Double Synchronous Primary Gynecologic Carcinoma of the Endometrium and Both Ovaries: A Rare Encounter
William L Ho1, Chien-Kuan Lee1, Chi-Feng Su2, Horng-Jyh Tsai2*
1Department of Pathology, Kuang Tien General Hospital, Taichung, Taiwan
2Department of Obstetrics and Gynecology, Kuang Tien General Hospital, Taichung, Taiwan
*Corresponding Author: Horng-Jyh Tsai, Department of Obstetrics and Gynecology, Kuang Tien General Hospital, 117 Sha-Tian Road, Shalu, Taichung, 433, Taiwan.
Received: 07 December 2019; Accepted: 16 December 2019; Published: 20 December 2019
Article Information
Citation:
William L Ho, Chien-Kuan Lee, Chi-Feng Su, Horng-Jyh Tsai. Double Synchronous Primary Gynecologic Carcinoma of the Endometrium and Both Ovaries: A Rare Encounter. Obstetrics and Gynecology Research 2 (2019): 108-112.
View / Download Pdf Share at FacebookKeywords
Endometrium, Seromucinous, Carcinoma
Endometrium articles Endometrium Research articles Endometrium review articles Endometrium PubMed articles Endometrium PubMed Central articles Endometrium 2023 articles Endometrium 2024 articles Endometrium Scopus articles Endometrium impact factor journals Endometrium Scopus journals Endometrium PubMed journals Endometrium medical journals Endometrium free journals Endometrium best journals Endometrium top journals Endometrium free medical journals Endometrium famous journals Endometrium Google Scholar indexed journals Seromucinous articles Seromucinous Research articles Seromucinous review articles Seromucinous PubMed articles Seromucinous PubMed Central articles Seromucinous 2023 articles Seromucinous 2024 articles Seromucinous Scopus articles Seromucinous impact factor journals Seromucinous Scopus journals Seromucinous PubMed journals Seromucinous medical journals Seromucinous free journals Seromucinous best journals Seromucinous top journals Seromucinous free medical journals Seromucinous famous journals Seromucinous Google Scholar indexed journals Carcinoma articles Carcinoma Research articles Carcinoma review articles Carcinoma PubMed articles Carcinoma PubMed Central articles Carcinoma 2023 articles Carcinoma 2024 articles Carcinoma Scopus articles Carcinoma impact factor journals Carcinoma Scopus journals Carcinoma PubMed journals Carcinoma medical journals Carcinoma free journals Carcinoma best journals Carcinoma top journals Carcinoma free medical journals Carcinoma famous journals Carcinoma Google Scholar indexed journals uterine bleeding articles uterine bleeding Research articles uterine bleeding review articles uterine bleeding PubMed articles uterine bleeding PubMed Central articles uterine bleeding 2023 articles uterine bleeding 2024 articles uterine bleeding Scopus articles uterine bleeding impact factor journals uterine bleeding Scopus journals uterine bleeding PubMed journals uterine bleeding medical journals uterine bleeding free journals uterine bleeding best journals uterine bleeding top journals uterine bleeding free medical journals uterine bleeding famous journals uterine bleeding Google Scholar indexed journals adenocarcinoma articles adenocarcinoma Research articles adenocarcinoma review articles adenocarcinoma PubMed articles adenocarcinoma PubMed Central articles adenocarcinoma 2023 articles adenocarcinoma 2024 articles adenocarcinoma Scopus articles adenocarcinoma impact factor journals adenocarcinoma Scopus journals adenocarcinoma PubMed journals adenocarcinoma medical journals adenocarcinoma free journals adenocarcinoma best