Prophylactic Internal Iliac Artery Balloon Occlusion (IIABO) in Placental Implantation Abnormalities
Ruben López Benítez1, Tomás Reyes del Castillo1*, Philipe S Breiding1, Markus Hodel2, Thiago VM Lima1, Levent Kara3, Magdalena Schmidt3, Justus E Roos1
1Department of Radiology and Nuclear Medicine, Luzerner Kantonsspital, Luzern, Switzerland
2Department of Obstetrics and Feto-Maternal Medicine, Luzerner Kantonsspital, Luzern, Switzerland
3Institute of Radiology and Nuclear Medicine, Triemli Hospital, Zurich, Switzerland
*Corresponding Author: Tomás Reyes del Castillo, Department of Radiology and Nuclear Medicine, Luzerner Kantonsspital, 6000 Luzern, Switzerland
Received: 30 November 2021; Accepted: 08 December 2021; Published: 10 February 2022
Article Information
Citation:
Ruben López Benítez, Tomás Reyes del Castillo, Philipe S Breiding, Markus Hodel, Thiago VM Lima, Levent Kara, Magdalena Schmidt, Justus E Roos. Prophylactic Internal Iliac Artery Balloon Occlusion (IIABO) in Placental Implantation Abnormalities. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 94-103.
View / Download Pdf Share at FacebookAbstract
Purpose: We aimed to evaluate the safety and efficacy of prophylactic internal iliac artery balloon occlusion (IIABO) in patients with placental implantation abnormalities.
Methods: The study consisted in a retrospective comparative analysis of 18 patients with placental implantation abnormalities, including four patients with placenta previa, eleven cases with placenta percreta and 3 cases with placenta percreta. 14 cases that underwent IIABO were allocated in the IIABO group, and 4 cases without endovascular intervention were classified as control group. The assayed variables were: estimated blood loss, number of red blood cell transfusions and length of the hospital stay for mother and child, intervention time, balloon inflation time, surgical time and fetal absorbed dose.
Results: The median estimated blood loss was similar in both groups (650ml ± 581ml vs 700 ± 475ml), the median number of red blood cell transfusion was slightly higher or the control group (0U ± 0.7U vs 0U ± 2U), three patients from the IIABO group required uterine artery embolization. The uterus was preserved in nine patients (64%) of the experimental group while three patients (75%) from the control group received an emergency hysterectomy. Average intra-hospitalary stay was significantly longer in the control group, especially for the child (6days vs 26.5 days). The average fetal radiation dose was 6.87 mGy. No complications were attributed to IIABO placement.
Conclusion: The prophylactic use of IIABO in placental implantation abnormalities is an effective and safe method of controlling perioperative rate of bleeding during cesarean section and hysterectomy. Indications should be strictly controlled, and interdisciplinary planning and management are mandatory.
Keywords
<p>Placenta Accreta, Placenta Previa, Postpartum Hemorrage, Hysterectomy, Radiation Dosage</p>
Article Details
Abbreviations:
IIABO: Internal iliac artery balloon-assisted occlusion; FAD: Fetal absorbed dose; MRI: Magnetic resonance imaging; UAE: Uterine artery embolization
1. Background
Placental implantation abnormalities remain among the major causes of massive postpartum hemorrhage and maternal mortality worldwide. The prevalence of these conditions is highly related to uterine scarring, multiple pregnancies, and advanced maternal age [1]. Morbidly adherent placenta occurs when the chorio-nic villi attach abnormally to the myometrium. Three grades of abnormal placental attachment have been defined according to the depth of attachment and invasion into the myometrium: accreta (chorionic villi attach to the myometrium), increta (chorionic villi invade the myometrium), and percreta (chorionic villi penetrate the uterine serosa with or without invasion of the vicinity organs). Postpartum hemor-rhage related to these conditions is particularly severe when the hypervascular placental bed is located at the poorly contractile lower uterine segment. This impli-es that hemostasis relies heavily on surgical techniq-ues such as the placement of hemostatic sutures, the use of intrauterine balloon tamponade, and the ligation of the uterine artery. If these conservative surgical approaches fail to control bleeding, the last resource is life-saving hysterectomy [2, 3].
