The SIRT1 Agonist SRT1720 Effectively Decreased Il-1β Levels in Cerebral Microvascular Endothelial Cells Caused By CMV Infection and Ox-LDL Treatment
Pian Ye2, Mengen, Zhu1, Shaoqiong, Zhou1, Lihua Liu1, Chibo Ai3, Xin Fang1*
1Department of Geriatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
2Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
3Department of Neurology, Yunyang County People’s Hospital, Chongqing, China
*Corresponding Author: Xin Fang, MD, Ph.D., Department of Geriatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430022, China.
Received: 31 October 2023; Accepted: 03 November 2023; Published: xx November 2023
Article Information
Citation: Pian Ye, Mengen, Zhu, Shaoqiong, Zhou, Lihua Liu, Chibo Ai, Xin Fang. The SIRT1 Agonist SRT1720 Effectively Decreased Il-1β Levels in Cerebral Microvascular Endothelial Cells Caused By CMV Infection and Ox-LDL Treatment. Archives of Internal Medicine Research. 6 (2023): 108-115
View / Download Pdf Share at FacebookAbstract
CMV infection may cause or worsen microvascular dysfunction induced by high cholesterol in mice, possibly through inflammation. Interleukin 1 Beta (IL-1β) can alter the permeability of brain endothelial cells. Sirtuin (SIRT1) inhibits the transcriptional activity of nuclear factor kappa B (NF-kB) as an upstream factor. Our objective is to verify the efficacy of correcting IL-1β levels by regulating the SIRT1/NF-κB pathway in brain microvascular endothelial cells (BMECs) induced by CMV infection and/ or ox-LDL treatment.
Methods:
bEnd.3 cells were categorized into four groups: Mock, CMV (infection multiple of 10), ox-LDL (100 μg/ml), and both. The cell viability was evaluated using the CCK-8 assay. The expression levels of SIRT1, acetylation and phosphorylation of p65 NF-κB were examined through Immunofluorescence staining or Western Blot. Moreover, IL-1β levels were measured using the ELISA method.
Results:
In BMECs, CMV infection or ox-LDL treatment increases IL-1β levels. Additionally, CMV or/and ox-LDL causes a decrease in SIRT1 levels and an increase in acetylation and phosphorylation of p65 NF-κB. Importantly, the administration of any NF-κB inhibitor or a SIRT1 agonist reduces the IL-1β induced by CMV and/or ox-LDL.
Conclusions:
Our findings offer novel insights into the impact of viral involvement in brain endothelial injury. It can be inferred that both the SIRT1 agonist SRT1720 and the NF-κB inhibitor helenalin effectively address the heightened IL-1β levels induced by CMV and/or ox-LDL in brain endothelial cells. Moreover, helenalin, as an NF-κB inhibitor, can reverse the reduced cell activity triggered by CMV and NF-κB, whereas SRT1720 fails to achieve this outcome.
Keywords
<p>CMV; ox-LDL; IL-1β; SIRT1; cerebral microvascular endothelial cells</p>
Article Details
Introduction
Cytomegalovirus (CMV) is a β-herpes virus that infects between 60% to 70% of adults in industrialized countries and close to 100% in emerging countries. Although CMV infection can be life-threatening in immunocompromised patients, it also may be asymptomatic in immunocompetent individuals. However, the virus often remains latent after infection, and it can reactivate when the individual's immune system is compromised [1]. CMV infection in healthy individuals has been shown to increase the risk of cardiovascular diseases [2].
The endothelium is an important site for CMV infection [3]. It has been demonstrated by Khoretonenko et al. that murine CMV infection can lead to microvascular dysfunction or worsen microvascular responses induced by high cholesterol in mice. Inflammation is considered a possible mechanism underlying these effects [4]. Interleukin-1β (IL-1β) is a potent pro-inflammatory cytokine that can induce alterations in brain endothelial cell permeability and mediate the inflammatory response to ischemic stroke [5,6] Sirtuin 1 (SIRT1), a sirtuin that localizes in the nucleus and cytoplasm, is involved in regulating cell survival in response to various stimuli, including metabolic factors and viruses [7]. It acts as an upstream impact factor by inhibiting nuclear factor kappa B (NF-kB) transcriptional activity [8-10]. Extensive evidence suggests that SIRT1 plays a role in endothelial dysfunction and subsequent cardiovascular diseases [7]. The absence of SIRT1 is believed to be a potential mediator of age-related cardiovascular diseases. Furthermore, it has been established that human CMV infection of endothelial cells results in reduced levels of SIRT1 protein at 24 h postinfection [11]. Therefore, it seems reasonable to speculate that CMV may augment IL-1β levels by suppressing SIRT1 expression, activating the NF-κB pathway, and exacerbating hypercholesterolemia responses. Based on this, our aim is to verify the efficacy of correcting IL-1β levels by regulating the SIRT1/NF-κB pathway in brain microvascular endothelial cells induced by CMV infection and/or oxidized low-density lipoprotein (ox-LDL, one of the main causative factors of atherosclerosis due to hypercholesterolemia) treatment.
