Variation in Extemporaneous Glucocorticoid Formulations and Doses for Asthma-like Symptoms in Children
Schmidt M1*, Haslund-Krog S1,4*, Kryger Jensen A2,3, Jorgensen IM4,5, Holst H1
1Department of Clinical Pharmacology, Bispebjerg University Hospital, Copenhagen, Denmark
2Biostatistics, Institute of Public Health, University of Copenhagen, Denmark
3Department of Clinical Research, Nordsjaellands Hospital, Hilleroed, Denmark
4Department of Paediatric and Adolescence Medicine, Nordsjaellands Hospital, Hilleroed, Denmark
5Institute for Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
*Authors who contributed equally to the paper
*Corresponding Author: Sissel Haslund-Krog, Department of Clinical Pharmacology, Bispebjerg University Hospital, Copenhagen, Denmark
Maria Schmidt, Department of Clinical Pharmacology, Bispebjerg University Hospital, Copenhagen, Denmark
Received: 08 July 2019; Accepted: 23 July 2019; Published: 09 September 2019
Article Information
Citation:
Schmidt M, Haslund-Krog S, Kryger Jensen A, Jorgensen IM, Holst H. Variation in Extemporaneous Glucocorticoid Formulations and Doses for Asthma-like Symptoms in Children. Journal of Pediatrics, Perinatology and Child Health 3 (2019): 104-114.
View / Download Pdf Share at FacebookAbstract
Treatment strategies for asthma-like symptoms are challenging with no international consensus on dose of systemic glucocorticoids. Despite, asthma-like symptoms remain one of the most common medical conditions seen in infants and preschool children. This is further complicated by limited licensed preparations and use of various extemporaneously prepared formulations in some countries. In Denmark, all extemporaneous formulations are produced according to GMP, which is different from other European countries. This retrospective cohort study examined the type and dose of extemporaneously prepared glucocorticoid formulations prescribed to infants and preschool children 0-5 years of age with acute asthma-like symptoms over a three-year period at three paediatric departments in the Capital Region of Denmark covering 1.8 mill inhabitants. In total 4877 patients were screened for eligibility. In addition, length of hospitalisation, prescribed co-medication, readmission, and adverse events were registered. Almost one in ten of the patients were treated with extemporaneous prednisolone. Eight different formulations were used interchangeably despite lack of studies supporting therapeutic equivalence between these formulations. Further, a higher dose did not result in shorter hospitalisation.
Keywords
<p>Paediatric, Pharmacology, Asthma, Wheezing, Extemporaneous formulations</p>
Article Details
1. Introduction
Asthma-like symptoms remain the most common medical condition seen in infants and preschool children [1]. The symptoms, such as wheezing, coughing and breathing difficulty, range from mild and self-limiting to severe and life-threatening and is one of the most frequent causes of hospital admissions in young children [2, 3]. The European Respiratory Society defined two pragmatic symptom-based classifications of wheezing in 2008: Episodic Viral Wheeze (EVW) and Multiple Trigger Wheeze (MTW) in the 0-5 year’s age group [4, 5]. These phenotypes cannot reliably be distinguished when the symptoms first appear, and vary comprehensively over time limiting the value in clinical practice [4, 6-8]. This heterogeneous group of patients is therefore diagnosed interchangeably with asthma, recurrent wheezing, and asthmatic bronchitis. In Denmark, the clinical diagnosis ‘asthmatic bronchitis’ is frequently used for patients who present with a viral-induced cough, wheezing, and dyspnoea, which diagnostically resembles EVW. In general, first line treatment of EVW is intermittent bronchodilator therapy, whereas the role of oral corticosteroids is debated [2-4, 9, 10]. As a result of limited data from randomised controlled trials and difficulties in applying precise diagnostic criteria, dosing recommendations, route and choice of glucocorticoids are inconsistent [2-4, 10]. Proposed dosing varies both internationally and nationally [2, 3]. It is important to use the lowest effective dose to reduce the risk of dose-dependent adverse effects [11, 12]. Especially adverse behavioural effects, e.g., anxiety and aggressive behaviour were found to occur more frequently with higher doses of prednisone or prednisolone [13].
