Ammonia-Nitrogen Adsorption by Melon Rind
Hikmatullah Ahmadi1*, Syed Sanaullah Hbibi2
1College Environmental Science and Engineering, Taiyuan University of Technology, 030024, Taiyuan, Shanxi, China
2Department of Agricultural Economics, Haryana Agricultural University, Hisar, India
*Corresponding Author: Hikmatullah Ahmadi, College Environmental Science and Engineering, Taiyuan University of Technology, 030024, Taiyuan, Shanxi, China
Received: 10 March 2022; Accepted: 18 March 2022; Published: 28 April 2022
Article Information
Citation: Hikmatullah Ahmadi, Syed Sanaullah Hbibi. Ammonia-Nitrogen Adsorption by Melon Rind. Journal of Analytical Techniques and Research 4 (2022): 60-70.
View / Download Pdf Share at FacebookAbstract
The presence of NH3-N in a watery arrangement is a major issue of environment, NH3-N is the significant water contamination. In this investigation, melon Rind for low-cost adsorbent instead of expensive technologies for removal of NH3-N from water arrangement, hence to increase the attractiveness of melon rind for absorption of NH3-N, Rind was treated with NaOH (0.3 M). To detect groups on the surface of the Rind were utilizing the Fourier Transform Infra-red (FTIR). SEM scanning electron microscopy was utilized morphological properties of the pre-arranged Melon Rind, Energy-dispersive X-beam spectroscopy (EDS) was utilized to research the Rind Surface Elements affecting adsorption are contact time, adsorbent dose, pH, NH3-N concentration, were investigated. The adsorption limit of NH3-N diminished as the adsorbent portion expanded. The Kinetic examinations showed best coordinated with the pseudo-first-order and pseudo-second-order models. The Freundlich and Langmuir isotherm models were utilized to depict the NH3-N adsorption onto the melon Rind adsorbent, the information gotten from the adsorption interaction were best agreeable with the Langmuir model with R20.9989 and a most qmax adsorption limit of 12.161mg/g.
Keywords
<p>Adsorption; NH3-N, Isotherm, Kinetic, Melon rind, EXD, SEM, FTIR</p>
Article Details
1. Introduction
Water contamination has become a significant overall natural issue annihilating sea-going biological systems, with the improvement of industrialization, different synthetic squanders from mechanical plants are pouring to ecological environments is one of the significant elements in water contamination [1] these squanders incorporate poisonous substances, muck and solvents. Sadly, alkali nitrogen is a typical water impurity in the solvents that affects the climate and represents a danger to human wellbeing [2]. What's more, smelling salts nitrogen is one of the significant nitrogen-containing toxins which may prompts the eutrophication and worsening algal blossoms in environments [3,4]. Nitrogen contamination incorporates a few structures and smelling salts nitrogen (NH3-N) is the average structure that comes from civil sewage, modern wastewater, landfill leachate and rural non-point source release [6]. Numerous methods are accessible for the expulsion of smelling salts nitrogen from water stage. Compound strategy anaerobic smelling salts expulsion [7] film distillation [8] alkali expulsion from watery arrangement by zeolite [9] of organic product, rinds based adsorbent for eliminating smelling salts nitrogen contaminations from water offers numerous alluring highlights like the exceptional adsorption limit and the way that these materials are minimal expense, non-poisonous and biocompatible [10]. In this examination, a new adsorbent was produced using melon Rind low cost to eliminate ammonia nitrogen from fluid water arrangement. Rind ware treated with NaOH to improve adsorbent adsorption capacity.
2. Material and Methods
2.1. Preparation of the Adsorbent
The melon Rind was gathered from TYUT organic product market, Shanxi/china. All synthetic substances utilized in this investigation are of standard scientific grade and were acquired from TYUT. The melon Rind were washed to eliminate dust utilizing faucet water, trailed by deionized, dried in a stove at 60°C for 6 days then, at that point cut it in ground into fine Nano-particles utilizing a processor, sieved. Take 10 g melon Rind 100 mL of a 0.3M arrangement of NaOH combination was mixed until uniform and drenched for 24 hours the biomass after treatment was washed with deionized water until the pH of the wash arrangement was close to the nonpartisan reach 7. After this, the adsorbent was dried at 60°C for 24 hours in a drying stove and put away dry until use, to be specific NaOH+ melon Rinds (NMRs).
