Functional Vitamin B2 Deficiency in Autism
Gregory Russell-Jones*
Department of Pharmacy, Mentor Pharmaceutical Consulting Private Limited, Sydney, Australia
*Corresponding Author: Gregory Russell-Jones, Department of Pharmacy, Mentor Pharmaceutical Consulting Private Limited, Sydney, Australia
Received: 10 May 2022; Accepted: 19 May 2022; Published: 17 July 2022
Article Information
Citation: Gregory Russell-Jones. Functional Vitamin B2 Deficiency in Autism. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 324-328.
View / Download Pdf Share at FacebookAbstract
Functional vitamin B2 levels were assessed in 600 children with autism aged between two and 30 years old. Every child assessed was found to have functional vitamin B2 deficiency. The deficiency appears to have stemmed from deficiencies in Iodine, Selenium and/or Molybdenum as was found in a previous report by this lab. Functional vitamin B2 is a known essential co-factor in the maintenance of the activity of vitamin B12, and potentially this would also mean that each child was deficient in functional vitamin B12 – a known predisposing factor for autism.
Keywords
<p>Autism, Organic Acids Test, Vitamin B2, Riboflavin</p>
Article Details
1. Introduction
Riboflavin, also known as vitamin B2, is known to play an essential role in health and metabolism. In order to do this, however, riboflavin must first be modified (activated) within the body. The activation of dietary or supplemental riboflavin involves contribution from Thyroid Stimulating Hormone (TSH), which stimulates the uptake of Iodine into the thyroid gland to make thyroid hormone (tetraiodothyronine, T4). T4 is in turn activated by removal of one Iodine to form T3 (triiodothyronine) by the Selenoprotein, iodothyronine deiodinase. T3 is then used in turning on the production of the first enzyme involved in riboflavin modification, riboflavin kinase. The resultant molecule, flavin mononucleotide (FMN), is further modified by the Molybdopterin-protein, FAD synthase, to form FAD. Hence, all three of Iodine, Selenium and Molybdenum are required for the complete activation of vitamin B2 to FMN and FAD. The activated FMN and FAD are used as co-factors in over 100 “flavoproteins” in the body. Several of these flavoproteins have important roles in the body in the activation of several vitamins, or in the maintenance of vitamin activity. Hence, FMN is a co-factor in the enzyme pyridoxine 5-phosphateoxidase, which converts pyridoxal to pyridoxal-phosphate, the active form of vitamin B6. FMN and FAD work together in several enzymes, including methionine synthase reductase (MTRR), a rescue enzyme in methyl B12 metabolism. FAD is also involved in the activity of methylene-tetrahydrofolate reductase (MTHR), an enzyme that is responsible for converting 5,10-methylenetetrahydro-folate to 5-methyltetrahydrofolate (5MTHF).
Vitamin B2, as FAD, is an essential co-factor for long, medium and short chain acylCoA-dehydrogenase [1, 2], and as such has a critical role in fat metabolism. Insufficient FAD in the womb, leads to a reduced capacity of the foetus, neonate or child to metabolize fat for energy, and can lead to many conditions including failure to thrive, metabolic acidosis, ketotic hypoglycemia, lethargy, hypotonia, developmental delay, seizure, myopathy and dystonia. FAD also is involved in a complex co-enzyme complex within pyruvate dehydrogenase interacting with thiamine-pyrophosphate (TPP) and alpha-lipoic acid (ALA), plus nicotinamide adenine dinucleotide and acetyl-CoA. Pyruvate dehydrogenase is effectively the last enzyme in glycolysis and lack of FAD reduces the conversion of pyruvate to acetyl-CoA. This then leads to the isomeric conversion of pyruvate to lactate, with the subsequent build-up of lactic acid. Genetic mutations in pyruvate dehydrogenase have been associated with elevated levels of lactic acid in serum, and a syndrome of neurologic signs, including hypotonia, epilepsy, metabolic abnormalities and microcephaly. Developmental delay is a nearly universal consequence of the condition [3]. FMN is also an essential co-factor for the citric acid cycle enzyme succinate dehydrogenase/respiratory complex II. The major enzyme that “bridges” Krebs cycle and the Electron Transport Chain. FMN, through its’ role in the activation of vitamin B6, is also required for the formation of Serotonin from 5-hyroxytryptophan by the P5P-dependent enzyme, hydroxytryptophan decarboxylase.