In Denmark, the licensed formulations of glucocorticoids are limited to oral tablet strengths inappropriate for children and intravenous preparations. In other EU countries, licensed oral glucocorticoid solutions for paediatric use are available. Instead, extemporaneous formulations are available in Denmark, manufactured under the Good Manufacturing Practice (GMP) regulations [14] by two specialized private pharmacies. The products are national formulations sold to public hospitals. Nonetheless, extemporaneous formulations often lack stability, efficacy, and safety studies [15]. Because of diagnostic challenges in preschool children with asthma-like symptoms and the extensive use of different extemporaneous prednisolone formulations, it is difficult to improve treatment strategies.
The objective of this study was to ascertain the type and dose of extemporaneously prepared glucocorticoid formulations administered to children 0-5 years of age with acute asthma-like symptoms at three different paediatric departments in the Danish Capital Region. Further, to evaluate if the selected dose affected the length of hospitalisation, prescribed co-medication, readmissions, and adverse events.
2. Methods
2.1 Design and settings
We conducted a retrospective cohort study on admitted patients or patients with an emergency visit at three paediatric departments at Copenhagen University Hospital Hvidovre, -Herlev and -Nordsjælland, Hillerød. All three hospitals were secondary hospitals with similar size reference areas in the Capital Region of Denmark. Each hospital had a paediatric emergency room and treated typical paediatric diseases. The reference areas encompass a broad spectrum of sociodemographic populations representing families attaining all educational levels. The study was approved by the Institutional Data Safety Committee, the Danish Data Protection Agency (BFH-2015-089 and I-Suite no.: 04284), and The Board for Patient Safety, Danish Health and Medicines Authority (ID: 3-3013-1457/1), waiving the need for patient consent. Data were retrospectively captured through chart review and extraction from electronic health records (EHR) from September 2016 to October 2017.
2.2 Study population
Infants and preschool children 0-5 years of age with a main diagnosis of asthma-like symptoms, defined by ICD (International Classifications of Diseases)-codes DJ20.9 and DJ45.9, and administered an extemporaneous glucocorticoid formulation were included between January 2013 and December 2015. Dispensed or administered doses of extemporaneous glucocorticoids were included in accordance with the Electronic Patient Medication Module (EPM). The treatment recommendations for oral systemic glucocorticoids for severe asthma-like symptoms differed in the three departments (2013-15): Hospital A: Initial dose of up to 2 mg/kg followed by 1 mg/kg for a total of 3 days; Hospital B and C: Initial and maintenance dose of 1 mg/kg for a total of 3 days.
2.3 Data extraction variables
Patient data were collected from the time of administration of the first dose of extemporaneous glucocorticoid formulation. Demographic data at baseline included the date of birth, weight, gender, diagnose: ICD codes J45.9 (Other and unspecified asthma) or J20.9 (Acute bronchitis, unspecified), comorbidity, length of hospitalisation in days, and prednisolone dose and formulation. Further, co-medication on and during admission (asthma medication/antibiotics), culture and sensitivity results from microbiology, adverse events and defined readmissions were registered. Adverse events (AEs) were captured by two reviewers when it was described as an adverse event in the chart and coded using the current version of the Medical Dictionary for Regulatory Activities, Version 19.0 (MedDRA MSSO, McLean, Virginia, USA). If the child was readmitted within seven days, this was regarded as a part of the recent admission and was excluded.
2.4 Data analysis
Continuous data were summarised as means with standard deviations or medians with range. Prednisolone doses were calculated as mg/kg using the dose administered at inclusion day and the body weight available in the EHR and EPM. The total dose dispensed or administered in the first 24 h was calculated, since the initial doses were expected to differ between hospitals. The estimated length of hospitalisation was the number of days calculated from the day where first dose prednisolone was administered to the day of discharge and an estimation of duration with symptoms needed to be treated in hospital. The relationship between the number of days of hospitalisation and the dose of extemporaneous prednisolone was estimated by a dual link Conway-Maxwell-Poisson (CMP) regression model where dose affected both the rate and dispersion parameters through separate log-linear models [16]. The CMP distribution was chosen to accommodate the under-dispersion of the observed data. The regression was adjusted for hospital and the significance of dose was assessed by a likelihood ratio test with two degrees of freedom. The effect of hospital on dose was assessed by a permutation-based ANOVA test and 95% confidence intervals for the pair-wise differences in means and medians, were calculated by non-parametric bootstrap using 105 resamples. The analyses were performed using statistical software packages SAS version 9.4 and R version 3.4.3. P-values below 5% were considered statistically significant.
