Comparison of Efficacy and Safety of Analog Insulins Vs Human Insulins-Basal Bolus Regimen and Premix Twice Daily Regimen in Indian Hyperglycemia in Pregnancy
Anand Shankar*
MBBS, Department of Medicine (MD), Shankar Diabetes Care Centre, Patna, Bihar, India
*Corresponding Author: Anand Shankar, MBBS, Department of Medicine (MD), Shankar Diabetes Care Centre, Patna, Bihar, India
Received: 25 August 2022; Accepted: 01 September 2022; Published: 03 October 2022
Article Information
Citation:
Anand Shankar. Comparison of Efficacy and Safety of Analog Insulins Vs Human Insulins- Basal Bolus Regimen and Premix Twice Daily Regimen in Indian Hyperglycemia in Pregnancy. Archives of Internal Medicine Research 5 (2022): 462-465.
View / Download Pdf Share at FacebookAbstract
Type 2 diabetes during the course of pregnancy is linked with an unfavourable outcome of maternal foetal. Human insulin is considered as the first choice of drug due to the province safety of use full stop however they are many questions regarding the use of insulin analogues during the time of pregnancy full stop the main objective of the study is to compare the safety and efficacy of human insulin and analogue insulin by understanding the premix daily regimen in hyperglycaemia during pregnancy. All the articles have been classified with an intermediate too high or moderate risk of biasness by showing the favourable results for using the insulin analogue and human insulin as an age of gestation. Therefore, the result evidence that "moderate to high risk of biasness does not allow the conclusion” that the insulin analogue is considered to be more effective while comparing it with the “human insulin for the treatment of pregnant women” suffering from “type 2 diabetes”.
Hyperglycemia articles, Pregnancy articles
Article Details
Introduction
“Diabetes mellitus (DM)” is a major issue in the public health care system in recent times. It can be estimated that there are more than 423 million of adults facing “DM worldwide” with an estimation of 620 million in the coming 2045. The objective of this present study is to identify the insulin analogues by comparing to “human insulin for the proper treatment of pregnant women with type 2 diabetes”. Diabetes mellitus is considered as the “measure of metabolic complication” during pregnancy and night occurs in different area of clinical approach such as it might develop during the pregnancy or the woman has been diagnosed with diabetes previously. Different studies of observation have indicated that hyperglycaemia without or with a history of diabetes is linked with some of the adverse effects that tends to increase the infection rate, mortality rate and Hospital stay.
The “basal- bolus regimen” for once in a “daily long-time insulin and rapid time insulin analogues” before having the mil is considered to be an effective for the improvement of glycaemic control and also reducing the complications in the hospital in terms of the “non- ICU patients suffering from type 2 diabetes”. Besides the benefits of the “basal bolus regimen”, there are also many health care experts who consider this method to be a difficult approach for implementing and is considered to be “inconvenient due to increasing number of injections and risk of hypoglycaemia”. Persistent hyperglycaemia is known as the harmful factor for all the women during pregnancy. Still, most of the patients having DM previously are facing a serious situation as hyperglycaemia might negatively influence the pregnancy since the duration of implantation and fertilisation. There are various complications that are possible for the child with “macrosomia, congenital malformations, spontaneous abortion traumas during the birth of the child, respiratory distress syndrome hypoglycaemia and many more”. However, the pregnant women can suffer from “premature rupture in terms of the amniotic membrane, polyhydramnios, premature birth, pregnancy toxaemia, high chances of caesarean section and mortality. Additionally, the worst situation of the chronic complications is already existing within diabetes such as nephropathy and retinopathy”.
Negative effects can also occur during the long run where both child and mother need to face increased obesity risk, “glucose intolerance and type 2 disease” for the child. On the other hand, the mother is more permitting in terms of “gestational DM type 2 diabetes, hypoglycaemia and systemic hypertension”. It is a significant approach to handle the glycaemic control during the time of pregnancy as it helps to improve the outcomes of foetal and mother. However, “insulin therapy” is the standard goal treatment and by using this human insulin during pregnancy is considered to be well established. The divergence may still exist in terms of the “use of insulin analogues” during the clinical situation as it conflicts with results which are previously found in the studies. Therefore, premixed insulin can be commonly prescribed by formulating to the “outpatient management of patients” suffering with “type 2 diabetes”. The “efficacy and safety” for the formation of premix insulin in the setting of a hospital is still not known.
