (KPROM) were tracked throughout the treatment program, monitoring the progress of mobility during individual study phases.
Participants resided in a skilled nursing facility in Singapore were recruited. The inclusion criteria for this study were as follows: participants were required to be elderly individuals aged 65 years or older, with the presence of contractures or a limitation of motion in the knee joint. Additionally, participants needed to be capable of providing informed consent and able to follow the study protocols throughout the duration of the trial. The exclusion criteria included individuals with severe comorbid conditions such as malignancy, severe cardiovascular disorders, or neurological conditions that could interfere with the study's objectives or pose a risk to the participant's health. Participants with pacemakers or other implanted electronic devices were also excluded due to potential safety concerns with the RPMS therapy. Furthermore, individuals with significant cognitive impairment or other conditions that could hinder their ability to participate fully in the study were not eligible to ensure accurate data collection and the safety of the participants. These criteria were designed to select a homogenous group of participants while minimizing potential risks and confounding factors that could affect the study's results.
Ethical considerations for this study were carefully followed to ensure the safety and well-being of all participants. Written informed consent was obtained from each participant before the study commences, ensuring they are fully informed about the study’s purpose, procedures, potential risks, and their rights. Additionally, the study received approval from ECON Healthcare Singapore Ethics Committee to ensure compliance with ethical standards and protect participant rights. Throughout the study, safety monitoring was an ongoing priority, with all adverse effects related to the RPMS therapy being promptly documented and addressed. Participants were instructed to immediately report any discomfort or issues, and appropriate actions were taken to ensure their safety during the study. These measures helped uphold the ethical integrity of the trial and safeguard the participants' health throughout the duration of the study.
The small sample size of 12 subjects was intentionally chosen to allow for detailed, individualized assessments and to keep the study manageable in scope. This sample size was appropriate for the ABA design, enabling a focused and in-depth analysis of each participant's response to the intervention.
The treatment program consisted of three 4-week phases: Phase A1, Phase B, and Phase A2. In Phase A1, participants received RPMS therapy twice weekly (23 minutes per session), totaling 8 sessions. Treatments followed the manufacturer’s guidelines for frequency, intensity, and duration, adjusted as needed for comfort and efficacy. The BTL 6000 Elite Super Inductive System was applied to the quadriceps using a muscle relaxation protocol (10 minutes) and to the hamstrings with a strengthening protocol (13 minutes). In Phase B, RPMS therapy was discontinued, and participants performed PROM exercises twice weekly. Data collection continued to evaluate whether the improvements from Phase A1 were maintained or reversed. Phase A2 reintroduced RPMS therapy alongside PROM exercises, following the same regimen as Phase A1, for another 8 sessions over four weeks. This phase aims to assess further improvements in knee range of motion (ROM) and enhance joint mobility beyond the gains achieved earlier.
The primary outcome measure, Knee Passive Range of Motion (KPROM), was assessed using a goniometer to evaluate knee flexibility and mobility. During testing, the clinician passively moved the participant's knee joint to measure the angle of movement. KPROM assessments at baseline and after each study phase identified significant changes in joint motion, reflecting the therapeutic impact of the intervention.
Data was processed and analyzed using Microsoft Excel 2016. As the Shapiro-Wilk test rejected the normality hypothesis, the non-parametric Wilcoxon Signed Rank test was applied. Statistical significance was defined as a probability (p-value) of less than 0.05 for changes between baseline and individual treatment phases.
3. Results
Of the 12 participants recruited, 9 completed the treatment program, with an average age of 83.3 ± 5.2 years, including 3 men. Three participants withdrew due to illness or other health-related issues. See Table 1 for detailed demographic information.
|
Subjects* |
Age |
Sex |
|
2 |
82 |
Male |
|
3 |
83 |
Male |
|
4 |
73 |
Male |
|
5 |
86 |
Female |
|
7 |
90 |
Female |
|
8 |
85 |
Female |
|
9 |
84 |
Female |
|
10 |
95 |
Female |
|
12 |
72 |
Female |
*Subjects 1, 6 and 11 have dropped out during the treatment program.
Table 1: Participants Demographic Profile.
