Peri-Partum Hysterectomy Over A 15 Year Period In A Tertiary Hospital; A Review And Discussion Of The Histopathological Diagnoses
Dr Katie Beauchamp1*, Dr Emma Doyle2
1Histopathology Specialist Registrar Rotunda Hospital, Dublin, Ireland.
2Consultant Histopathologist Rotunda Hospital, Dublin, Ireland.
*Corresponding author: Dr Katie Beauchamp, Histopathology Specialist Registrar Rotunda Hospital, Dublin, Ireland.
Received: 17 July 2025; Accepted: 24 July 2025; Published: 04 August 2025
Article Information
Citation: Dr Katie Beauchamp, Dr Emma Doyle. Peri-Partum Hysterectomy Over A 15 Year Period In A Tertiary Hospital; A Review And Discussion of The Histopathological Diagnoses. Obstetrics and Gynecology Research. 8 (2025): 115-121.
View / Download Pdf Share at FacebookAbstract
Peri-partum hysterectomy (PH) is a life-saving procedure for massive obstetric haemorrhage. It is most commonly performed at the time of delivery in the setting of placenta accreta spectrum (PAS). The number of PH is increasing, likely secondary to the increasing rates of PAS and increasing caesarean section rates. Other causes of postpartum haemorrhage include uterine atony, vascular injury, retained placenta and subinvolution. Meticulous macroscopic and microscopic examination along with appropriate clinical details are essential in the diagnoses in these often-complex specimens.
Keywords
<p>Pperipartum hysterectomy; Placenta accreta spectrum; Postpartum haemorrhage; Pathology</p>
Article Details
INTRODUCTION
Peri-partum hysterectomy (PH) is a life-saving procedure in the setting of massive obstetric haemorrhage (MOH). It is most commonly performed for placenta accreta spectrum (PAS) disorders, but can also be required for treatment of other causes of primary and secondary post-partum haemorrhage (PPH) including atony, vascular injury, coagulation disorders, retained placenta and sub-involution [1]. Previous caesarean section is the most commonly associated risk factor for development of PAS and hence the incidence of PAS is increasing secondary to the rising number of caesarean sections (CS). The worldwide prevalence of PH is 1 in 1000 deliveries however is even lower in Ireland with an incidence of 1 in 3000 deliveries [2,3].
While the numbers of PH in Ireland are low, we have noticed an increase in PH within our unit. As a tertiary maternity hospital in Ireland with an average of 8692 deliveries a year over the last 15 years, we review the PH performed in our institution over this time period and discuss the pathological diagnoses in these cases.
Methods
A search of the laboratory information system was conducted between January 2009 and December 2023 using SNOMED codes. 65 peri-partum hysterectomies were identified over this 15-year period. The histology reports were retrospectively reviewed, and histological diagnoses along with associated risk factors were assessed.
Results
During this 15-year period, there were 130,383 number of deliveries with an average of 8692 per year. Most recently in 2023, there were 8283 deliveries. The CS rate has increased from 28.5% in 2009 to 40% in 2023.
65 peripartum hysterectomies were performed in our unit during this time with a rate of 0.5 per 1000 deliveries. Of these 87.7% (57/65) were caesarean hysterectomies and 12.3% (8/65) were postpartum hysterectomies. The hysterectomy specimens included total hysterectomies, subtotal and a hemiuterus specimen.
Maternal age ranged from 21-46 years with 63% (41/65) being of advanced maternal age (35 years or more). Parity ranged from 1 to 6.
The gestational age ranged from 18+1 weeks to 41+4 weeks gestation. 69.2% (45/65) required preterm delivery (<37 weeks gestational age).