Methods
In the prospective and randomised study, the patients suffering from type 2 diabetes during pregnancy have been recruited who were being admitted to surgery services and general medicine. The prescription of the patients indicating the entire information who are suffering from diabetes during their pregnancy. However, a systematic review of the original article is performed to determine the use of insulin analogues for the “diabetic pregnant women” treatment. The method to use in the current study is followed by the recommendations to conduct a systematic review which is opined by Janez [1]. The recommendation is the part of this technique which helps to create protocol and is available to all the public by disclosing after the registration of the database. In this manner, the protocol can be developed and be available if the public indicates some interest. The paper has excluded the patients suffering from type 1 diabetes, along with hyperglycaemia without the diagnosis of diabetes previously [2]. The ethics committee from the participating institution has approved the protocol of study. Moreover, informed consent has been obtained from the patients by informing about the objective potential risk and nature of the study the following study is conducted with treatment and randomisation assignment.
Results
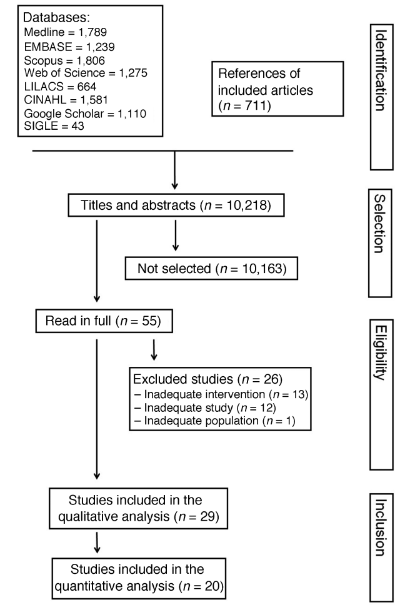
There are several additional articles that are identified on the database as well as the articles that are excluded and included from the original papers are presented as a systematic review in the below Figure 1 [3]. The articles that have been selected with the help of such strategies of which 11 of them were randomised trials of clinical approach and 18 studies of observation. Data extracted from the search strategy have been analysed by characterising the included studies which is shown in the Table 1.
|
Database |
Search strategy |
|
Scopus |
Gestation, insulin and diabetes or regular insulin |
|
Pubmed |
Gestational, diabetes, or gestational diabetes, and insulin, or isophane insulin. |
|
Google scholar |
Insulin, pregnancy |
|
Embase |
Gestational diabetes, aspart, NPH insulin and lispro. |
Table 1: Search strategy of database.
Sensitivity analysis has been planned due to the bias risk in the study that are consist in the current study. However, this paper has not been conducted since all the original articles have been categorized as having a “high or moderate risk of bias” [4]. The sensitivity analysis has not been performed as it was not considered to be a proper fit to the previous criteria. The main eligible study is related to blinding as all of them are considered to be open trials. Statistical biases are present in the study due to the “absence of calculation of sample size” in most of the cases.
Discussion
Insulin is considered as the choice of drug for the “treatment of type 2 diabetes during pregnancy” since it will never cross the obstacles of placental criteria in appropriate volume. “The use of insulin analogues for a proper treatment of "diabetic pregnant women" is being determined and is not entirely distributed within the clinical approach besides the evidence connected with the safety area” [5]. There are still some preferences for using human insulin during the period of gestation. The requirement for this “type of insulin” is to be used appropriately during the time of gestation in order to maximise the resistance of insulin that might start with a “dose of 0.5 U per kg”. However, it is highly recommended to use a small portion of net amount of dose as a base insulin in a daily purpose (<50%) and high proportion (>50%) as an insulin. This keep might be used in multiple doses or continuous infusion on a daily basis. Moreover, adjustment should be conducted according to the observing of "capillary blood glucose".
The analysis of results on the current study is classified by having a high or moderate risk of biasness. The evidence that is determined on the current review can be easily classified as low. Accordingly, this paper is consistent with the main limitation of the process of review where the studies are involved with a high level of risk of biases that tends to present a positive outcome which may lead the reviews with an inadequate conclusion [6]. The planning of sensitivity analysis has been performed but was not conducted due to the proper eligible articles considered to have a high or moderate risk of biasness. Some reasons also prevent the “inclusion of the studies that present the meta-analysis” such as the different concepts for maternal hypoglycaemia and neonatal hypoglycaemia. Additionally, there are several ways to “present the results or the absence of gross outcomes” which has been reported in the study.