KPROM values measured before and after the individual treatment phases are summarized for each study participant in Table 2. Range of motion was assessed for both limbs - the limb that showed better ROM at baseline was designated Dominant, while the limb showing greater limitation was designated Non-dominant.
|
Subjects* |
Baseline |
Phase A1 |
Phase B |
Phase A2 |
||||
|
D |
ND |
D |
ND |
D |
ND |
D |
ND |
|
|
2 |
130 |
95 |
130 |
105 |
125 |
110 |
135 |
115 |
|
3 |
110 |
80 |
130 |
70 |
130 |
70 |
120 |
105 |
|
4 |
125 |
105 |
115 |
80 |
130 |
95 |
130 |
110 |
|
5 |
125 |
105 |
130 |
105 |
130 |
100 |
135 |
105 |
|
7 |
100 |
55 |
110 |
110 |
110 |
85 |
125 |
90 |
|
8 |
100 |
90 |
95 |
80 |
100 |
100 |
100 |
100 |
|
9 |
20 |
10 |
90 |
60 |
55 |
35 |
85 |
25 |
|
10 |
110 |
90 |
105 |
120 |
130 |
120 |
145 |
130 |
|
12 |
75 |
75 |
130 |
70 |
105 |
95 |
110 |
95 |
|
Mean (SD) |
99.44 (34.2) |
78.33 (30) |
115 (16) |
88.89 (21.3) |
112.78 (24.8)** |
90 (25) |
120.56 (19.1)** |
97.22 (29.5)** |
*Subjects 1, 6 and 11 have dropped out during the treatment program.
**The difference from baseline values is statistically significant with p<0.05.
IQR: Interquartile range, D: Dominant, ND: Non-dominant
Table 2: KPROM values obtained before the start of the study program and after the completion of each phase.
Significant variability in ROM between limbs was observed among participants before the start of the treatment program. Subject 2 demonstrated significantly greater ROM in the dominant knee (130°) compared to the non-dominant knee (95°). In contrast, Subject 9 exhibited pronounced limitations, with the dominant knee measuring 20° and the non-dominant knee 10°. Conversely, Subject 12 displayed relatively balanced knee ROM (75° for both knees), indicating moderate flexibility compared to more severely affected participants. These findings underscore the substantial variability in baseline knee ROM, ranging from severe stiffness to relatively higher mobility. This data provides a critical reference for evaluating the intervention's impact on improving joint flexibility and reducing contractures in elderly patients.
After Phase A1, many participants showed notable improvements in knee ROM, particularly those with initially limited mobility, such as Subjects 9, 7, and 12. However, the intervention's effects varied, with some participants showing minimal changes or declines (e.g., Subjects 4 and 8). Asymmetrical responses were also observed, with some participants improving in one knee while the other showed little or no change, or even deterioration.
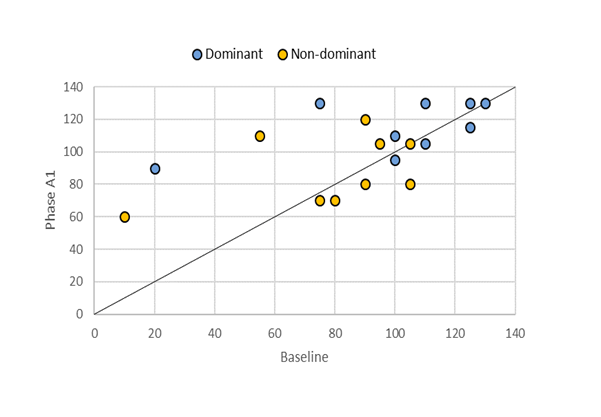
Figure 1 presents a graphical representation of KPROM development for individual limbs. While mobility deterioration showed minor changes, improvements were relatively significant compared to baseline. However, despite these notable enhancements in both limbs, the changes did not achieve statistical significance when compared to baseline KPROM values (Table 2).
Figure 1: A scatter plot illustrating the evolution of KPROM values for dominant and non-dominant limbs during Phase A1. Points below the diagonal line indicate limbs with deterioration compared to baseline, points on the line represent no change, and points above the line reflect improvement during Phase A1.
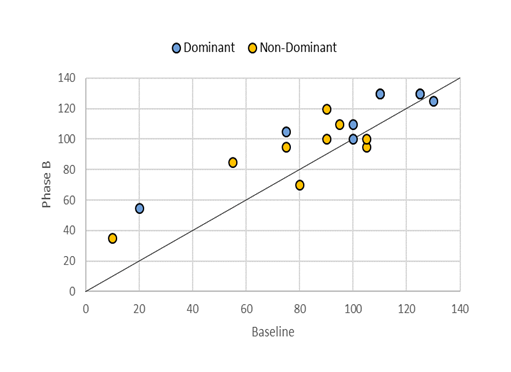
Phase B data shows that the effects of RPMS therapy on knee ROM varied among participants after therapy withdrawal. Some maintained their ROM (e.g., Subjects 3 and 5), while others even showed improvements (e.g., Subjects 4, 8, and 10). However, several participants experienced a decline (e.g., Subjects 7, 9, and 12). Figure 2 graphically illustrates the development of limb mobility compared to baseline. Although the improvement trend was slower than in Phase A1, further progress was observed, with nearly all limbs showing improvements. Statistical analysis confirmed this finding, revealing a statistically significant change in the KPROM of the dominant limb compared to baseline (Table 2).