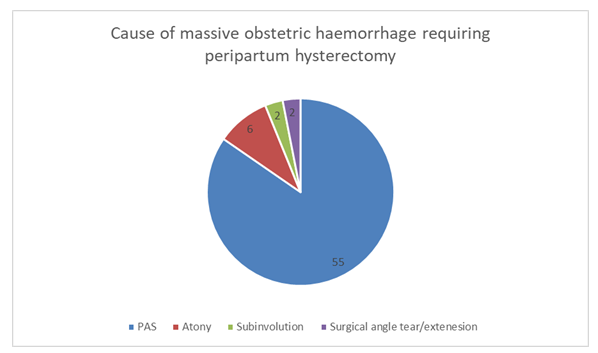
Placenta accreta spectrum (PAS) was the most common pathology with 84.6% (55/65) of cases performed for PAS. One of these cases also had an amniotic fluid embolism, a second case had a scar ectopic showing focal PAS and a third case had implantation over previous scar which dehisced along with PAS.
Of these PAS cases, 98.2% (54/55) had a known risk factor for PAS. Risk factors included: previous caesarean section (48/55) with a range of 1-5 previous cesarean sections. Previous uterine instrumentation (14/55) (including; dilatation and curettage, manual removal of placenta (MROP), evacuation of retained products of conception (ERPC), termination of pregnancy). One patient had a congenitally abnormal uterus (uterus didelphys). One patient had a previous myomectomy. One patient had a diagnosis of Asherman syndrome. Five had previously documented PPHs. At least six patients had treatment for infertility. Five patients had a history of preeclampsia (PET). Only one patient had no known risk factors.
Atonic uterus was the second most common cause of PH with 9.2% (6/65) performed for management of uterine atony. One of these cases also had retained placental membranes.
Subinvolution was found in 3% (2/65) of cases. These cases occurred on day 8 and day 13 respectively. Surgical angle tears/extensions requiring hysterectomy occurred in 3% (2/65).
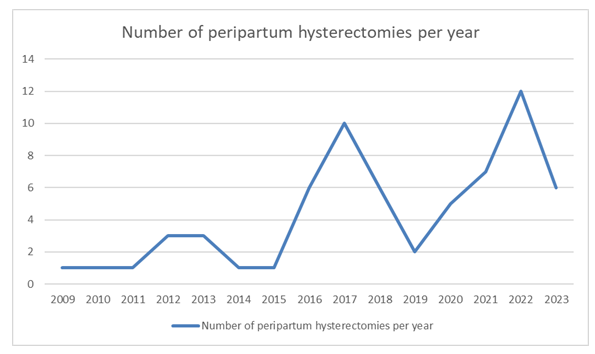
The number of PH varied each year but the trend has been steadily increasing over this 15-year period. This trend is demonstrated by figure 2 below showing the number of PH per year from January 2009 - December 2023.
Discussion:
Massive obstetric haemorrhage (MOH) is a life-threatening complication of pregnancy and delivery, which often requires blood transfusion and can ultimately require lifesaving hysterectomy. The prevalence of PH worldwide is almost 1 in 1000 deliveries and in Ireland the incidence is 1 in 3000 deliveries. While there are many causes of MOH necessitating PH including uterine atony, retained placenta, coagulation and bleeding disorders, PAS is the cause most commonly associated with PH.
Placenta Accreta Spectrum:
PAS refers to a form of abnormal placentation resulting in partial or complete retention of the placenta at the time of delivery [4]. The leading hypothesis regarding the aetiology of PAS is that defects of the endometrial-myometrial interface from uterine scarring leads to a failure of normal decidualisation, which allows abnormally deep anchoring of the placental villi. Previous CS is the most commonly associated risk factor for PAS and with a rising CS rate worldwide, we are seeing an increase in incidence. This is demonstrated by the rise in incidence of PAS in patients with one previous CS 0.24% rising to 6.74% in those with 6 or more CS [4]. Other risk factors include any disruption to the endo-myometrial layer (including endometrial curettage, removal of polyps and leiomyomas, ERPC, MROP), advanced maternal age, high parity along with conditions associated with abnormal trophoblast implantation such as preeclampsia (PET) [2].
The prevalence of PAS is increasing worldwide from 1 in 2610 -4017 in the 1970s and 1980s to 1 in 533 in 2002. This has increased again more recently with an incidence of 1 in 272 in the USA in 2016 [4] PAS can be diagnosed antenatally by ultrasound with a sensitivity of 92.72% and specificity of 96.94% [4] Clinicians should have a high index of suspicion for PAS in those patients with the above associated risk factors as well as those with placenta praevia.