journals adenocarcinoma top journals adenocarcinoma free medical journals adenocarcinoma famous journals adenocarcinoma Google Scholar indexed journals squamous differentiation articles squamous differentiation Research articles squamous differentiation review articles squamous differentiation PubMed articles squamous differentiation PubMed Central articles squamous differentiation 2023 articles squamous differentiation 2024 articles squamous differentiation Scopus articles squamous differentiation impact factor journals squamous differentiation Scopus journals squamous differentiation PubMed journals squamous differentiation medical journals squamous differentiation free journals squamous differentiation best journals squamous differentiation top journals squamous differentiation free medical journals squamous differentiation famous journals squamous differentiation Google Scholar indexed journals endometrial cancer articles endometrial cancer Research articles endometrial cancer review articles endometrial cancer PubMed articles endometrial cancer PubMed Central articles endometrial cancer 2023 articles endometrial cancer 2024 articles endometrial cancer Scopus articles endometrial cancer impact factor journals endometrial cancer Scopus journals endometrial cancer PubMed journals endometrial cancer medical journals endometrial cancer free journals endometrial cancer best journals endometrial cancer top journals endometrial cancer free medical journals endometrial cancer famous journals endometrial cancer Google Scholar indexed journals ovarian articles ovarian Research articles ovarian review articles ovarian PubMed articles ovarian PubMed Central articles ovarian 2023 articles ovarian 2024 articles ovarian Scopus articles ovarian impact factor journals ovarian Scopus journals ovarian PubMed journals ovarian medical journals ovarian free journals ovarian best journals ovarian top journals ovarian free medical journals ovarian famous journals ovarian Google Scholar indexed journals endometrial tumor articles endometrial tumor Research articles endometrial tumor review articles endometrial tumor PubMed articles endometrial tumor PubMed Central articles endometrial tumor 2023 articles endometrial tumor 2024 articles endometrial tumor Scopus articles endometrial tumor impact factor journals endometrial tumor Scopus journals endometrial tumor PubMed journals endometrial tumor medical journals endometrial tumor free journals endometrial tumor best journals endometrial tumor top journals endometrial tumor free medical journals endometrial tumor famous journals endometrial tumor Google Scholar indexed journals gastrointestinal articles gastrointestinal Research articles gastrointestinal review articles gastrointestinal PubMed articles gastrointestinal PubMed Central articles gastrointestinal 2023 articles gastrointestinal 2024 articles gastrointestinal Scopus articles gastrointestinal impact factor journals gastrointestinal Scopus journals gastrointestinal PubMed journals gastrointestinal medical journals gastrointestinal free journals gastrointestinal best journals gastrointestinal top journals gastrointestinal free medical journals gastrointestinal famous journals gastrointestinal Google Scholar indexed journals
Article Details
1. Case Report