To avoid severe hemorrhage, prophylactic manage-ment techniques, such as perioperative internal iliac artery balloon occlusion (IIABO), can minimize blood loss and facilitate surgical performance throu-gh reductions in the rate of uterine perfusion [4, 5]. However, the existing literature on this approach is limited and contradictory. Some authors have confir-med that balloon occlusion is effective in reducing blood loss and transfusion requirements [5]. How-ever, other authors have not found that this method induced any differences in estimated blood loss, transfusion demand, the durations of postoperative hospital stays, or hysterectomy requirement [6]. The failure of IIABO to reduce blood loss may occur due to extensive intrapelvic vascular anastomoses (e.g., the ovarian arteries) [6].
Another controversial aspect of IIBAO is fetal radiation exposure. Normally, the adequate position-ing of intra-arterial occlusion balloons is confirmed prior to their use by fluoroscopy with or without a contrast medium; this exposes the fetus to the negative effects of variable doses of radiation. Theoretically, IIABO is associated with a low radiation dose, with a fetal absorbed dose (FAD) of approximately 12.8–31.5 mGy per procedure [7, 8]. The present study aimed to evaluate the efficacy and safety of prophylactic IIABO placement by an interventional radiologist before cesarean delivery in patients with placental implantation abnormalities.
2. Materials and Methods
We performed a retrospective comparative analysis of the medical and radiological records of eighteen (n = 18) pregnant patients with placental disorders between May 2017 and May 2021. These placental disorders included placenta previa in four cases (n = 4), placenta increta in eleven cases (n = 11), and placenta percreta in three cases (n = 3). Fourteen (n=14) cases received prophylactic IIABO while four (n=4) cases did not received endovascular inter-vention. Cesarean section was performed as an elective surgery in all cases and hysterectomy was planned and performed in six (n = 6) cases. Preoperative diagnoses were confirmed using transabdominal and transvaginal Doppler sonography and/or magnetic resonance imaging (MRI).
The typical ultrasound findings were based on the detection of at least two of the following chara-cteristics:
- Loss/irregularity of the hypoechoic area between the uterus and the placenta.
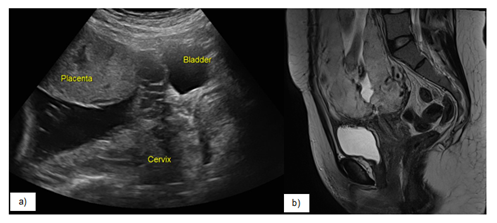
- Thinning/interruption of the uterine serosa-bladder wall interface (Figure 1).
- Myometrial thickness < 1 mm.
- Turbulent placental lacunae with high-velocity flow (>15 cm/seg).
- Increased vascularity of the uterine serosa-bladder wall interface.
- Irregular intraplacental vascularization.
The MRI findings were based on the detection of at least two of the following characteristics:
- Demonstration of uterine bulging and loss of the normal uterine contour.
- T2 sagittal images showing partial or total insertion of the placenta into the lower uterine segment (Figure 2).
- T2 sagittal images showing focal thinning of the myometrium and interruption of the junctional zone.
All included patients were beyond 28 gestational weeks and were discussed by all involved teams in a roundtable meeting two weeks prior to the cesarean appointment. Gynecologists, interventional radiologi-sts, anesthetists, pediatricians, and nurses planned every case in two possible scenarios (planned vs. emergency surgery) and discussed the step-by-step setting before, during, and after the cesarean section with or without prophylactic IIABO. All patients were fully informed of the study and surgical procedures by their doctors and then signed informed consent forms. This research project was approved by the biomedical ethics committee of the hospital.
2.1 Interventional balloon placement technique
All interventions were performed in sterile preope-rative conditions under standby local anesthesia with the assistance of the anesthesiology team (prior to the cesarean surgical event). First, a bilateral common femoral arterial puncture and access was performed with 7-Fr vascular sheaths (35-cm Super-Arrow Flex®, Athlone, ROI) under local anesthesia. Both internal iliac arteries were sequentially (first right and then left) cannulated using a crossover approach with a 4-Fr Cobra C2 catheter (Cordis™, California, USA). In one case (n = 1), it was necessary to employ a 4-Fr Omniflush catheter for the crossover approach due to a sharp aortic bifurcation. After selective catheterization of the anterior branch of the internal iliac artery, approximately 15 mm after its origin, a 145-cm 0,035" stiff wire (Amplatz Super Stiff, USA) was placed and the 4-Fr catheter was exchanged for an 8.5-mm/11.5-mm x 80-cm occlusion balloon catheter (Berenstein, Boston Scientific, USA). Then, the wire was removed.