Diagnosis of PAS depends on macroscopic and microscopic examination of the placental bed and must show absence of decidua between the placenta and myometrium. A new three-tiered grading system has been devised by the Federation of Gynaecology and Obstetrics (FIGO) which quantifies histologically the percentage of depth of invasion and aims to introduce a more reproducible grading system among histopathologists [5]. It is based on the macroscopic examination of the depth of invasion of the placenta and tissue destruction.
PAS Grade 1 is defined as non-invasive and includes those with grossly adherent placenta on manual palpation. Microscopy shows a smooth placenta-myometrial interface and uniform myometrial thickness without thinning. PAS Grade 2 is defined as superficial invasion without involvement of the outer myometrium but with preservation of at least 25% of the wall thickness relative to the uninvolved myometrium. PAS Grade 3A is deep invasion with involvement of the outer myometrium and with preservation of less than 25% of the wall thickness relative to the uninvolved myometrium with intact serosa. PAS Grade 3D includes deep invasion with disruption of the serosa and PAS Grade 3E includes deep invasion with adherent extra uterine structures such as extrauterine fibroadipose tissue or most commonly the bladder. Microscopy of PAS Grade 2-3 all show an irregular placental-myometrial interface [5] PAS can only be diagnosed on hysterectomy specimens or partial myometrial resections. Abnormal placentation seen in delivered placentas or curettings are not included under the PAS umbrella but in a separate category basal plate myometrial fibres (BPMF) which is not covered in this discussion [5].
PAS can have devastating effect on the patient and hence early clinical diagnosis is essential for appropriate elective management of these cases when possible.
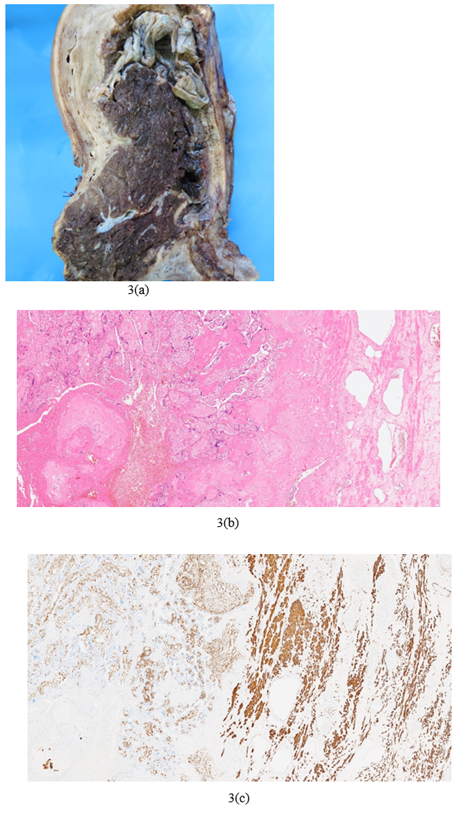
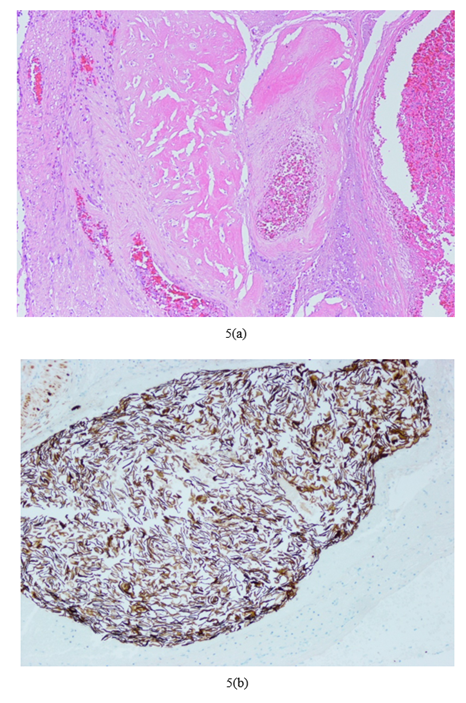
Figure 3: Peripartum hysterectomy showing placenta accreta spectrum FIGO Grade 3D: (a)Macroscopic image (b)Microscopic image haematoxylin and eosin (H&E) showing direct apposition of chorionic villi with underlying myometrium (c)Immunohistochemistry (IHC) stain desmin highlights myometrium and shows apposition of villi with myometrium
Uterine atony:
Uterine atony is the most common cause of postpartum haemorrhage. It is a clinical diagnosis made once other causes have been excluded. The vast majority of cases are treated successfully, with uterotonic medications +/- surgical interventions (not discussed in this paper). However, this is not always successful leading to a PH. Gross examination of an atonic postpartum uterus shows a large, poorly contracted or “boggy” uterus. Evidence of prior surgical interventions may be present including an intrauterine balloon and B-Lynch sutures. The presence of myometrial or surface bleeding may also be noted, however this is not specific to uterine atony. Microscopic findings are non-specific and may include dilated and partially thrombosed maternal vessels at the implantation site. The pathological diagnosis of uterine atony is one of exclusion and can only be made, once other causes e.g., retained placenta, PAS, disrupted blood vessels etc. have been discounted.