In the study reported here, Organic Acids Test data obtained from children with a diagnosis of Autism Spectrum Disorder (ASD) has been used to ascertain if there is an alteration in the accumulation of various markers associated with functional B2 deficiency, including glutaric acid, fatty acids, lactic acid, Quinolinic Acid:Kynurenic Acid, and oxalic acid, in these individuals.
2. Methods
2.1 Study sample
Data analysis was carried out under the Australian National Health and Medical Research Council guidelines (NHMRC). Under these guidelines, all data was deidentified and steps were taken to ensure the anonymity and confidentiality of the data. Deidentification has consisted of absolute anonymity and confidentiality of the data, such that no specifics such as gender, ethnicity, Country of Origin, etc is associated with any data point in the study. As such per the NHMRC guidelines:
- The research does not carry any risk to the participants.
- The benefits of the research are many and will be of considerable benefit to any past, current or future participants, and as such represent no harm.
- The data is from over 600 participants collected over 6 years and as such it would be impracticable to obtain consent from the participants. Further the participants had been notified at the time of analysis that data presented for analysis might potentially be used in research – but would be totally de-identified (which it has been).
- There is no known reason why any of the participants would not have consented if they had been asked.
- Given the total de-identification of the data, there is absolute protection of their privacy.
- Data is only housed in one location and has only been assessed by one person, and as such the confidentiality of the data can be assured.
- No financial benefits from the data is anticipated, rather the data will be used to help prevent and treat those to whom the data applies.
- The waiver is not prohibited by State, Federal or International Law.
A retrospective analysis was performed upon data submitted to us for analysis from a cohort of 600 children and adults who had been diagnosed with ASD from countries including USA, Canada, United Kingdom, Ireland, Germany, Spain, France, Italy, Bulgaria, India, Sweden, Bulgaria, Serbia, Dubai, Croatia and Australia. Age distribution of the 600 individuals was 1-5 (358); 6-10 (172); 11-15 (46); 16-20 (9); >21 (16). No selection was made in the acceptance of data, with no data being rejected. We were not made privy to either the methods of assessment nor of the severity of the Developmental Delay in the Children. Data is presented regardless of sex, or age. Ages varied from 1 year old to thirty-four years old. Organic Acid Test Data (600 sets, Great Plains Laboratories, Lenexa, KS, USA), which had been submitted to us for interpretation, by parents of children with autism spectrum disorder, (ASD) was compared to that from persons who were healthy, and who had no previously identified health condition (NT). Individual data is plotted as Scattergrams (see Graphs 1 to 3). Organic Acid Test Data (OAT) (600 sets, Great Plains Laboratories, Lenexa, KS, USA), from children with ASD, was compared to that from persons who were healthy, and who had no previously identified health condition, and was plotted as Scattergrams (see Graphs 1 to 8). Data is represented as mmol/mol creatine for NT (n=20), and ASD (n=600)
3. Results
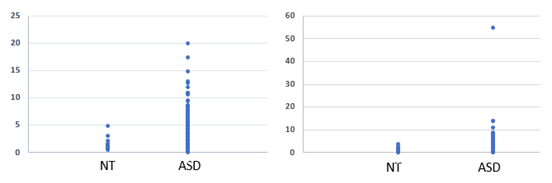
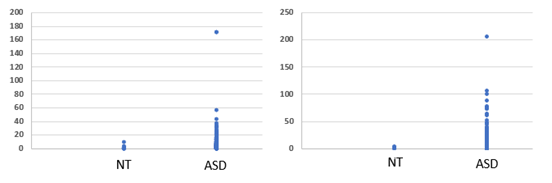
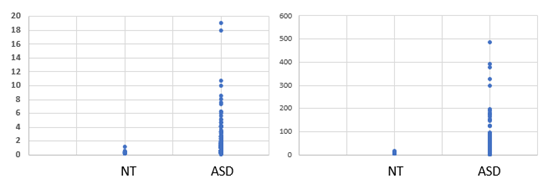