3. Results
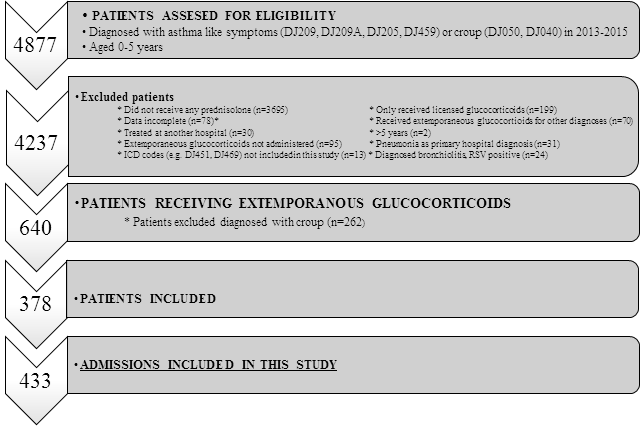
Four thousand eight hundred and seventy-seven patients were screened for eligibility. In total, 378 patients diagnosed with asthma-like symptoms were treated with extemporaneously glucocorticoid preparations (8%) (Figure 1 and Table 1). Overall, they accounted for 433 admissions. Table 1 outlines the anthropometric and clinical characteristics of the admissions in the three hospitals by calendar-year. There were no statistically significant differences between the hospital populations (A, B, C). Thirty-seven percent were females. Twenty-three readmissions within seven days were registered at all three hospitals and excluded (Table 1).
*e.g. no current weight listed in the medical records
3.1 Type of formulations and doses of extemporaneous glucocorticoids
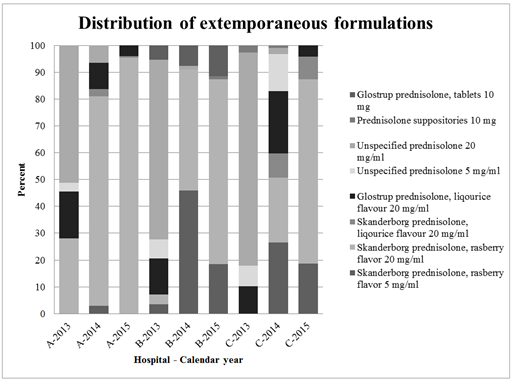
Eight different types of extemporaneous formulations (tablets, solutions, and suppositories) were recorded. Oral formulations were most frequently administered to patients in all three departments, but different oral formulations
were used interchangeably (Figure 2). The formulations include different excipients, see Supplementary Table S2.
Table 1 outlines demographic data for the three different hospitals (A-C) and calendar-year (2013-2015).
*Mean ± SD, **Median [range]
Table 1: Demographic and clinical characteristics.
Figure 2 shows the eight different extemporaneous formulations used in the different hospitals (A, B and C) and calendar year (2013-2015).
The unspecified preparations are either one of the above formulations, just not assigned correctly in Electronic Patient Medication Module (EPM) or an extemporaneous formulation which no longer exists at the Danish market.
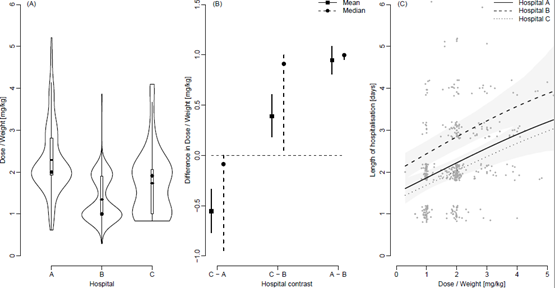
Figure 3A shows violin plots of the distributions of doses stratified by hospital with added boxplots and mean values (straight lines). The percentage of patients that received exactly 1 mg/kg was 4%, 21% and 18% for Hospitals A, B and C respectively. For 2 mg/kg the percentages were 15%, 10% and 12% respectively. The pair-wise differences in doses mg/kg between Hospitals summarized through means and medians are shown in Figure 3B along with 95% confidence intervals. Figure 3C shows the length of hospitalisation in days and the dose of prednisolone at the day of inclusion in mg/kg for Hospital A, B and C along with the estimated average relationship for each hospital and 95% point-wise confidence intervals. Jitter has been added to the length of hospitalisation solely for illustrative purposes.