Methods
Cell Culture
NIH/3T3 cells were obtained from Wuhan Punuosai Life Science and Technology Co., Ltd. and cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) under controlled conditions of a 37°C incubator with a humidified atmosphere saturated with 5% CO2. The cells in the logarithmic growth phase were used for both passaging and experimental procedures to ensure optimal conditions for their proliferation and study.
bEnd.3 cells were also obtained from Wuhan Punuosai Life Science and Technology Co., Ltd. and cultured in DMEM medium containing 10% FBS. The cells were seeded into 96-well cell culture plates at a density of 2000 cells per well and divided into four groups: Mock, CMV, ox-LDL, and CMV + ox-LDL. The Mock group served as the control. The CMV group was then treated with the K181 strain of mouse CMV (mCMV) at a multiple of infection (MOI) of 10 for 2 hours, followed by washing with PBS and culturing with complete medium. Next, the ox-LDL group was treated with 100 μg/ml ox-LDL (H7950-2mg, Solarbio, Beijing, China) for 48 hours. In the CMV + ox-LDL group, the cells were first infected with mCMV at a MOI of 10 for 2 hours, and after 5 days of infection, 100 μg/ml ox-LDL was added for 48 hours. At day 7 post-infection, cells in each group were harvested and pelleted for subsequent testing. In the inhibition experiment, a complete medium containing 10 μM SRT1720 (SIRT1 agonist, Target, T2412) or 10 μM helenalin (NF-κB inhibitor, Abcam, Ab146197) was used to replace the cell medium. At day 5 post-infection, some infected and uninfected cells were treated with 100μg/ml ox-LDL. After 7 days of infection, all cells in each group underwent follow-up tests after the respective treatment periods.
Determination of viral infection titer
The NIH/3T3 cells in the logarithmic phase were detached by 0.25% trypsin. The cell concentration was adjusted to 3x105/ml with DMEM culture solution. Then, the cell culture (1.5 ml/well) was added to 6-well cell culture plates. Incubation was carried out at 37°C in a 5% CO2 incubator for 24 hours to achieve 80% cell density. After aspirating the culture solution from each well, 1.5 ml of viral supernatant was taken from a 109 concentration. It was then diluted to a 101 concentration and inoculated into three wells for each concentration of the K181 strain of mCMV virus. The plates were then incubated at room temperature for 2 hours. Following aspiration of the supernatant, the cells were treated with 1% methylcellulose-containing medium. After a 10-20 minute incubation to solidify the medium, the plates were placed at 37°C in a 5% CO2 incubator for 7-10 days. Morphological changes of the cells were observed daily under an optical inverted phase contrast microscope, and the number of characteristic cytopathic wells was recorded. The plaques on the counting plate were fixed with 0.5% crystal violet and counted once there was no change. Finally, the TCID50 (viral titer pfu/ml = 1/dilution multiple multiplied by the number of plaques divided by the inoculation volume per well) was calculated.
The activity of bEnd.3 cells
Cell viability was evaluated using a Cell Counting Kit-8 (CCK-8, Dojindo, CK04) according to the manufacturer’s instructions when the cell culture of the above groups reached the predetermined time. Briefly, 100 μl medium was replaced in each well. Subsequently, 10 μl of CCK8 solution was added to each well, and the cells were treated for 4 hours at 37 °C. Afterward, 10 μl of termination solution was added to each well and mixed immediately. Data analysis was performed using a microplate reader OD450, with the blank culture medium serving as the background. Cell viability was calculated as a percentage.
ELISA assay
Cell grouping and treatment were performed as previously described. After treatment, the cell supernatants were collected and centrifuged at 3000 rpm for 30 minutes. The contents of inflammatory factor IL-1β in the cell culture supernatants of each group were then detected using specific ELISA kits (Cloud-Clone Corp.) according to the manufacturer’s instructions. Briefly, the collected cell supernatants were added to 96-well plates coated with the indicated antibodies. After the reaction between the enzyme and substrate, the absorbances of the samples were measured at 450 nm using a microplate reader (Thermo Fisher Scientific, USA). To determine the concentration of each sample, a standard curve was drawn based on the standard solution. The results were expressed in ng/mL.