Analysis of OAT data from 600 children diagnosed with ASD revealed each and every child had a functional deficiency in vitamin B2 (see Figures 1 to 4), as defined by elevation in levels of Organic Acids. Thus, there was reduced activity of acyl-CoA-reductase (Figures 1, 2), leading to elevations in Ethylmalonic acid, Methylsuccinic acid, adipic acid and suberic acid, as well as reduced activity of the FAD-dependent enzyme, Glutaryl-CoA reductase, leading to elevated glutaric acid (Figure 3). In addition, there was reduced activity of the FAD/TPP/lipoate- dependent enzyme pyruvate dehydrogenase, leading to elevated lactic acid (Figure 4 Left Panel). These large elevations in organic acids, reflect energy loss from the metabolism of fats and sugars, and as a result in order to obtain sufficient energy, the ASD individuals revert to the catabolism of proteins, yielding glucogenic and ketogenic amino acids. Part of this catabolism is the metabolism of hydroxy-proline, to yield glyoxylate, which is then converted to Glycolate via the FAD-dependent enzyme, glyoxylate reductase. In FAD-deficiency, however, glyoxylate is oxidized to yield oxalic acid, via lactate dehydrogenase and glyoxylate oxidase. In concordance with the elevations in other FAD-deficiency markers, levels of oxalic acid were elevated in persons with ASD (Figure 4. Right Panel). Conversion of kynurenin to kynurenic acid is dependent upon the vitamin B6 dependent enzyme kynurenin amino transferase, and in functional FMN deficiency, there is reduced production of the active form of vitamin B6, P5P, resulting in an increased ratio of QA:KA (Table 1). The degree of functional B2 deficiency was highly variable between ASD individuals as is reflected in the high standard deviation from the mean values (Table 1)
|
Marker |
NT |
ASD |
|
Ethyl Malonic Acid |
1.53 +/- 1.12 |
3.10 ± 2.3 |
|
Methyl Succinic Acid |
1.25 ± 1.02 |
3.11 ± 3.1 |
|
Adipic Acid |
1.45 ± 1.2 |
7.12 ± 15.2 |
|
Suberic Acid |
1.77 ± 2.29 |
6.0 ± 11.9 |
|
Lactate |
12.9 ± 15.6 |
29.1 ± 40.28 |
|
QA:KA |
3.12 ± 2.05 |
5.32 ± 12.89 |
|
Succinate |
4.48 ± 3.14 |
28.5 ± 44.8 |
|
Glutarate |
0.27 ± 0.24 |
1.25 ± 1.77 |
|
Oxalate |
71.73 ± 50.6 |
269 ± 231 |
Table 1: Functional Differences in Vitamin B2 -deficiency related markers between neurotypical (NT) persons and those with autism (ASD). Data is presented as Mean ± STD
4. Discussion
In a previous article [4] it was found that all children with ASD that were tested were deficient in one of more of Iodine, Selenium and Molybdenum, each of which is involved in the activation of vitamin B2 (riboflavin) to FMN and FAD. The current study has extended those findings and has shown that the previously prescribed mineral deficiencies, are accompanied by an expected deficiency in functional vitamin B2. This deficiency then affects the metabolism of fatty acids, glucose and amino acids. Functional B2 deficiency also affects the activity of succinate dehydrogenase – the linking enzyme between energy production in Krebs cycle and electron transfer in the Electron Transport Chain. In addition, functional B2 (as FMN) is also required for the activation of vitamin B6. Given that it is known that vitamin B2, in its active forms, FMN and FAD, is involved as a co-factor in over 100 enzymes, these findings have profound implications for energy production in the brain of the children and for the activation and control of neurotransmitters, amongst other reactions.
The observed deficiency in activation of vitamin B2, is accompanied by a reduced ability to burn fat for energy, and so may explain the observed higher rate of obesity in persons with autism [5-11], and the higher rate of obesity in mothers of children with autism [15-17]. Functional vitamin B2 deficiency, also affects the ability of the enzyme pyruvate dehydrogenase to process pyruvate, which effectively blocks the glycolysis pathway leading to elevated lactic acid. Persons with mutations in the pyruvate dehydrogenase enzyme have been shown to have very elevated lactic acid and neurodevelopmental delay is a universal finding in these individuals [18]. Elevated lactic acid is one of the features of diabetes, lack of activity of pyruvate dehydrogenase may explain the higher rate of diabetes seen in persons with autism [19, 20] as well as the known association of gestational diabetes in mothers and the subsequent development of autism in the children [21-28]. The reduced ability to produce energy from glucose, the preferred energy source in the brain, would in turn necessitate the catabolism of protein to form ketogenic amino acids, and would explain the elevated levels of glutaric acid and oxalic acid observed in these individuals.