3.3 Effect of dose of prednisolone on length of hospitalisation
The Conway-Maxwell-Poisson regression showed a significant and positive association between the length of stay and the total dose of extemporaneous prednisolone even when adjusting for hospital. A higher dose increased the expected length of stay (p<0.001), Figure 3C. The effect of hospital was included in the regression model as a factor-valued covariate allowed to affect both the average length of stay as well as the variance of the length of stay as a function of dose. To account for a potential difference in disease severity between hospital’s Paediatric Early Warning Scores (PEWS) were randomly collected from 20, 20 and 18 patients in 2015 from Hospital A, B and C respectively. PEWS is a recognized tool to detect deterioration in hospitalised children [17] and was the only available parameter registered in all hospitals and by same regional procedure, but only electronically available from January 2015. A one-way ANOVA analysis showed no difference in average PEWS score between the three hospitals (p=0.23).
3.4 Co-medication
In almost all (94%) admissions (409 out of 433) patients were treated with beta-2-agonists in nebulised form, as recommended in the guidelines, ranging from 1 to 18 doses at the day of inclusion (Supplementary Table S3). Ipratropium bromide was primarily used in Hospital B. In seventy-nine admissions, patients received antibiotics (18%), amoxicillin was used most frequently. Treatment with inhaled fluticasone varied between hospital and calendar-year with more patients receiving fluticasone in Hospital A. In total, fluticasone was administered in 111 admissions (26%).
3.5 Adverse events
In total 12 AEs were captured in the medical charts from 2013-2015 i.e. agitation, leucocytosis and hyperglycaemia.
4. Discussion
Almost one in 10 children admitted with asthma-like symptoms over a three-year period were treated with extemporaneous prednisolone. Both 1 and 2 mg/kg were used as initial dose in Hospital A-C despite differences in local guidelines, most likely reflecting the severity of symptoms assessed by the prescribing physician. In total, eight different extemporaneous formulations were used reflecting the need for different formulations in different age groups and different preferences of the child. These findings are in line with a variety of unlicensed and untested captopril formulations used interchangeably for children with heart failure in the UK [18]. Likewise, we found that oral solutions were the most used formulation, also confirmed by Lucas-Bouwman et al. [19]. However, no studies support the interchangeable use of various prednisolone formulations, as rate and extent of prednisolone absorption could vary and be dependent of the excipients used [20]. Other drawbacks of extemporaneously prepared formulations are limited shelf-life, safety-profile, taste etc. [21-23]. Unavailability of licensed drugs appropriate for children in countries with small populations makes it challenging to avoid the use of extemporaneous formulations [24, 25]. For example, marketed prednisolone products are available in UK, Germany, The Netherlands, France and Italy, interestingly with different dose recommendations for treatment of acute asthma, see supplementary Table S1.
The initial dose of prednisolone varied significantly (p<0.001) between hospitals. However, treatment strategies included maintenance dosages that were at least twice as high as in adults. Treating children with higher doses per kilo is noteworthy, but may partly be explained by a higher hepatic clearance of glucocorticoids in young children, caused by a higher liver size relative to body weight [26]. The evidence of the superiority of any treatment regime is sparse in preschool children which was reflected in the inconsistency in doses administered in this study both between hospitals and within the same paediatric department in the same region [27].