Subinvolution:
Subinvolution is failure of the maternal spiral arteries to return to a pre-pregnancy state, which is a normal postpartum physiologic change [6]. This results in secondary postpartum haemorrhage most commonly in the second week post-delivery.
Physiologic changes to the maternal spiral arteries are seen as early as six weeks gestation including extravillous trophoblast (EVT) invading and plugging maternal spiral arteries. From the second trimester these EVT replace endovascular lining of maternal spiral arteries and express endothelial type markers and induce angiogenesis. Interstitial and endovascular EVT cause remodelling of the spiral arteries seen histologically as hyaline fibrinoid material replacing the arterial media and loss of elastic fibres. These changes ensure a high flow, low resistance blood flow through large calibre arteries to supply the placenta [6].
Involution is the physiologic return of the spiral arteries to their pre-pregnancy state in an attempt to reduce haemorrhage at and post-delivery. It usually commences in the third trimester when the endovascular EVT are replaced by maternal endothelial cells. Within 24 hours after delivery histological changes include occlusive fibro-intimal thickening, endarteritis, thrombosis, regeneration of the internal elastic lamina and disappearance of the endovascular and interstitial EVT. When this normal physiological process goes awry the result is subinvolution with secondary post-partum haemorrhage, often requiring life-saving hysterectomy [6].
The exact cause remains undetermined however it is believed to be an immunologic phenomenon of abnormal cellular interaction between the EVT and the maternal tissue [6] Andrew et al observed the absence of immunoglobulins and complement proteins in the walls of sub-involuted vessels that are observed in involuted vessels [6,7] EVT apoptosis is believed to play a role in subinvolution with increased EVT expression of antiapoptotic factor Bcl-2. While preeclampsia involves poor spiral artery remodelling early in pregnancy, subinvolution is thought to be on the opposite end of the spectrum to preeclampsia. EVT in pre-eclampsia cases have loss of antiapoptotic Bcl-2 expression while cases of subinvolution have increased Bcl-2 expression [6]
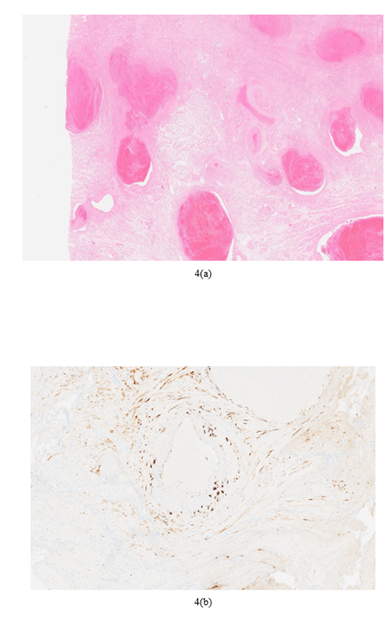
The diagnosis of subinvolution can be made on curettage specimen if the placental site is sampled and is an important diagnosis as a cause of secondary post-partum haemorrhage which excludes iatrogenic causes. The key histological features for a diagnosis are large patent dilated superficial myometrial vessels with pink hyaline material replacing the media and partial intravascular thrombosis of variably aged thrombi. Usually, these vessels are clustered together and may be admixed with normally involuted vessels [6] Interstitial and or endovascular EVT should also be identified. Other features of subinvolution include lack of internal elastic lamina duplication, partial or complete absence of a true endothelial lining and immunohistochemical (IHC) overexpression of Bcl-2. Other IHC stains can be used to identify EVT including “low molecular weight cytokeratin, inhibin alpha, Mel-CAM and human placental lactogen” and elastin stains to delineate the vessel wall [6]. Without the key histological features a diagnosis of subinvolution can be suggested in the correct clinical setting as a possible diagnosis. A diagnosis of subinvolution should not be made even if these key histological features are seen in a specimen of less than 24 hours post-partum as this should be interpreted as normal physiology for this time [6].