More importantly, the measured association of deficiencies of Iodine, Selenium and/or Molybdenum and functional B2 deficiency in those with autism also provides a potential mode of prevention and treatment of autism. In addition, the same approach has potential application to weight loss in obesity and glucose reduction in diabetes. In separate studies, not reported here, we have examined the levels of functional vitamin B12, using Urinary Organic Acids Tests, and in these studies, every child who was found to be functionally deficient in vitamin B2 was also functionally deficient in vitamin B12. In this regard is has been known for over 40 years that vitamin B12 deficiency in the neonate is associated with developmental delay in children [29-34]. This then poses the theory that the dramatic increase in the rate of autism over the past 20 years could be due to decreases in the intake of Iodine, Selenium and/or Molybdenum in countries, in which soil deficiencies are now more common. Hence lack of one single nutrient could be the precipitating event that results in the spectrum of developmental delays such as those seen in Autism Spectrum Disorder.
5. Conclusions
Dietary deficiency of Iodine, Selenium and/or Molybdenum in children with autism, results in insufficient activation of dietary vitamin B2. This then leads to a functional deficiency in vitamin B2, thereby resulting in reduced capacity of the child to gain energy from the metabolism of fats, sugar and protein. In addition, functional B2 is an essential requirement for the metabolism of vitamin B12, and hence a deficiency in functional vitamin B2 would potentially lead to the accumulation of inactive vitamin B12, in these individuals, a known predisposing factor for developmental delay.
Acknowledgements
Acknowledgements go to all of those parents who have contributed their data to the preparation of this manuscript in the hope that the manuscript will be of benefit to those who have children with autism.
Compliance with Ethical Standards
- We declare that there are no potential conflicts of interest.
- Research did not involve human participants and/or animals.
- No Informed consent is required, all data is “blinded” and as such is anonymous.
References
- Gregersen N. Riboflavin-responsive defects of beta-oxidation. Journal of inherited metabolic disease (1985): 65-69.
- Tanaka K, Yokota I, Coates P M, et al. Mutations in the medium chain acyl-CoA dehydrogenase (MCAD) gene. Human mutation 1 (1992): 271-279.
- Wolfe L, Jethva R, Oglesbee D, et al. Short-Chain Acyl-CoA Dehydrogenase Deficiency. In M. P. Adam (Eds.) et. al., GeneReviews®. University of Washington, Seattle (2011).
- Russell-Jones GJ. Deficiency of the essential metals, Iodine, Selenium and Molybdenum in individuals with Autism. Autism Research (2021).
- Dhaliwal K K, Orsso C E, Richard C, et al. Risk Factors for Unhealthy Weight Gain and Obesity among Children with Autism Spectrum Disorder. International journal of molecular sciences 20 (2019): 3285.
- Srinivasan S M, Pescatello L S, Bhat A N. Current perspectives on physical activity and exercise recommendations for children and adolescents with autism spectrum disorders. Physical therapy 94 (2014): 875-889.
- Kahathuduwa C N, West B D, Blume J, et al. The risk of overweight and obesity in children with autism spectrum disorders: A systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity 20 (2019) 1667-1679.
- Hill A P, Zuckerman K E, Fombonne E. Obesity and Autism. Pediatrics 136 (2015): 1051-1061.
- Jones R A, Downing K, Rinehart N J, et al. Physical activity, sedentary behavior and their correlates in children with Autism Spectrum Disorder: A systematic review. PloS one 12 (2017): e0172482.
- Pham D, Silver S, Haq S, et al. Obesity and Severe Obesity in Children with Autism Spectrum Disorder: Prevalence and Risk Factors. Southern medical journal 113 (2020): 168-175.
- Li Y J, Xie X N, Lei X, et al. Global prevalence of obesity, overweight and under-weight in children, adolescents and adults with autism spectrum disorder, attention-deficit hyperactivity disorder: A systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity 21 (2020): e13123.