2.2. Preparation of stock solution
Engineered NH3-N arrangements were utilized all through the adsorption tests. Alkali chloride salt (NH4Cl) with determined weight was disintegrated in 1L of refined water for wanted focus. The underlying pH worth of arrangement was changed in accordance with 7.0 utilizing weaken arrangement of 0.1 M of hydrochloric corrosive HCL and sodium hydroxide (NaOH).
2.3. Batch Adsorption test
Batch experiment solutions were engendering by diluting the stock solutions in deionized water. The adsorption investigation of NH3-N on NMRs by using the following operating parameters: pH (3-9), biosorbent amount (0.1-1 g/L), concentration initial (0.4-10 mg/L), adsorption period (5 min-90min) at 25oC. The effect of pH on adsorption of NH3-N was investigated using 0.1 g/L biosorbent and initial concentration of 10 mg/L in a 50 mL suspension volume. At optimal pH 7, the effect of biosorbent amount on NH3-N adsorption was examined by biosorbent increasing from 0.1g/L to 1g/L, at 25oC, the fancy volume of 50 mL was shaken at 180 rpm. aim of the kinetic study was to find how long it takes to reach equilibrium using the real amount of biosorbent concentration initial 0.4 to 10 mg/L were used in equilibrium adsorption tests, Optimal dose of adsorbent of 0.1 g/L, using 0.1M HCl and 0.1M NaOH solutions for pH adjustment. The NMPs were isolated from the medium using filtration after equilibration, measure of NH3-N in the arrangements were dissected in nurse’s region at a frequency of 420 nm by the (PerkinElmer spectrophotometer Spector quant Nova 60). At last, the NH3-N harmony was determined as displayed in Equation the measure of NH3-N adsorbed was resolved utilizing the condition underneath eq1:
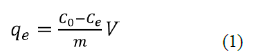
Where C0 and Ce are NH3-N focuses (mgL-1) before and after the fact adsorption, V is the volume of adsorbate (L) and m is the heaviness of the adsorbent (g).
2.4. Adsorbent characterizations
The characteristics of NMRs such as surface morphology, surface functional group, chemical characteristics were determined through laboratory diagnostic techniques using scanning electron microscopy (SEM)(EDX), Fourier transform infrared (FTIR)
3. Results
3.1. Characterization of Biosorbent
The obtained results of FTIR spectra showed that the surface of NMRs had the different functional groups. The peaks showed in (Figure 1a) for NMPs are related to O-H groups, carbonyl, C-H, hydroxyl and carboxyl. (Figure 1b) In the SEM micrographs, surface of NMPs showed many pours on the surface of sorbent it suitable for NH3-N adsorption. (Figure 1c) shows EDX analysis which was performed for NMRs in order to determine the chemical compound of adsorbent. The obtained results indicate that the adsorbent, in addition to main composition, has different amounts of other chemical compounds.
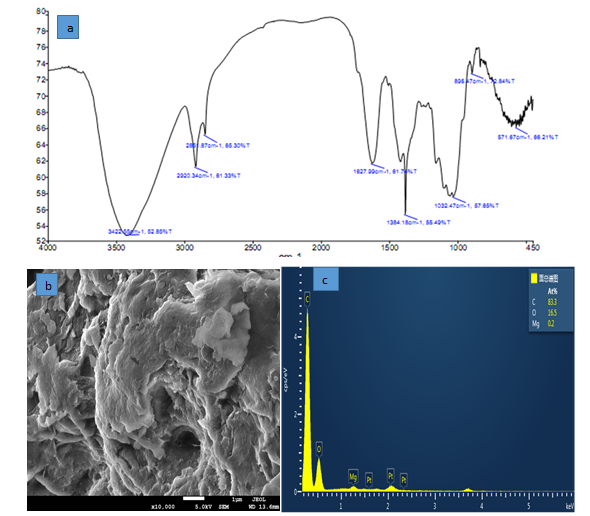
Figure 1: FTIR (a) SEM image (b), EDS spectrum (c)
3.2 Effects of parameters on adsorption process
3.2.1. Effect of initial pH: The underlying pH arrangement is perhaps the most basic controlling boundaries in adsorption measures. The impact of introductory pH on the adsorption cycle was seen by taking the normal pH upsides of water where the worth lies in scope of 3 to 11. The adsorption limit of ammonia nitrogen qe (mg/g) at various pH is displayed in figure 2 for the underlying arrangement centralization of 10 mg/L. The adsorption limit of NMPs on the NH3-N measure shows least at esteem at pH 3 and 11(0.17 and 0.45), expanded to 0.56 mg/g at pH 5 and arrived at greatest adsorption limit (0.745mg/g) at pH 7. The adsorption esteems diminished to 0.665 mg/g as the pH worth of 9.