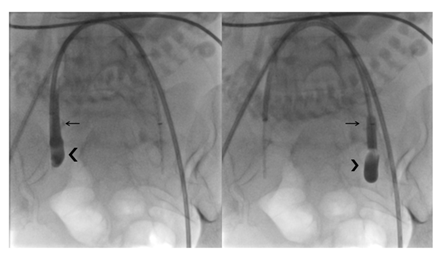
A balloon occlusion test was performed with 1.5 ml of diluted contrast media in each balloon, always unilaterally (never both balloons at the same time) and always under continuous monitoring of the fetal heart rate. The correct position and function of each balloon (positive occlusion balloon test) was determined if a column of contrast media was visible above the filled occlusion balloon in the internal iliac artery, indicating complete occlusion of the blood flow distal to the balloon (Figure 2). During the procedure, we paid particular attention to the minimi-zation of fetal radiation exposure using intermittent low-dose fluoroscopy. Both introducer/catheter systems were secured to the patient’s legs with steri-lized transparent dressing tape (3M® USA), making them visible and accessible for further injection.
2.2 Cesarean surgery and balloon inflation
After a surgical time-out with all teams present, the surgery was started with the interventional radiologist beside the surgical team in sterile conditions. Imme-diately after the cesarean delivery of the child, the balloons were inflated at the time of cord clamping to minimize the risk of fetal hypoxia with 1.5 ml fluid and without the use of fluoroscopy. The balloons remained inflated constantly throughout the surgery until no more bleeding was present. During this period, the interventional radiologist stayed in the operating room, monitoring distal leg pulses (dorsal pedis or tibialis posterior) with a portable Doppler probe. Balloon deflation was performed sequentially when bleeding risk was not more present by report of the surgical and anesthesiology teams; during this period, potential transvaginal bleeding was controlled for two minutes. If no further bleeding was observed, the balloons were not reinflated. When the surgery was finished, balloon catheter extraction was perfo-rmed and closure devices (Proglide™, Abbott Vas-cular, USA, or Mynx™, Cardinal Health, USA) were used to ensure hemostasis of the common femoral arteries. If the patient presented with continuous bleeding after the surgery, the balloons remained inflated until another management action, such as uterine artery embolization (UAE), was taken.
The assayed variables of this study were:
- Estimated blood loss, the number of red blood cell transfusions, and the length of the hospital stay for mother and child.
- Reported and evaluated intervention time, balloon inflation time, surgical time, and fetal absorbed dose.
The degree of intraoperative blood loss was estimated with reference to the amount of blood collected by the suction system and the weight and number of surgical pads. This estimation was performed by anesthetists who were not part of the research team. We also reviewed the rates of emergency UAE and conservation of the uterus. The FAD and the mother’s effective dose were calculated with PCXMX version 2.0 [9] based on individual procedure conditions (dose area product/DAP and source-to-surface distance) and patient information (height and weight). Average field sizes (0.09 ± 0.01 m2) were used for all estimations. After the aforementioned data were entered into the program, the radiation dose absorbed by the uterus was calculated and used to represent the fetal absorbed dose. For statistical analysis, continuous variables were presented as means ± SDs. The main characteristics and outcomes are presented in Table 1.
|
Characteristic |
IIABO Group (n=14) |
Non-IIABO Group (n=4) |
|
Age (years) |
36 ± 4.1 |
33 ± 3.4 |
|
Pregnancy length (week) |
34.3 ± 1.8 |
31.3 ± 2.4 |
|
Prior Cesarean Section |
1 ± 1.5 |
0.5 ± 0.5 |
|
Estimated Blood Loss (ml) |
650 ± 581.8 |
700 ± 475 |
|
Packed RBC Transfused (u) |
0 ± 0.7 |
0 ± 2 |
|
Placenta previa |
3 |
1 |
|
Placenta increta |
8 |
3 |
|
Placenta percreta |
3 |
0 |
|
Interventional time (min) |
21 ± 7.5 |
- |
|
Ballon Inflation time (min) |
33 ± 34.1 |
- |
|
Surgery time (min) |
69 ± 59.4 |
70 ± 25 |
|
Uterus preservation |
8 |
1 |
|
Mother effective dose (mSv) |
2.2 ± 3.4 |
- |
|
Fetal radiation dose (mGy) |
6.4 ± 12.1 |
- |
|
Mothers hospital length of stay (days) |
6 ± 3.2 |
12 ± 17 |
|
Childs hospital length of stay (days) |
7 ± 6.7 |
26.5 ± 17.2 |
Table 1: Patient characteristics and outcomes.