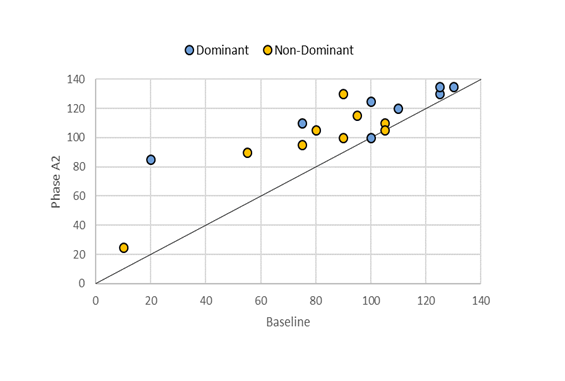
The reintroduction of RPMS therapy combined with PROM exercises in Phase A2 led to overall improvements in KPROM for most participants. While all subjects showed progress, two participants (Subjects 3 and 9) experienced slight worsening in one limb, accompanied by substantial improvement in the other. Figure 3 highlights the positive trend in limb mobility throughout the treatment program, showing that no limb experienced a decrease in KPROM compared to baseline. Statistical analysis further confirmed significant improvements in KPROM for both limbs during the course of the program (Table 2).
Figure 2: A scatter plot depicting the changes in KPROM values for dominant and non-dominant limbs during Phase B. Points located below the diagonal line represent a decline in KPROM compared to baseline, points on the line show no change, and points above the line indicate improvement during Phase B.
Figure 3: A scatter plot showing the progression of KPROM values for both dominant and non-dominant limbs during Phase A2. Points falling below the diagonal line represent a decline in KPROM compared to baseline, points on the line indicate no change, and points above the line show an improvement during Phase A2.
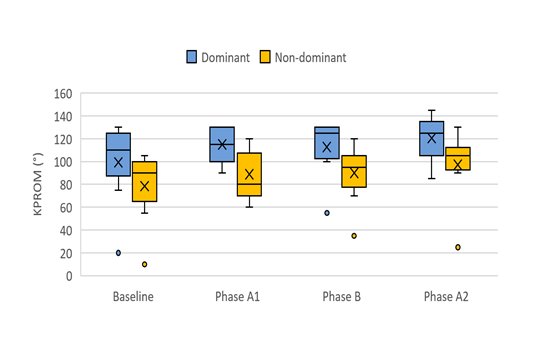
Figure 4 presents a box plot illustrating the distribution and progression of KPROM data throughout the study. A gradual improvement is evident across the individual phases for both the dominant and non-dominant limbs.
Figure 4: Box plot graphs depicting the distribution and evolution of KPROM data throughout the treatment program. The plot includes the minimum and maximum values (whiskers), the first quartile (lower edge of the box), the third quartile (upper edge of the box), the median (line within the box), and the mean (cross).
4. Discussion
Current evidence highlights the potential of RPMS therapy in managing contractures among geriatric patients within eldercare settings. Older individuals often experience diminished joint flexibility due to factors such as aging, reduced physical activity, and musculoskeletal conditions. In geriatric populations, particularly among those in their 80s and 90s, limited knee mobility is commonly observed, reflecting severe contractures or stiffness. These conditions are frequently associated with arthritis, muscle atrophy, or prolonged immobility. The current study demonstrated that a treatment protocol combining RPMS therapy and PROM exercises resulted in an average improvement of approximately 20° in knee joint ROM for both dominant and non-dominant limbs over three months. This finding aligns with limited existing evidence supporting RPMS's efficacy in older adults. For example, Kamiue et al. [12] reported significant positive effects of RPMS therapy on maximum voluntary contraction, RPMS-induced torque, and motor performance in older patients, assessed through the Timed-Up-and-Go Test and the 30-second chair-stand test. Similarly, Leonardo et al. found notable improvements in muscle strength and motor skills in sarcopenic older adults using the Timed-Up-and-Go Test [13]. While the present study uniquely focused on PROM monitoring, limiting direct comparability, previous research has established that enhancing ROM in older individuals promotes greater physical independence, reduces disability, and improves quality of life. Moreover, improved ROM can lower the risk and fear of falls, contributing to better physical and mental well-being in geriatric populations [14,15].
The current analysis reveals that while combining RPMS therapy with PROM exercises significantly improves knee ROM in elderly participants, the magnitude of KPROM changes varies. Some participants exhibited substantial and sustained improvements, while others experienced fluctuations across different phases, resulting in only modest overall progress. Evaluating the specific contribution of RPMS therapy to the combined protocol remains complex, though average KPROM values suggest higher effectiveness during RPMS-inclusive phases. The small sample size and variability in responses highlight the need to consider factors potentially influencing treatment outcomes, including individual reactions to RPMS. For some participants, the effects of RPMS may persist for several weeks post-application, whereas for others, the benefits might be temporary. Additionally, factors such as age, baseline mobility, and underlying health conditions likely play a critical role in influencing outcomes. To better understand the long-term efficacy of this therapeutic approach and to establish optimal protocols, further research with larger sample sizes and rigorously controlled methodologies is essential.