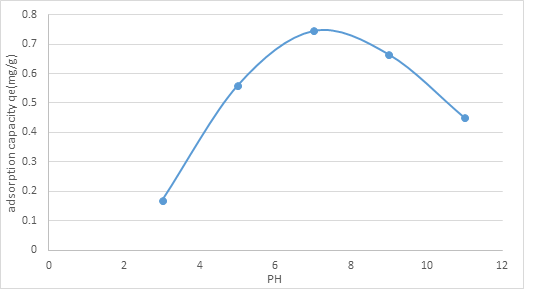
Figure 2: Effect of initial pH on the adsorption capacity of NMPs.
3.2.2. Effect of adsorbent dosage: As shown in figure 3, the adsorption limit of (NMRs) diminished with the expansion of adsorbent dose from 0.1g to 1g. This impact can be credited to a lessening number of dynamic surface locales for the NH3-N subsequently essentially decreasing the adsorption limit. This was principally because of the covering of adsorbent layers.
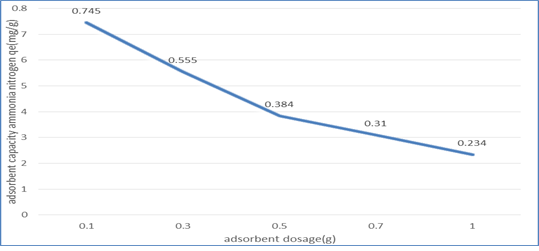
Figure 3: Graph of Adsorption Capacity of (NMRs), qt (mg/g) against Adsorbent Dosage (g)
3.3.3. Effect of contact time of adsorption: As delineated in figure 4, it tends to be seen that the adsorption limit was quick at the main phase of adsorption (0-20 minutes), then, at that point gradually expanded with time (20-an hour), and getting practically steady following an hour (an hour onwards). This marvel was because of the great accessibility of empty destinations in the primary phase of adsorption. The adsorption was then trailed by an expansion in shocking powers because of the presence of the adsorbed particles. The adsorption cycle proceeded to a point where the adsorbent couldn't adsorb the encompassing adsorbate because of the absence of empty locales. In this examination, the ideal contact time was an hour.
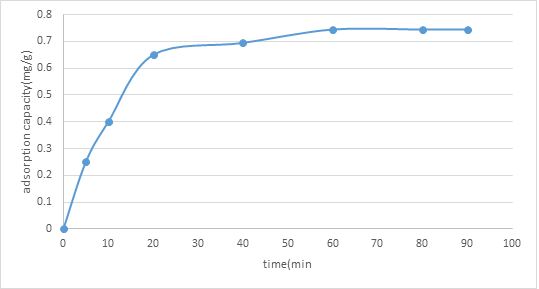
Figure 4: Graph of Adsorption Capacity of (NMRs), qt (mg/g) against Contact Time (min)
3.2.4. Effect of initial concentration of NH3-N solution: From figure 5, clearly by expanding the underlying convergence of NH3-N arrangement from 0.4 mg/l to 10mg/l, the adsorption limit of the (NMRs), expanded from 0.0283 mg/g to 0.745 mg/g. It was on the grounds that higher NH3-N fixation gave a more noteworthy main impetus to adsorption as more NH3-N particles can be adsorbed by the (NMRs).