The "gestational diabetes mellitus" (GDM) is “accounting for 90% of the cases where the pregnancy is considered to be complicated by diabetes.” GDM may have high effects that include an increasing subsequent “risk of developing type 2 diabetes for both child and mother”. Moreover, the goal for the “GDM management” is to maintain and control the maternal mean “fasting plasma glucose (FPG) concentration” with 90 mg per dl and "postprandial plasma glucose (PPG) concentration with 120 mg per dl” to make sure that the "neonatal birth weight” is appropriate for the age of gestation [7]. Although, the effectiveness of improving the "postprandial glycaemic control" vote on "fast acting insulin analogue and soluble human insulin" is needed to administer before the mail and additionally the basal insulin should be required to regulate the level of "fasting plasma glucose". This results with a complexity of "basal-bolus regimen" that requires at least four injections on a daily basis. The previous study of poly pharmacy in women with “type 2 diabetes” are found to have an in which relationship among the regimen complexity and treatment adherence. With an unmanaged GDM it can be consequently profound for both “child and mother and is vital to clarify the treatment as much as possible”.
The formulation of “premixed insulin” might provide an effective and simple alternative that combines a long acting and rapid acting element to control both FPG and PPG respectively with a “single formulation of convenience”. This provides a potential way to achieve the control on glycaemic by reducing the frequency number of injection [8]. However, the benefits of “insulin analogues” are “administration during the morning controls of post breakfast and lunch, administration during the evening controls in post dinner as well as FPG, reducing the risk of hypoglycaemia, improving HbAlc and simplifying dose by reducing the amount of injection per day”. By using the premix human insulin frequently can comprise 70% of intermediate acting of human insulin and 30% of short acting that can be “administered twice depending on the requirement of the patient on a daily basis. The capacity of BHI 30 to imitate the natural insulin of physiology release profile can be compromised by the properties of pharmacokinetic in terms of the insulin components” [2]. Besides, the potential benefit of premix insulin throughout India within the management of GDM various studies have been assessing the “safety and efficacy of either BIAsp 30 or BHI 30” in pregnant women. This paper performs with different results that involves the obstetric characteristics within the pregnant women and facing difficulties with type 2 diabetes.
Conclusion
The study including the matter analysis indicates that there is available evidence to understand the high or moderate risk of biasness and does not support the conclusion that insulin analogues can be considered to be more effective while comparing it to the human insulin for a proper treatment of the pregnant women suffering from “type 2 diabetes”. The findings of the study also indicate that the insulin is effective in reducing the concentration of postprandial glucose which results in a significant way of lowering the glucose without the use of insulin. Regular use of “human insulin” failed to create an appropriate impact on reducing the concentration of “postprandial glucose” as the insulin utilised can be measured as both endogenous and exogenous insulin with a contribution of “endogenous insulin to PPG profile” within ascertained by comparing it to FPG. However, the analogue of premix insulin is also considered to be a useful formulation for the clinical use of both in pregnant women and diabetic population with abnormal tolerance of glucose. The pregnant women have found “BIAsp to be convenient as the preparation allows flexibility during the male time by dozing insulin and does not disturb their pattern of routine”. Additionally, BIAsp is found to be safer by using it during the course of pregnancy. Therefore, this is the prospective randomised trial that compares the safety and efficacy of basal bolus regimen in terms of insulin on a daily basis and with the premix regiment of human insulin to the patients suffering from type 2 diabetes.
References
- Janez A, Guja C, Mitrakou A, Lalic N, Tankova T, Czupryniak L, Tabák AG, Prazny M, Martinka E, Smircic-Duvnjak L. Insulin therapy in adults with type 1 diabetes mellitus: a narrative review. Diabetes Therapy 11 (2020): 387-409.
- Murphy HR, Howgate C, O'Keefe J, Myers J, Morgan M, Coleman MA, Jolly M, Valabhji J, Scott EM, Knighton P, Young B. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. The lancet Diabetes & endocrinology 9 (2021): 153-164.
- Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. Cmaj 180 (2009): 385-397.
- Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, Pieber TR. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Archives of Internal Medicine 165 (2005): 1337-1344.
- Pettitt DJ, Ospina P, Kolaczynski JW, Jovanovic L. Comparison of an insulin analog, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes care 26 (2003): 183-186.
- Seshiah V. Premixed Insulin Analogues in Diabetes and Gestational Diabetes Mellitus (2016).
- Santos LL, Santos JL, Barbosa LT, Silva ID, Sousa-Rodrigues CF, Barbosa FT. Effectiveness of insulin analogs compared with human insulins in pregnant women with diabetes mellitus: systematic review and meta-analysis. Revista Brasileira de Ginecologia e Obstetrícia 41 (2019): 104-115.
- Guney Z. Insulin and its analogues–what are they for? (pros and cons). Trends in Diabetes and Metabolism 2 (2019): 1-2.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 