3. Results
3.1 Maternal demographics
The study group included eighteen (n = 14) patients ranging in age from 29 to 41 years, with a mean age of 36 ± 4.1 years. The number of gestational weeks ranged from 33 + 1 weeks to 40 + 3 weeks, with a mean of 34.3 ± 1.8 weeks. The number of previous C-sections ranged from one to six, with a mean of 1 ± 1.5. The control group included four (n=4) patients ranging ng age from 30 to 38 years, with a mean age of 33 years. The number of gestational weeks ranged from 28 + 1 to 34 + 1 weeks, with a mean of 31.3 ± 2.4 weeks. The number of the previous C-section ranges from zero to one, with a mean of 0.5 ± 0.5
3.2 Operating room and intervention times
Fourteen IIABO placements were performed, including twelve (n = 12) in the hybrid operating room and two (n = 2) in the interventional suite. The intervention duration ranged from 17 to 38 minutes, with a median of 20.5 ± 7.2 minutes.
3.3 Radiation-Related factors
The fluoroscopy time ranged from 180 to 482 seconds, with a mean of 294.5 ± 101.3 sec. The DAP ranged from 2.3 to 42.5 Gycm2, with a mean of 6.87 ± 13.2 Gycm2. The mother's effective dose ranged from 0.58 to 11.87 mSv, with a mean of 2.2 ± 3.47 mSv. The uterus dose ranged from 1.78 to 14.09 mGy, with a median of 4.67 ± 7.50 mGy.
3.4 Inflation and surgery times
For all IIABO procedures, the inflation time ranged from 25 to 38 min, with a mean of 38 ± 43.8 min. The surgery time ranged from 35 to 300 min, with a mean of 77 ± 81.6 min. None of the patients had vascular complications related to the internal iliac occlusion or the femoral access (e.g., embolic comp-lications, vascular dissections, or gluteal ischemia). The surgery time of the control group was 70min ± 25min.
3.5 Intraoperative blood loss and transfusions
The estimated blood loss during IIABO ranged from 400 to 2500 ml, with a mean of 700 ± 1312.6 ml, while the control group was 700ml ± 475ml. The number of red blood cell transfusion packages ranged from 0 to 2, with a mean of 0U ± 1.4U for the IIABO group and 0U ± 2U for the control group.
3.6 Uterine artery embolization (UAE) and emergency hysterectomy
In the three patients who presented with continuous bleeding (n = 3), the balloons remained inflated until UAE had been performed. The procedure was executed on the same day using 700-um particles and Spongostan, without any further complications. None of the patients required emergency hysterectomy (n = 0).
3.7 Length of hospital stay for mother and child
The length of the mother's hospital stay after C-section and prophylactic IIABO ranged from 3 to 14 days, with a mean of 6 ± 3.09 days while the control group ranged from 8 to 46 days with a mean of 12 days ± 17 days. The length of the child's hospital stay ranged from 3 to 29 days; with a mean of 7 ± 6.5 days while the control group range from 20 to 58 days, with a mean of 26.5 ± 12.7 days. Factors that increased the likelihood of a prolonged hospital stay included secondary injuries to the bladder causing hematuria or urine leakage and emergency hyste-rectomy.