In the present study, a higher dose was associated with an increase in the expected length of stay (p<0.001). This could be confounding by indication, as a high initial dose may reflect severity of symptoms or a previous complicated medical history. For example, more patients in Hospital A received fluticasone, which may indicate intermittent asthma-like symptoms (MTW), which cannot be accounted for in this study. Severity of symptoms is included as an important factor in the assessment of wheezing in the ERS update [4] with the possibility of a more individual approach. Nevertheless, children with acute asthma-like symptoms despite severity might not have a therapeutic advantage of 2 mg/kg compared with 1 mg/kg nor shorter recovery as reported by Langton et al. [28].
Adding to the heterogeneity some clinical studies suggest viral-induced wheezing is partly resistant to corticosteroid treatment and the specific virus causing the symptoms should be assessed [29]. In the present study available microbiology results for each patient were collected. Only 26 patients had a positive laryngeal sample where different patterns of viruses and bacteria were identified. These possible clinical infections could also explain some of the differences in length of stay. Notably, Farber et al. found that overprescribing of oral corticosteroids in children with viral respiratory infections is still ongoing [30]. In our study, children with bronchiolitis or tested positive for respiratory syncytial virus were excluded due to potential resistance to steroid treatment [29].
Consistent with current local hospital guidelines nearly all patients received inhaled beta-2-agonists as first-line treatment. The 6% that did not receive beta-2-agonists, could have received inhaled saline as first-line treatment, which is not recorded in the EPM. The number of beta-2-agonists doses varied considerably between hospitals (i.e. 1 to 18 doses/ 24 hr). This remains the treatment of choice, though the evidence is limited [4]. Hospital A used the highest doses of prednisolone but the lowest number of beta-2-agonist doses, however, that prednisolone could be beta-2-agonist sparring is not consistent with the findings of Langton et al. Eighteen percent received antibiotics primarily beta-lactams. A study by De Boeck et al. shows that co-administration of asthma medications and antibiotics is very likely OR 1.90 95% [CI 0.89-1.90] [31]. The authors postulate that this finding may be due to respiratory tract infections treated with antibiotics despite viral origin or underlying secondary infection [31]. These patients were not excluded since beta-lactams have no documented effect on the duration of symptoms in contrast to azithromycin treatment [32].
Only a few AEs were captured i.e. agitation, leucocytosis and hyperglycaemia, which corresponds to very common AE’s [33]. Confounding of glucocorticoid behavioural adverse effects may arise due to the potential for co-administered beta-2-agonists to also result in similar effects, e.g. agitation. Further, as the hospitalisation is relatively short, side effects may be seen at home.
5. Limitations
This study relied on correct ICD code registration. The number of patients eligible for inclusion was similar over the three-year period, except for Hospital B where only one third were identified in 2015. Despite several efforts, it was not possible to explain this variation. It might be due to shift in the ICD coding practice. Furthermore, registration of extemporaneous formulations is not 100% complete in EPM. The frequency may therefore be slightly underestimated. The length of hospitalisation was calculated in days, which may be less precise if the child was admitted close to midnight. Counted by hours from admission-note to discharge-note, would also be misleading due to delays in registration or discharging. Two researchers conducted the data collection to increase consistency. To assess severity, we did a random sample of the PEWS score from each hospital in 2015. However, the recording of the PEWS score was noted in the chart within a variable period from treatment.
6. Conclusion
Considerable variability in choice of extemporaneous prednisolone formulations for asthma-like symptoms were seen between Hospitals A, B and C within the same region. The initial prednisolone dose differed significantly between hospitals, yet a higher dose did not result in a shorter duration of hospitalisation. Studies on bioequivalence between formulations are lacking. In addition, studies confirming which children benefit from oral glucocorticoids are warranted.
Funding
During her six months of research MS received a grant from the Augustinus Foundation.
Acknowledgements
We sincerely thank the paediatric departments, especially the secretaries who procured the patient lists. In addition, Glostrup and Skanderborg Pharmacies for contributing with the monographs.
References
- Kwong CG, Bacharier LB. Management of Asthma in the Preschool Child. Immunol Allergy Clin North Am 39 (2019): 177-190.
- British Thoracic Society, Scottish Intercollegiate Guidelines Network British guideline on the management of asthma: a national clinical guideline. SIGN, Edinburgh (2016).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2016).
