Insights into the Menstrual Pain Experience: Results from Quality Improvement Surveys of American Women
Margo S Harrison1*
1Wave Bye Inc, USA.
*Corresponding Author: Margo S Harrison, 275 S Dahlia St, Denver CO, 80246.
Received: 20 January 2024; Accepted: 29 January 2024; Published: 02 February 2024
Article Information
Citation: Margo S Harrison. Insights into the Menstrual Pain Experience: Results from Quality Improvement Surveys of American Women. Obstetrics and Gynecology Research. 7 (2024): 06-07.
View / Download Pdf Share at FacebookAbstract
Objective:
The objective of these quality improvement surveys was to improve business practices based on consumer feedback about menstrual pain.
Methods:
All de-identified data was collected through an online survey platform called Survey Monkey and no personal health information as defined by HIPAA regulations was collected.
Results:
For the cohort of women initially surveyed (n = 103), to believe in the legitimacy of a new menstrual pain medication, they rely largely on doctors (n = 28), clinical research (n = 31), and testimonials from other women (n = 20). For a second cohort asked about success of treatment, they defined it as a reduction in pain (72%), and if the treatment failed they would be worried about side effects, wasting money, and disappointment that the treatment was not effective.
Conclusion:
Women have negative menstrual experiences, rely on doctors, research, and each other to develop trust in menstrual treatments, and define success of a menstrual treatment by its ability to reduce pain.
Keywords
<p>Menstrual pain; American women</p>
Article Details
Introduction
In the United States, there have been calls for more research about the menstrual experience.[1] Identified areas of interest include gaps in menstrual education, research, and cultural acceptance of menstruation.[2] Wave Bye Inc (WBI) is a company that specializes in over the counter medications and dietary supplements focused on managing menstrual pain and symptoms. WBI has conducted a number of quality improvement surveys to improve business practices based on consumer feedback. This work was not hypothesis driven and was designed as part of a program to implement, test, and evaluate our products. Because of the cited lack of information about the North American menstrual experience, we are sharing the results of our quality improvement work, though it cannot contribute to generalizable knowledge as it is not research.
Material and Methods:
In this analysis we present the results of a few different quality improvement projects all directed towards the same aforementioned purpose. They did not meet the definition of research per DHHS regulations. All de-identified data was collected through an online survey platform called Survey Monkey and no personal health information as defined by HIPAA regulations was collected.
Participation was voluntary and all participants are expected to benefit from the collection of quality improvement data as it will improve WBI products targeted directly towards the menstrual experience of women. Survey Monkey recruitment and methodology practice can be found, here: www.surveymonkey.com/mp/audience. As multiple questionnaires were sent as part of this quality improvement exercise, there were no structured primary or secondary outcomes. However, areas of interest included evaluating consumer experience of their menstrual period, education, trust, and valuation of medical research.
This analysis presents the results of two quality improvement surveys of a population of women participating in Survey Monkey surveys. STATA software version 15.2 (StataCorp LP, College Station, TX, USA) was used for analysis.
Results:
The first survey conducted focused on evaluating consumer education, trust, and opinions of medical research. Out of 103 self-identifying women surveyed, 26% were between the ages of 18 and 29, 58% were between the ages 30 - 44, and the remainder were older than 45. Women were asked to use a single word to describe their period, the results of which are shown in Figure 1; not a single positive word was used. Among this cohort, the leading sources of information about new medications were the internet (n = 64) and physicians (n = 35). For these women to believe in the legitimacy of a new medication, they relied largely on doctors (n = 28), clinical research (n = 31), and testimonials from other women (n = 20). Similar results were seen with respect to trusting a new product and believing it is medically valid, with recommendations from doctors, clinical research, and testimonials driving the results for both questions.