This study has several limitations that should be considered when interpreting the findings. A primary limitation is its non-randomized design, which, combined with the small sample size, may introduce bias and restrict the generalizability of results to a larger population. The absence of randomization increases the risk of selection bias, where specific participant characteristics could influence outcomes. Additionally, the lack of blinding may have introduced biases related to both participant and researcher expectations. Participants' preconceived beliefs about treatment efficacy might have influenced their responses or self-reported outcomes, while researchers' awareness of the treatment could have inadvertently affected the assessment or interpretation of results. These factors could compromise the internal validity of the study and should be carefully considered in the evaluation of its conclusions.
Despite the limitations mentioned, this study provides valuable insights suggesting potential benefits of combining RPMS and PROM therapy in geriatric patients within eldercare settings. These findings could serve as a foundation for further research and may be useful for physiotherapists working with elderly populations in clinical practice.
5. Conclusions
The present study demonstrated the potential of a combined RPMS and PROM protocol to improve ROM in geriatric patients, with an average increase of 20° over three months. However, due to the small sample size, further large-scale studies are needed to confirm these findings and establish general conclusions. For elderly patients with contractures, this approach holds promise for enhancing quality of life, reducing the risk and fear of falls, and improving overall physical and mental health. Additionally, implementing such a non-invasive intervention in routine nursing home care could significantly improve mobility and independence for this vulnerable population.
References
- Muller M, Fischer U, Bartoszek G, et al. Impact of joint contractures on functioning and social participation in older individuals – development of a standard set: study protocol. BMC Geriatrics 13 (2013):
- Tariq H, Collins K, Tait D, et al. Factors associated with joint contractures in adults: A systematic review with narrative synthesis (2022).
- Wang F, Zhang QB, Zhou Y, et al. The mechanisms and treatments of muscular pathological changes in immobilization-induced joint contracture: A literature review. Chinese Journal of Traumatology 22 (2019): 93-98.
- Kelly RR, Sidles SJ, LaRue AC. Effects of neurological disorders on bone health. Frontiers in Psychology 11 (2020): 612366.
- Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Research Reviews 47 (2018): 123-132.
- Pabon-Porras MA, Molina-Rios S, Florez-Suarez JB, et al. Rheumatoid arthritis and systemic lupus erythematosus: Pathophysiological mechanisms related to innate immune system. SAGE Open Medicine 7 (2019): 2050312119876146.
- Grimmer M, Riener R, Walsh CJ, et al. Mobility related physical and functional losses due to aging and disease – A motivation for lower limb exoskeletons. Journal of NeuroEngineering and Rehabilitation 16 (2019).
- Chen YC, Lin KC, Yeh SH, et al. Associations among quality of life, activities, and participation in elderly residents with joint contractures in long-term care facilities: A cross-sectional study. BMC Geriatrics 22 (2019): 197.
- Skalsky AJ, McDonald CM. Prevention and management of limb contractures in neuromuscular diseases. Physical Medicine and Rehabilitation Clinics of North America 23 (2013): 675-687.
- Ross CL, Zhou Y, McCall CE, et al. The use of pulsed electromagnetic field to modulate inflammation and improve tissue regeneration: A review. Bioelectricity 1 (2019): 247-259.
- Vadala M, Morales-Medina JC, Vallelunga A, et al. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Medicine 5 (2016): 3128-3139.
- Kamiue M, Tsubahara A, Ito T, et al. Effects of repetitive peripheral magnetic stimulation on knee joint extensor strength in older persons receiving day services. Japanese Journal of Comprehensive Rehabilitation Science 15 (2024): 49-57.
- de Almeida GCT, dos Santos JB, Gomes AM, et al. Effects of pulsed electromagnetic field in reducing pain and increasing the range of motion in patients with knee osteoarthritis: A systematic review. MTP & Rehab Journal (2023).
- Bacanoiu MV, Danoiu M. New strategies to improve the quality of life for normal aging versus pathological aging. Journal of Clinical Medicine 11 (2022): 4207.
- Kalideen L, Govender P, van Wyk JM. Standards and quality of care for older persons in long-term care facilities: A scoping review. BMC Geriatrics 22 (2022):

 Impact Factor: * 1.3
Impact Factor: * 1.3 Acceptance Rate: 73.39%
Acceptance Rate: 73.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 