Amniotic Fluid Embolism:
AFE is a rare complication of pregnancy whereby amniotic fluid enters the maternal pulmonary circulation leading to maternal cardiovascular collapse. AFE is extremely rare with an estimated incidence between 1.9-7.7 / 100,000 (8). The exact incidence is unknown due to the rare nature of the condition and the difficulty in diagnosing in non-fatal cases. While rare, it remains one of the leading causes of direct maternal mortality in developed countries. (8) Clinical diagnosis is one of exclusion, however histopathologic diagnosis requires the presence of fetal squames, fetal hair or other debris within the maternal circulation. (9) The exact pathophysiology remains unclear however the effect is that of compliment activation and anaphylactoid reaction rather than a circulatory obstruction. (9) The release of thrombogenic factors triggers a disseminated intravascular coagulation (DIC) which in turns causes massive haemorrhage, contributing to the maternal circulatory collapse. AFE most commonly occurs intrapartum or immediately postpartum however can also occur in the second trimester or up to 48 hours post-delivery. (9)
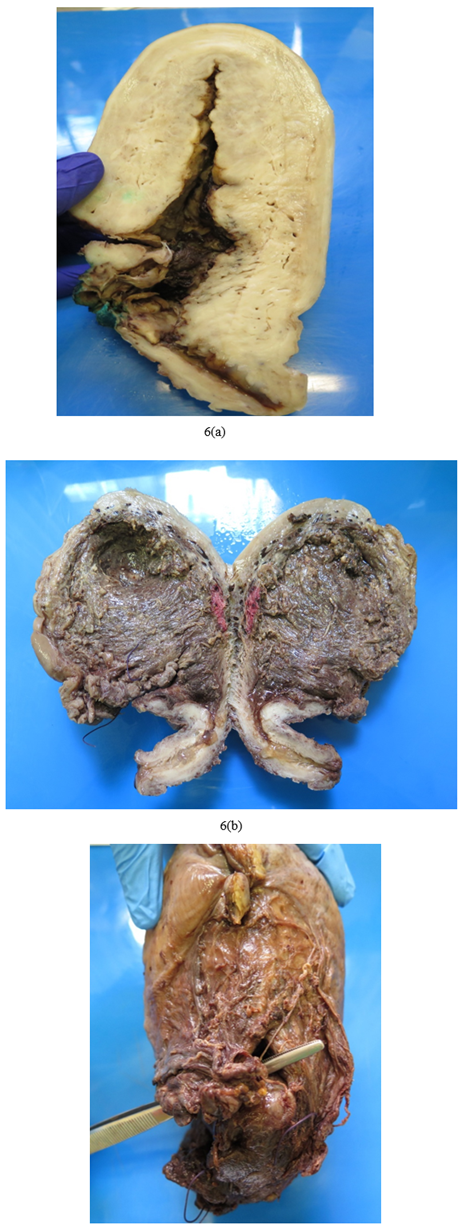
Macroscopy:
On receipt of a PH specimen, it is important to ensure adequate formalin fixation. The policy in our unit is to open the uterus sagittally along the midline dividing the uterus into right and left halves to allow fixation with sufficient formalin for at least 48 hours. As with most specimens, a detailed gross examination of a PH specimen with accurate clinical details is essential. A caesarean hysterectomy will likely have different pathologies to a peripartum hysterectomy performed two weeks after delivery. The presence of a placenta (intact or piecemeal), praevia position and adherence of the placenta to the uterus indicates PAS in the setting of a caesarean hysterectomy. A large, poorly contracted uterus with or without a fresh caesarean section scar indicate uterine atony. These cases may contain an intrauterine balloon device or have large “brace” or B-Lynch sutures attempting to contract the uterus, again indicating uterine atony refractory to medical and other surgical interventions. A uterus containing small ragged fragments of placenta or amniotic membranes indicate retained placenta, which may be accompanied by endometritis. The presence of large dilated myometrial blood vessels is suggestive of subinvolution. The presence of a uterine wall defect can indicate either trauma at the time of caesarean section or a uterine rupture.