4. Discussion
Placental implantation abnormalities are potentially life-threating conditions, and management strategies for these scenarios vary depending on local expertise and access to specialist multidisciplinary teams. Early reports on placental implantation abnormalities have estimated maternal mortality rates of approximately 7%, reaching 30% in the absence of prenatal diag-nosis. Recent data suggest that mortality rates in the range of 0.05% are achievable when prenatal diagnosis and multi-professional expert management are available [10, 11]. The clinical outcomes of placental implantation abnormalities are directly related to the depth and topography of the placenta. When there is widespread placental invasion into the myometrium, the risk of life-threatening hemorrhage and hysterectomy is increased [12, 13]. Perioperative prophylactic occlusion of the internal iliac arteries through balloon placement prior to cesarean delivery is an emerging conservative strategy for the preven-tion of severe hemorrhage in these patients. One of the biggest drawbacks of the regular employment of IIABO is the lack of information about the existence and effectiveness of this technique within the obstetric community. However, there is consistent evidence that compared to women managed with standard obstetric care, patients who are prenatally diagnosed with placental disorders and managed by a multidisciplinary team are less likely to require emergency hysterectomy, large-volume blood trans-fusion, and reoperation for bleeding complications within seven days of delivery [14, 15]. In a recent systematic review, D'Antonio et al. stated that interventional radiology procedures beneficially reduced blood loss and need for transfusion in women undergoing surgery for PAS. This benefit persisted in a sample consisting only of women undergoing hysterectomy and those affected by placenta percreta [15].
In the present study, IIABO effectively controlled perioperative bleeding in 12 cases (80%), with a mean estimated blood loss of 700 ml. Only three cases required additional red blood cell transfusions. The present series is not the first to demonstrate that IIABO is a helpful technique for the reduction of operative blood loss and transfusion requirements in patients undergoing hysterectomy for invasive placenta [5, 6]. Other investigations, such as that conducted by Liu et al., have suggested that other techniques (e.g., intra-aortic balloon occlusion) can also effectively reduce bleeding rates in patients with placenta accreta; nevertheless, this study reported higher blood loss (>1900 ml) compared to the present study. In addition, Peng et al. stated that IIABO placement did not reduce intraoperative bleeding or the hysterectomy rate and resulted in longer operation times and postoperative hospital stays [4, 7] In the present study, the use of a hybrid operating room enabled easier transition between procedures, with a median intervention time of 25 min and a median surgical time of 69 min. After IIABO placement, the surgical group was able to operate without inter-ruption or concerns about high-frequency bleeding. The presence of an interventional radiologist thro-ughout the entire procedure allowed for effective interdisciplinary communication and prompt action if an emergency embolization was required.
In a randomized controlled trial, Chen et al. reported that days of hospitalization did not significantly differ between patients who underwent emergency cesarean hysterectomy with and without prophylactic place-ment of intravascular balloon catheters (four to seven days). In the present series, the average maternal intra-hospitalary stay duration was 6 ± 3.09 days. The presence of hematuria and possible bladder invasion/ resection were the main reasons for longer in-hospital observation days. Complications directly related to the IIABO technique have been reported to affect between 1.7% and 16% of cases; the most common of these complications are hematomas at the puncture site, arterial dissection, and vascular thrombosis [16, 17]. An additional concern about endovascular procedures performed during pregnancy is fetal radiation exposure. The probability of the occurrence of a radiation-induced fetal effect is related to the amount of accumulated radiation and the gestational age at which the radiation is applied. In the present study, the risk of fetal effects caused by ionizing radiation (e.g., carcinogenesis and DNA injury) related to the IIABO procedure could be considered minimal, as the average registered fetal absorbed dose was 6.87 mGy. This amount of radiation is very unlikely to induce stochastic or deterministic effects, as fetal abnormalities have only been reported with radiation doses above 50 mGy [18, 19]. Interven-tional radiologists should use all available techniques to minimize fetal radiation exposure while main-taining interventional accuracy. Blind procedures involving the placement of balloon occlusion catheters in the iliac arteries are not recommended because these techniques could increase the risk of vascular damage (e.g., arterial dissections) or ineffective occlusion and hemorrhage control.
The present study had several limitations, including the retrospective, single-center nature of the investigation and the limited number of analyzed patients. There is a real need for prospective, randomized, multi-center controlled trials with larger numbers of patients to support wider acceptance of this method. However, ethical factors are the biggest limiting factors because studies such as this demonstrate that certain benefits are associated with the use of IIABO. In conclusion, the prophylactic use of IIABO in placenta previa and placenta accreta spectrum is an effective and safe method of controlling bleeding during cesarean section and hysterectomy. However, indications should be strictly controlled, and interdisciplinary planning and management are mandatory.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by our institutional Review Board.