- Brand PLP, Caudri D, Ernst Eber, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur Respir J 43 (2014): 1172-1177.
- Brand PLP, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J 32 (2008): 1096-1110.
- Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy 63 (2008): 5-34.
- Spycher BD, Cochrane C, Granell R, et al. Temporal stability of multitrigger and episodic viral wheeze in early childhood. Eur Respir J 50 (2017).
- Raaymakers MJA, Brand PLP, Landstra AM, et al. Episodic viral wheeze and multiple-trigger wheeze in preschool children are neither distinct nor constant patterns. A prospective multicenter cohort study in secondary care. Pediatr Pulmonol (2019).
- De Benedictis FM, Bush A. Infantile wheeze: rethinking dogma. Arch Dis Child 102 (2017): 371-375.
- Beigelman A, Bacharier LB. Management of preschool recurrent wheezing and asthma: a phenotype-based approach. Curr Opin Allergy Clin Immunol 17 (2017): 131-138.
- Schäcke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96 (2002): 23-43.
- Raissy HH, Kelly HW. Benefits and Risks of Long-Term Asthma Management in Children: Where Are We Heading? Drug Saf 40 (2017): 201-210.
- Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. Chest 122 (2002): 624-628.
- Danish Medicines Agency. Danish Medicines Agency Departmental order on Danske Lægemiddelstandarder (2017).
- Nahata MC, Allen LV. Extemporaneous drug formulations. Clin Ther 30 (2009): 2112-2119.
- Sellers K, Shmueli G. A Flexible Regression Model for Count Data. Annals of Applied Statistics 4 (2010): 943-961.
- Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care 13 (2009): 135.
- Mulla H, Tofeig M, Bu’Lock F, et al. Variations in captopril formulations used to treat children with heart failure: a survey in the United kingdom. Arch Dis Child 92 (2007): 409-411.
- Lucas-Bouwman ME, Roorda RJ, Jansman FG, et al. Crushed prednisolone tablets or oral solution for acute asthma? Arch Dis Child 84 (2001): 347-348.
- Merke DP, Cho D, Calis KA, et al. Hydrocortisone suspension and hydrocortisone tablets are not bioequivalent in the treatment of children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86 (2001): 441-445.
- Mulla H, Hussain N, Tanna S, et al. Assessment of liquid captopril formulations used in children. Arch Dis Child 96 (2011): 293-296.
- Standing JF, Tuleu C. Paediatric formulations--getting to the heart of the problem. Int J Pharm 300 (2005): 56-66.
- Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr. 92 (2003): 486-490.
- Bajcetic M, Kearns GL, Jovanovic I, et al. Availability of Oral Formulations Labeled for Use in Young Children in Serbia, Germany and the USA. Curr Pharm Des 21 (2015): 5668-5673.
- Haslund-Krog SS, Rolighed Christensen H, Bjerager M, et al. Development of one paediatric and one neonatal formulary list in hospital settings. Br J Clin Pharmacol 84 (2018): 349-357.
- Hill MR, Szefler SJ, Ball BD, et al. Monitoring glucocorticoid therapy: a pharmacokinetic approach. Clin Pharmacol Ther 48 (1990): 390-398.
- Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev (2016): CD011801.
- Langton Hewer S, Hobbs J, Reid F, et al. Prednisolone in acute childhood asthma: clinical responses to three dosages. Respir Med 92 (1998): 541-546.
- Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: Systematic review with meta-analysis. Pediatr Pulmonol 51 (2016): 868-876.
- Farber HJ, Silveira EA, Vicere DR, et al. Oral Corticosteroid Prescribing for Children With Asthma in a Medicaid Managed Care Program. Pediatrics 139 (2017).
- Kris De Boeck, François Vermeulen, Isabelle Meyts, et al. Coprescription of antibiotics and asthma drugs in children. Pediatrics 127 (2011): 1022-1026.
- Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 4 (2016): 19-26.
- Danish Medicines Agency. Summary of Product Characteristics. Prednisolon ‘DAK’ (2017).


 Impact Factor: * 4.8
Impact Factor: * 4.8 Acceptance Rate: 69.70%
Acceptance Rate: 69.70%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 