A 51-year-old nulligravida presented with some irregular abnormal uterine bleeding on and off, spotting in nature, of a few months duration, and was referred to our hospital for management. Her medical history reviewed well controlled chronic hypertension of 15 years duration, and fairly controlled diabetes mellitus with hyperlipidemia of 6 years duration. Surgical history showed anal hemorrhoid, and had been treated in the clinic. Physically, her body mass index was 25 kg/m2, and otherwise she was relatively healthy apparent. The first gynecologic ultrasound examination detected her endometrium 0.8 cm, uterine myomas 3.0 cm at anterofundal area and 3.7 cm at posterior wall. One month prior to the admission, a Pap test reported adenocarcinoma; then diagnostic D&C showed endometrial adenocarcinoma, well to moderately differentiated, with squamous differentiation. Inmunohistochemistry stain of tumor cells showed P16 (equivocal), ER (+, 80%), PR (+, 70%), vimentin (+) and CEA (equivocal). Tumor markers reported CA125 84.74 U/mL, CA-199 440.3 U/mL and CEA 2.61 ng/ml. Magnetic resonance imaging confirmed an1. 3 cm endometrial tumor compatible to endometrial cancer, uterine myomas, and a right ovarian cystic lesion (Figure 1a). She underwent gynecologic cancer staging operation, including total abdominal hysterectomy, bilateral salpingo-oophorectomy, lymphadenectomy and omentectomy. Uterine myomas , a small endometrial lesion, a small right ovarian cystic lesion of 24 mm and a small left ovarian lesion of 15 mm were found [Figure 1b]. The rest of the gross findings were unremarkable. Microscopically, the borderline tumor component shows a complex branching papillary architecture, with the epithelial lining resembling “endocervical-type” mucinous or serous epithelium, which is different from the “gastrointestinal- type” epithelium seen in ovarian mucinous tumors. High grade nuclear atypia is noted at the intraepithelial carcinoma component, including high Nucleus-Cytoplalsm ratio, vesicular chromatin and nucleoli. The invasive carcinoma area is represented by small nests of atypical epithelial cells surrounded by desmoplastic stroma. Therefore, the Pathology reported endometrioid carcinoma of the endometrium, well differentiated (FIGO grade 1), and the tumor invaded to superficial myometrium , FIGO stage IA; seromucinous carcinoma of the right ovary, limited to the ovary (Figure 2a, b), FIGO stage IA and seromucinous borderline tumor of left ovary, with microinvasion and intraepithelial carcinoma, and the tumor limited to the left ovary (Figure 3). The tumor cells are immunoreactive to the ER (>95%) and PAX8 and negative for p16 (negative, patchy), WT-1 and napsin A. The expression of p53 is wild type. The rest of the microscopic feature was unremarkable. Early double primary carcinoma is verified, but not metastatic cancer. Post-operative course was rather smooth without any complications. She was in remission at the time of this report and for continuing follow-up.
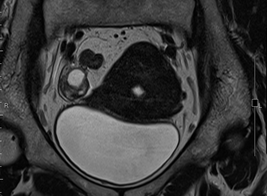
Figure 1a: MRI showing 1.3 cm endometrial tumor and right ovarian cystic lesion.
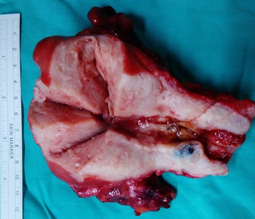
Figure 1b: Gross finding of uterine myomas and small endometrial tumor, and small ovarian cystic lesion.
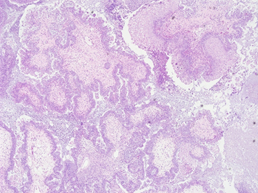
Figure 2a: Microscopic section of seromucinous carcinoma of right ovary (H&E 40X).
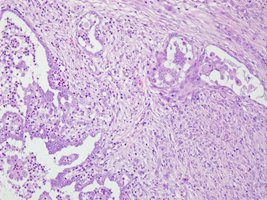
Figure 2b: Right ovary with invasive carcinoma in desmoplastic stroma (H&E 200X).
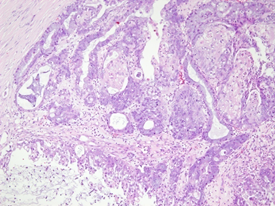
Figure 3: Microscopic section of borderline tumor of left ovary showing similar morphology to that of right ovarian tumor (H&E 100X).