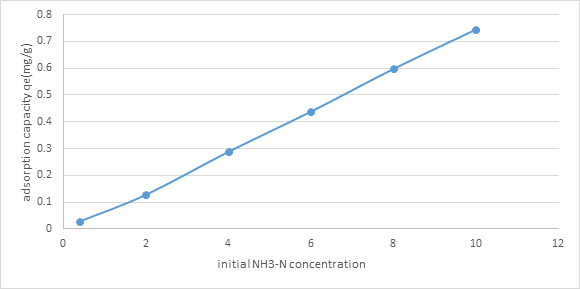
Figure 5: Graph of Adsorption Capacity of (NMRs), qe (mg/g)
3.3. Kinetic study
The adsorption motor was performed by shifted adsorption contact time which was 0, 10, 20, 30, 40, 60, 80, and an hour and a half to continue active fitting and decide adsorption rate. The two diagrams of pseudo-first-request and pseudo-second-request show various practices. The worth of R2 of pseudo second-request modular was 0.9905 which closer to 1 contrasted with pseudo-first-request, 0.9106. Consequently, pseudo-second-request model was more ideal for adsorption dynamic which the worth of R2 is closer to 1 that show a decent numerical fit (figures 6,7).
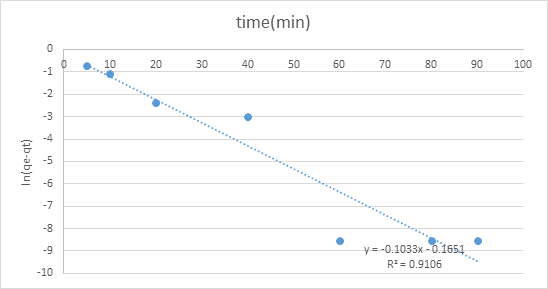
Figure 6: Graph of pseudo-first-order.
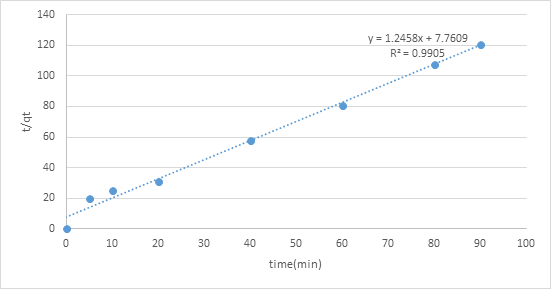
Figure 7: Graph of pseudo second order.
3.4. Adsorption isotherm
The harmony adsorption information was depicted by two of the most much of the time utilized isotherm models: Langmuir and Freundlich isotherm models. The Langmuir isotherm model can be communicated in a linearized condition as eq2:
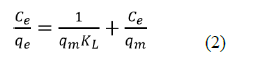
Where ce is the harmony centralization of NH3-N (mg/L), qe is the measure of NH3-N adsorbed at balance (mg/g), qm is the greatest adsorption limit (mg/g) and KL is the Langmuir isotherm consistent identified with the free energy of adsorption (L/mg). Figure 8 showed the plot of 1/qe against beginning focuses (1/Ce) which gave a straight-line diagram. The Langmuir consistent (KL) and most extreme adsorption limit were acquired from the incline and capture of the chart individually. Table 1 likewise showed a Langmuir plot with a relationship coefficient of 0.9991, which was more prominent than 0.99, in this way, demonstrating that the information acquired for the adsorption interaction fitted well into the Langmuir Isotherm Model.
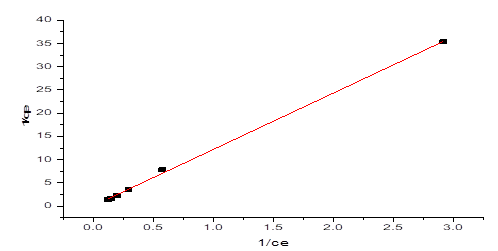
Figure 8: Langmuir Isotherm graph on NH3-N adsorption by NMPs
Freundlich equation can be expressed as:
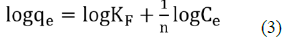
Where, KF is the constant related to adsorbent capacity (mgg-1) and is an empirical parameter related to the sorption intensity of the adsorbent. The plot of logqe against logCe gave a straight-line graph as seen in Figure 9, with a slope of and an intercept of KF. The Freundlich Isotherm model plot of data obtained from the NH3-N adsorption onto NMPs with gave a correlation coefficient of table 2, R2 = 0.9971. The values of KF and obtained were 0.080707 And 1.02897 respectively.