Western blot
The cells of each group were collected and homogenized in ice-cold lysis. After centrifugation at 12,000 rpm for 10 min at 4°C, the supernatant was collected. The concentration of acquired protein was quantified using the BCA Protein Assay Kit (Jiangsu Keygen Biotech Corp.,Ltd.). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate and load equivalent protein (30 µg). 0.45 μM polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA) were then used to transfer the separated proteins. The membranes were blocked with 5% bovine serum albumin (BSA, BIOSHARP) for 1 h at room temperature. Afterwards, they were incubated overnight at 4°C with affinity-purified antibodies against the following proteins: Sirt1 (#ab189494, Abcam, 1:1000), phospho-NF-κB p65 (#3031, CST, 1:1000), acetyl-NF-kappaB p65 (Lys310, #AF1017, Affinity, 1:1000), NLRP3 [#DF7438, Affinity, 1:1,000], caspase-1 [AF5418,Affinity, 1:1,000], and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ATPA00013Rb, 1:5,000, Proteintech Group, United States). The membranes were washed and then incubated with secondary antibodies including HRP-labeled Goat Anti-Rabbit IgG (H + L) (1:5000 dilution) [Zhongshan Jinqiao Biological Technology Co., Ltd., Beijing, China] or HRP-labeled Goat Anti-Mouse IgG (H + L) (1:5000 dilution) [Zhongshan Jinqiao Biological Technology Co., Ltd., Beijing, China] antibodies for 1.5 hours. Finally, the immunoreactive bands were visualized with chemiluminescence using the Bio-rad ChemiDoc™XRS+ system, and the OD value was analyzed with the Image-Pro Plus 6.0 software (Bio-Rad, Hercules, CA, USA).
Measurement of caspase-1 activity
The pyropptosis/caspase-1 assay kit (Immunochemistry Technologies, Bloomington, USA) was used to detect caspase-1 viability. The cells were trypsinized to create suspensions and labeled with the FLICA (FAM-YVAD-FMK) probe according to the manufacturer's instruction. Finally, the stained cells were observed under an Olympus BX53 fluorescence microscope.
Statistical processing
Statistical analysis was performed using SPSS 25.0 software. Data were expressed as mean ± standard deviation (mean±SD). One-way ANOVA was initially conducted to compare means across multiple groups, and the Bonferroni method was employed for subsequent two-to-two comparisons. A statistically significant difference was considered when p<0.05. GraphPad Prism 7.0 software was utilized for plotting.
Results
Plaque assays and viral infectivity titers
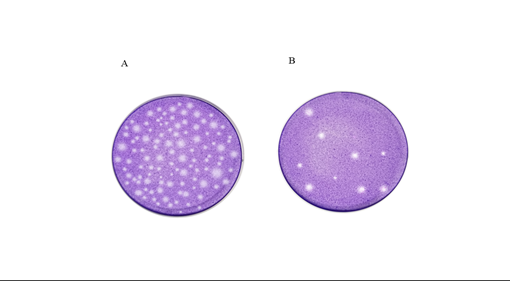
Measurement of plaques under the inverted microscope began after 7-10 days of cultivating NIH/3T3 cells infected with mouse K181CMV, once the plaques reached a stable state. Plaques were not detectable when the dilution ratio of mCMV was lower than 1.0×105 or higher than 1.0×106. At a dilution ratio of 105, mCMV formed widespread and stable plaques throughout the culture dish (Figure 1A), whereas only a few scattered plaques were present at a dilution ratio of 1.0×106 (Figure 1B). The virus titers were 7.1×106 pfu/ml and 7.8×106 pfu/ml when the dilution ratio of mCMV was 1.0×105 and 1.0×106, respectively.
The viability of bEnd.3 cells was decreased by CMV infection and/or ox-LDL treatment
In this study, CCK-8 was used to measure cell proliferation and activity. Cell viability was assessed 7 days after infection, with a ten MOI of CMV and/or intervention with ox-LDL conducted 48 hours before cell examination. As demonstrated in Figure 2A, cell viability decreased to approximately 34% after 7 days of culture following ten MOI infection, showing a significant statistical difference compared to the Mock group (66.11% vs. 100%, p<0.01). Similarly, when ox-LDL intervention was administered 48 hours prior to cell examination, a significant decrease in cell viability was observed compared to the control group, with a decline similar to that caused by CMV intervention (66.20% vs. 100%, p<0.05). Furthermore, after 7 days of culture following 10 MOI infection and a 48-hour ox-LDL intervention before cell examination, cell viability was further reduced to 57.55% compared to the control group (42.45% vs. 100%, p<0.01). These results indicate that both CMV infection and ox-LDL intervention have an additive effect in reducing cell viability.