Synchronous primary cancers of the endometrium and ovary are not uncommon, and they are found in 10% of women with ovarian cancer and 5% of women with endometrial cancer [1]. A retrospective observational study examining Surveillance, Epidemiology, and End Results Program (SEER) between 1973 and 2013, concluded that synchronous ovarian cancer has decreased among endometrial cancer whereas synchronous endometrial cancer has increased among epithelial ovarian cancer [2]. It is known that women with synchronous primary cancers of the endometrium and ovary are young, obese, nulliparous, and premenopausal. In a study with a series of 84 patients, 68% of them (57) had endometrioid histology of both their endometrial and ovarian cancers; and the patients with concordant endometrioid histology had a favorable prognosis [1]. Seromucinous carcinoma of the ovary is an uncommon tumor. Given the characteristic admixture of cell types and the overlapping morphologic features with low-grade serous, mucinous, and endometrioid neoplasms; they are a new category of ovarian epithelial tumor in the revised World Health Organization Classification of Tumors of the Female Reproductive Organs. Borderline carcinomas are well described, but there have been few reports of seromucinous carcinomas [3]. Among 19 patients of ovarian seromucinous carcinoma, a synchronous uterine endometrioid adenocarcinoma was reported in 1 case [3]. Since the most appropriate categorization of seromucinous carcinomas remained uncertain, they are probably best regarded as a distinct type of ovarian epithelial malignancy, and are most likely behavior similar to endometrioid adenocarcinomas [3]. But, the controversy regarding the terminology of non-serous borderline tumors, or so-called "atypical proliferative tumor", in view of their largely benign behavior, has not been resolved. For seromucinous borderline tumor of left ovary of our patient, the concepts of intraepithelial carcinoma and/or microinvasion shall evolve even more in further studies, as their presence appears to have no prognostic impact; and also it is a subject to considerable inter-observer variability [4]. In a retrospective case-control study examining SEER program between 1973 and 2013, survival of women with stage I endometrioid endometrial cancer with stage I endometrioid ovarian cancer [EOC] (n=839) were compared to women with stage I EOC without synchronous ovarian cancer (n=123,692), a survival outcome was similar [5]. Although a retrospective analysis (in China) of 63 pathologically proven patients of synchronous primary endometrial and ovarian cancer reported lymphovascular space invasion (LVSI), non-endometrioid type ovarian cancer and stage of ovarian cancer above stage I were independent prognostic factors for progression-free survival (PFS); while LVSI, high grade of endometrial cancer, and stage of ovarian cancer above stage I were independent prognostic factors for 5-year overall survival [6]. In another multivariate analysis of 65 women with known lymph node status revealed two prognostically distinct groups: a high-risk group comprising endometrial endometrioid carcinomas (EECs) with ≥ 50% myometrial invasion irrespective of lymph node status (n?=?21; median PFS 12.7?months, 95% CI, 9.24-19.8); and a low-risk group consisting of all endometrial non-endometrioid endometrial carcinomas (ENECs), as well as lymph node-negative ENECs with <50% myometrial invasion (n?=?44, median PFS not reached) [7]. Therefore, in conclusion, we expect our patient although with non-concordant early double primary gynecologic carcinoma, not metastatic, will have a good prognosis.
2. Conflicts of Interest
The authors declare no conflicts of interest.
References
- Soliman PT, Slomovitz BM, Broaddus RR, Sun CC, Oh JC, Eifel PJ, et al. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol 94 (2004): 456-462.
- Matsuo K, Machida H, Blake EA, Holman LL, Rimel BJ, Roman LD, et al. Trends and outcomes of women with synchronous endometrial and ovarian cancer. Oncotarget 9 (2018): 28757-28771.
- Taylor J, McCluggage WG. Ovarian seromucinous carcinoma: report of a series of a newly categorized and uncommon neoplasm. Am J Surg Pathol 39 (2015): 983-992.
- Hauptmann S, Friedrich K, Redline R, Avril S. Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch 470 (2017): 125-142.
- Matsuo K, Machida H, Frimer M, Marcus JZ, Pejovic T, Roman LD, et al. Prognosis of women with stage I endometrioid endometrial cancer and synchronous stage I endometrioid ovarian cancer. Gynecol Oncol 147 (2017): 558-564.
- Wang Y, Yu M, Yang JX, Cao DY, Zhang Y, Shen K , et al. [Clinicopathologic and survival analysis of synchronous primary endometrial and ovarian cancer.] Zhonghua Fu Chan Ke Za Zhi 53 (2018): 816-822.
- Turashvili G, Gómez-Hidalgo NR, Flynn J, Gonen M, Leitao MM Jr, Soslow RA, et al. Risk-based stratification of carcinomas concurrently involving the endometrium and ovary. Gynecol Oncol 152 (2019): 38-45.


 Impact Factor: * 3.2
Impact Factor: * 3.2 Acceptance Rate: 76.63%
Acceptance Rate: 76.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 