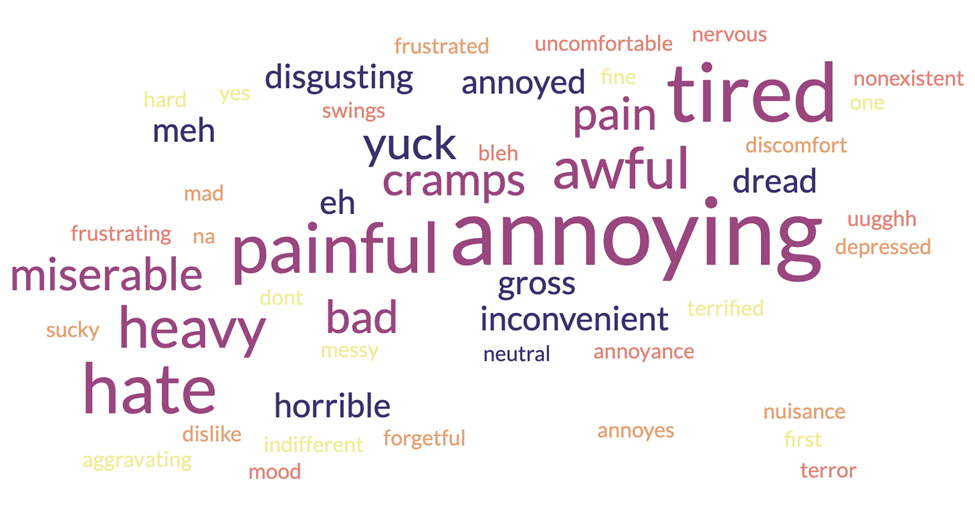
Figure 1: Word cloud of words women used to describe their menstrual experience (n = 103).
In our second survey, 100 self-identifying women participated, 17% of whom were aged 18 - 29, and 83% of women were between the ages of 30 - 44. This survey focused more on the pains they experienced during menstruation and the gains they would see if their menstrual experience were improved by medication. When asked to identify the source of their menstrual problems, most respondents said complaints were physical (n = 64), the next highest response was mental (n = 23), and 10 women said they have no menstrual complaints. 72% of women defined success of a menstrual treatment as a reduction in pain. When asked what they would be most disappointed about if a menstrual treatment was not successful, 38 women responded they would be worried about side effects with no benefit, 16 said they would be disappointed it was not effective, and 30 women said they would be annoyed by having spent money on the product.
Discussion:
These surveys were not prospectively designed research endeavors driven by hypotheses grounded in prior research, and were only intended to inform WBI business decisions and operations; as such, they are extremely limited and the findings cannot be considered research findings. That being acknowledged, the quality improvement work that WBI performed through consumer surveys suggests that women have negative menstrual experiences, rely on doctors, research, and each other to develop trust in menstrual treatments, and define success of a menstrual treatment by its ability to reduce pain. As the literature on the menstrual experience of American women is not robust, WBI will continue to share its findings in order to give some insight into the lived experience of its consumers.
Author Contributions:
MSH designed surveys, analyzed and interpreted them, and wrote this quality improvement manuscript.
Funding:
There was no funding mechanism for this quality improvement work.
Conflict of Interest:
The author is the founder, majority equity owner, and CEO of Wave Bye Inc and wishes to disclose this information, though it should not influence the objectivity of this paper and its review, as it was not conducted in reference to any existing products and was performed in order to better understand the menstrual experience of American women. The findings presented in this paper represent the views of the named authors only, and not the views of their institutions or organizations.
Ethical Statement:
This work met quality improvement characteristics as determined by the Colorado Multiple Institutional Review Board checklist.
References
- Olowojesiku R, Shim DJ, Moppins B, et al. Menstrual experience of adolescents in the USA: protocol for a scoping review. BMJ Open 11 (2021): e040511.
- Ramaiyer M, Lulseged B, Michel R, Ali F, Liang J, Borahay MA. Menstruation in the USA. Curr Epidemiol Rep 10 (2023): 186-195.


 Impact Factor: * 3.2
Impact Factor: * 3.2 Acceptance Rate: 76.63%
Acceptance Rate: 76.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 