- Matheson B E, Eichen D M. A Review of Childhood Behavioral Problems and Disorders in the Development of Obesity: Attention Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, and Beyond. Current obesity reports 7 (2018): 19-26.
- Walls M, Curtin C, Phillips S, et al. Developmental-Behavioral Pediatricians' Diagnosis and Coding of Overweight and Obesity in Children with Autism Spectrum Disorder. Journal of developmental and behavioral pediatrics: JDBP 41 (2020): 258-264.
- Healy S, Aigner C J, Haegele JA. Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism: the international journal of research and practice 23 (2019): 1046-1050.
- Edlow A G. Maternal obesity and neuro-developmental and psychiatric disorders in offspring. Prenatal diagnosis 37 (2007): 95-110.
- Sanchez C E, Barry C, Sabhlok A, et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity 19 (2018): 464-484.
- Contu L, Hawkes C A. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. International journal of molecular sciences 18 (2007): 1093.
- Sofou K, Dahlin M, Hallböök T, et al. Ketogenic diet in pyruvate dehydrogenase complex deficiency: short- and long-term outcomes. J Inherit Metab Dis 40 (2017): 237-245.
- Tanaka K, Yokota I, Coates P M, et al. Management and quality indicators of diabetes mellitus in people with intellectual disabilities. Journal of intellectual disability research : JIDR 57 (2013): 1152-1163.
- Shedlock K, Susi A, Gorman G H, et al. Autism Spectrum Disorders and Metabolic Complications of Obesity. The Journal of pediatrics 178 (2016): 183-187.e1.
- Wang X, Lu J, Xie W, et al. Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dis-mutase 2. Proceedings of the National Academy of Sciences of the United States of America 116 (2019): 23743-23752.
- Aviel-Shekler K, Hamshawi Y, Sirhan W, et al. Gestational diabetes induces behavioral and brain gene transcription dysregulation in adult offspring. Translational psychiatry 10 (2020): 412.
- Cafiero P J, Krochik G. Diabetes materna y trastornos del neurodesarrollo en los hijos [Maternal diabetes and neurodevelopmental disorders in offspring]. Medicina 80 (2020): 685-695.
- Xiang A H, Wang X, Martinez M P, et al. Association of maternal diabetes with autism in offspring. JAMA 313 (2015): 1425-1434.
- Rotem R S, Chodick G, Shalev V, et al. Maternal Thyroid Disorders and Risk of Autism Spectrum Disorder in Progeny. Epidemiology (Cambridge, Mass.) 31 (2020): 409-417.
- Aguilar Cordero A M J, Baena García L, Rodríguez Blanque R, et al. Maternal Diabetes Mellitus and its Impact on Child Neurodevelopment; Systematic Review. Nutricion hospitalaria 32 (2015): 2484-2495.
- Kong L, Nilsson I, Brismar K, et al. Associations of Different Types of Maternal Diabetes and Body Mass Index with Offspring Psychiatric Disorders. JAMA network open 3 (2020): e1920787.
- Wan H, Zhang C, Li H, et al. Association of maternal diabetes with autism spectrum disorders in offspring: A systemic review and meta-analysis. Medicine 97 (2018): e9438.
- Hall C A. Function of vitamin B12 in the central nervous system as revealed by congenital defects. American journal of hematology 34 (1990): 121-127.
- Dror D K, Allen L H. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutrition reviews 66 (2008): 250-255.
- Kocaoglu C, Akin F, Caksen H, et al. Cerebral atrophy in a vitamin B12-deficient infant of a vegetarian mother. Journal of health, population, and nutrition 32 (2014): 367-371.
- Bousselamti A, El Hasbaoui B, Echahdi H, et al. Psychomotor regression due to vitamin B12 deficiency. The Pan African medical journal (2018).
- Bravo J P, Ibarra C J, Paredes M M. Hematological and neurological compromise due to vitamin B12 deficit in infant of a vegetarian mother: case report. Revista chilena de pediatria 85 (2014): 337-343.
- Chalouhi C, Faesch S, Anthoine-Milhomme M C, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatric emergency care 24 (2008): 538-541.


 Impact Factor: * 4.8
Impact Factor: * 4.8 Acceptance Rate: 69.70%
Acceptance Rate: 69.70%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 