Consent for publication
Consent for publication was obtained for every individual persons data included in the study.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was not supported by any funding.
References
- Giampaolino P, Della Corte L, Apparente P, et al. Uterine arteries prophylactic occlusion balloon placement in pregnancies with placenta praevia. Minim Invasive Ther Allied Technol 23 (2019): 1-5.
- Cho SB, Hong SJ, Lee S, et al. Preoperative prophylactic balloon-assisted occlusion of the internal iliac arteries in the management of placenta increta/percreta. Medicina (Kaunas) 56 (2020): 368.
- Yu SCH, Cheng YKY, Tse WT, et al. Perioperative prophylactic internal iliac artery balloon occlusion in the prevention of postpartum hemorrhage in placenta previa: a randomized controlled trial. Am J Obstet Gynecol 223 (2020): 117.e1-117.e13.
- Liu J, Wang Y, Jiao D, et al. Prophylactic occlusion balloon placement in the abdominal aorta combined with uterine or ovarian artery embolization for the prevention of cesarean hysterectomy due to placenta accreta: a retrospective study. Cardiovasc Intervent Radiol 42 (2019): 829-834.
- Picel AC, Wolford B, Cochran RL, et al. Prophylactic internal iliac artery occlusion balloon placement to reduce operative blood loss in patients with invasive placenta. J Vasc Interv Radiol 29 (2018): 219-224.
- Salim R, Chulski A, Romano S, et al. Precesarean prophylactic balloon catheters for suspected placenta accreta: a randomized controlled trial. Obstet Gynecol 126 (2015): 1022-1028.
- Peng Y, Jiang L, Peng C, et al. The application of prophylactic balloon occlusion of the internal iliac artery for the treatment of placenta accreta spectrum with placenta previa: a retrospective case-control study. BMC Pregnancy Childbirth 20 (2020): 349.
- Nieto-Calvache AJ, Salas LF, Duran EJ, et al. Estimation of fetal radiation absorbed dose during the prophylactic use of aortic occlusion balloon for abnormally invasive placenta. J Matern Fetal Neonatal Med 21 (2019): 1-6.
- Kai K, Hamada T, Yuge A, et al. Estimating the radiation dose to the fetus in prophylactic internal iliac artery balloon occlusion: three cases. Case Rep Obstet Gynecol 2015 (2015): 170343.
- Shrivastava V, Nageotte M, Major C, et al. Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol 197 (2007): 402.e1e5.
- Fonseca A, Ayres de Campos D. Maternal morbidity and mortality due to placenta accreta spectrum disorders. Best Pract Res Clin Obstet Gynaecol (2020): S1521-6934(20)30121-8.
- Li K, Zou Y, Sun J, et al. Prophylactic balloon occlusion of internal iliac arteries, common iliac arteries and infrarenal abdominal aorta in pregnancies complicated by placenta accreta: a retrospective cohort study. Eur Radiol 28 (2018): 4959-4967.
- Richa A, Amita S, Bala VN, et al. Morbidly adherent placenta: a critical review. J Obstet Gynaecol India 62 (2012): 57e61.
- Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol 217 (2017): 27-36.
- D'Antonio F, Iacovelli A, Liberati M, et al. Role of interventional radiology in pregnancy complicated by placenta accreta spectrum disorder: systematic review and meta-analysis. Ultrasound Obstet Gynecol 53 (2019): 743-751
- Chen M, Liu X, You Y, et al. Internal iliac artery balloon occlusion for placenta previa and suspected placenta accreta: a randomized controlled trial. Obstet Gynecol 135 (2020): 1112-1119.
- Chen L, Wang X, Wang H, et al. Clinical evaluation of prophylactic abdominal aortic balloon occlusion in patients with placenta accreta: a systematic review and meta-analysis. BMC Pregnancy Childbirth 19 (2019): 1e8.
- Communities European. Council directive 97/43/EURATOM of 30 June 1997 on health protection of individuals against the dangers of ionizing radiation in relation to medical exposure. J Eur Commun 180 (1997): 22-27.
- Committee Opinion No. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol 130 (2017): e210-e216.


 Impact Factor: * 4.8
Impact Factor: * 4.8 Acceptance Rate: 69.70%
Acceptance Rate: 69.70%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 