Meticulous examination, documentation and sampling of the placenta, placental bed site, endometrium, myometrium, and any uterine wall defects is essential. The microscopy as described above, will confirm the diagnosis in conjunction with the macroscopic examination.
Conclusion:
PH is a life-saving intervention in the setting of MOH. A rise in PH is following the trend of increasing CS rates. The vast majority of these PH are in the setting of PAS, however other causes of MOH include atony, vascular injury, coagulative disorders, subinvolution, retained products of conception and rarely AFE. On receipt of a PH specimen, careful gross examination by the pathologist is key to determine the location and adherence of any residual placental tissue and to exclude uterine tears and vascular injury. This will determine appropriate sampling and microscopic examination should then confirm the working diagnosis. Thorough examination is essential, not only in ensuring the correct diagnosis but can also form an important basis in medico-legal cases. With a rising CS rate leading to increasing PAS, we will continue to see more of these cases and a methodical approach with accurate diagnoses is important.
Acknowledgements:
The manuscript was prepared solely by the two named authors who wrote, reviewed, approved the manuscript for submission.
Conflicts of Interest:
We have no conflicts of interest. We received no funding.
References
- Huque S, Roberts I, Fawole B, et al. Risk factors for peripartum hysterectomy among women with postpartum haemorrhage: Analysis of data from the WOMAN trial. BMC Pregnancy Childbirth 18 (2018): 1-8.
- Van Den Akker T, Brobbel C, Dekkers OM, et al. Prevalence, Indications, Risk Indicators, and Outcomes of Emergency Peripartum Hysterectomy Worldwide: A Systematic Review and Meta-analysis. Obstet Gynecol 128 (2016): 1281-1294.
- Campbell SM, Corcoran P, Manning E, et al. Peripartum hysterectomy incidence, risk factors and clinical characteristics in Ireland. Eur J Obstet Gynecol Reprod Biol 207 (2016): 56-61.
- Cahill AG, Beigi R, Heine RP, et al. Placenta Accreta Spectrum. Am J Obstet Gynecol [Internet] 219 (2018): B2–16. Available from: https://doi.org/10.1016/j.ajog.2018.09.042
- Hecht JL, Baergen R, Ernst LM, et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: recommendations from an expert panel. Mod Pathol [Internet] 33 (2020): 2382-2396. Available from: http://dx.doi.org/10.1038/s41379-020-0569-1
- Weydert JA, Benda JA. Subinvolution of the Placental Site as an Anatomic Cause of Postpartum Uterine Bleeding A Review. Arch Pathol Lab Med 130 (2006).
- Andrew A, Bulmer JB ML. Subinvolution of the uteroplacental arteries: an immunohistochemical study. Int J Gynecol Pathol 12 (1993): 28-33.
- Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, et al. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: A population-based cohort and nested case-control study. Vol. 123, BJOG: An International Journal of Obstetrics and Gynaecology. (2016): p. 100–109.
- Kaur K, Bhardwaj M, Kumar P, Singhal S, Singh T, Hooda S. Amniotic fluid embolism. Vol. 32, Journal of Anaesthesiology Clinical Pharmacology. Medknow Publications (2016): p. 153–159.


 Impact Factor: * 3.2
Impact Factor: * 3.2 Acceptance Rate: 76.63%
Acceptance Rate: 76.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 