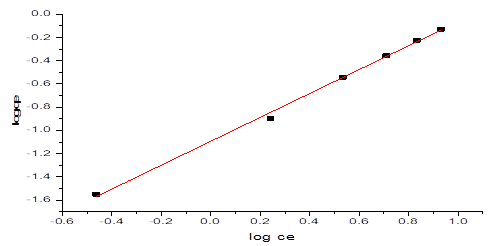
Figure 9: Freundlich Isotherm graph on NH3-N adsorption by NMPs
|
Intercept |
Slope |
qmax(mg/g) |
KL |
RL |
R2 |
|
0.08223 |
12.14519 |
12.1610118 |
0.00677058 |
0.93658757 |
0.9989 |
Table 1: Langmuir Parameters
|
Intercept |
Slope |
1/n |
Kf |
R2 |
|
-1.09309 |
1.02897 |
1.02897 |
0.080707 |
0.9971 |
Table 2: Freundlich Parameters
4. Conclusion
This Research was done to investigate biosorbent melon Rinds, as a new biosorbent, for treating NH3-N from watery arrangement. The exploratory outcomes showed that melon Rind treated by 0.3M NaOH (NMRs) can significantly further develop the sorption expulsion for NH3-N Chemicals alteration for melon Rinds and pH showed substantial impact on NH3-N evacuation, while contact time introduced brief impact The energy study showed that the sorption conduct better fit the pseudo-second-model condition than the Langmuir and Freundlich equation<(99). The FTIR retention range investigation of melon Rinds and NMPs prior and then afterward sorption uncovered that the movement deferent gatherings at the outside of the melon Rinds SEM and XRD portrayal tests completed on the (NMRs) to see better adsorbent surface. In light of the adsorbent the most appropriate to be utilized for NH3-N adsorption measure. In this investigation, 0.1 g of (NMRs) adsorbent was demonstrated the most ideal dose for the adsorption of NH3-N. Concerning contact time examination, an hour was the most ideal contact time for adsorption of NH3-N. 10mg/L of NH3-N arrangement was the most proficient starting focus for adsorption.
Conflicts of Interest
The authors declare that they have no conflicts of interest
Availability of data and materials
All data, models, and code generated or used during the study appear in the submitted article.
Acknowledgment
The author(s) wish to acknowledge Hikmatullah Ahmadi Microbiology Laboratory, Taiyuan University of Technology, Taiyuan, Shanxi China, for providing the facilities needed for this research work.
References
- Shavisi Y, Sharifnia S, Zendehzaban M, et al. Application of solar light for degradation of ammonia in petrochemical wastewater by a floating TiO2/LECA photocatalyst. J Ind Eng Chem 20 (2014): 2806-2813.
- Lee J, Park H, Choi W. Selective photocatalytic oxidation of NH3 to N2 on platinized TiO2 in water, Environ. Sci. Technol. 36 (2002): 5462-5468.
- Zheng, Y. Chen, R. Wu, Long-Term Effects of Titanium Dioxide Nanoparticles on Nitrogen and Phosphorus Removal from Wastewater and Bacterial Community Shift in Activated Sludge. Environ Sci Technol 45 (2011): 7284-7290.
- Xiao S, Qu J, Zhao X, et al. Electrochemical process combined with UV light irradiation for synergistic degradation of ammonia in chloride-containing solutions. Water Res 43 (2009): 1432-1440.
- Huang J, Kankanamge NR, Chow C, et al. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J Environ Sci 63 (2018): 174-197.
- Ibrahim A, Yusof L, Beddu N, et al. 2016 IOP Conf. Series: Earth Environ Sci 36 (2016): 012-020.
- Walker M, Iyer K, Heaven S, et al. Ammonia removal in anaerobic digestion by biogas stripping: An evaluation of process alternatives using a first order rate model based on experimental findings. Chemical Engineering Journal 15 (2011): 14-24.
- Worawit Intrchom, Sagar Roy, Somenath Mitra. Functionalized carbon nanotube immobilized membrane for low temperature ammonia removal via membrane distillation. Separation and Purification Technology 235 (2020): 78-85.
- Min W, Ruzhen X, Yao C, et al. A novel mesoporous zeolite-activated carbon composite as an effective adsorbent for removal of ammonia-nitrogen and methylene blue from aqueous solution 268 (2018): 726-732.
- Austine O, Patrick H, James MR, et al. Pineapple peel biochar and lateritic soil as adsorbents for recovery of ammonium nitrogen from human urine. Journal of Environmental Management 293 (2021): 52-60.


 Impact Factor: * 2.8
Impact Factor: * 2.8 Acceptance Rate: 77.30%
Acceptance Rate: 77.30%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 