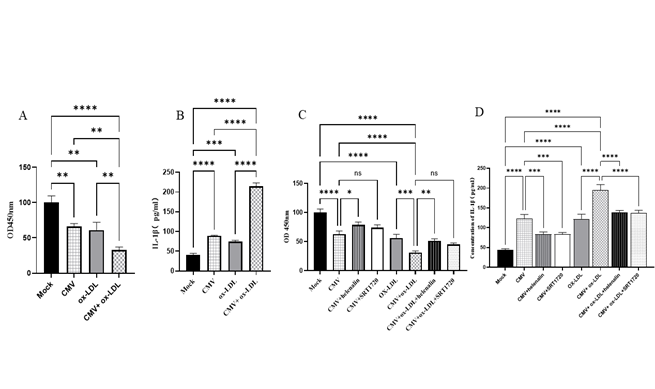
Figure 2: Cell viability with CCK-8 and IL-1β secretion in supernatant of bEnd.3 cell with ELISA analysis (mean ±SEM, n=3). (A) CMV infection or/and ox-LDL treatment decreased cell viability. (B) CMV infection or/and ox-LDL treatment increased IL-1β secretion. (C) The effect of SRT1720 or helenalin on cell viability. (D) SRT1720 or helenalin corrected IL-1β secretion. *p<0.05, **p<0.01, *** p<0.001, and ****p<0.0001 compared with indicated group
CMV and/or ox-LDL reduced SIRT1, activated NF-kB pathway, and elevated IL-1β levels
Figure 2B shows the results of using Elisa to detect IL-1β in the supernant of bEnd.3 cells in our study. Our findings revealed a 2.2-fold increase in the protein expression levels of IL-1β in the CMV infection group compared to the normal control group (P<0.0001). Furthermore, the protein expression levels of IL-1β in the CMV infection group were found to increase by 1.8-fold compared to the normal control group (P=0.0002). Moreover, after the intervention of the two factors, the expression of IL-1β protein increased by 5.29 times compared to the Mock group (P<0.0001), which was significantly higher than any two single-factor interventions (P<0.0001, respectively).
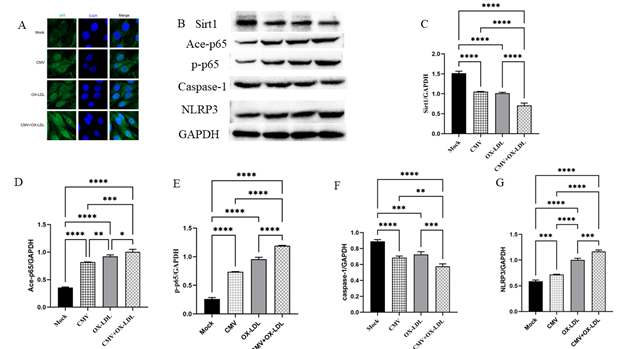
We employed Western blot to examine SIRT1 expression in bEnd.3 cells (Figure 3A-C). The results revealed a significant decrease in SIRT1 protein levels of 31% in the CMV infection group and 33% in the ox-LDL treatment group, compared to the mock group. The combined effect of both factors led to a remarkable reduction of 53.2% compared to the control group, and a decrease of about 30% compared to the single factor (all p<0.0001). These findings indicate that both CMV and ox-LDL contribute to the reduction of SIRT1 in bEnd.3 cells, as well as the increase in IL-1β production.
Figure 3: Immunofluorescence staining and protein bands and quantified evaluation A. Representative microphotographs of immunofluorescence staining of SIRT1 (green) and the nuclei (DAPI, blue) in bEnd.3 cells (400×). B-G. Representative protein bands and quantified evaluation of SIRT1, acetylation and phosphorylation of p65 NF-κB, caspase-1, NLRP3 (n=3). *p<0.05, **p<0.01, *** p<0.001, and ****p<0.0001 compared with indicated group
Next, we examined the expression of acetylation (Ace-p65) and phosphorylation of p65 NF-κB (p-p65). CMV infection or ox-LDL induced an elevation of Ace-p65 and p-p65 proteins compared to the control group (Figure 3B, D and E). The levels of Ace-p65 induced by CMV infection were increased by 2.3 times, which was significantly higher than the control group (p<0.0001). Similarly, the levels of Ace-p65 induced by ox-LDL were increased by 2.6 times compared to the control group (p<0.0001). Notably, the levels of Ace-p65 induced by both CMV infection and ox-LDL were 2.8 times higher than the control group (p<0.0001). Furthermore, the levels of p-p65 proteins induced by CMV infection were increased by 2.8 times, while those induced by ox-LDL were increased by 3.6 times compared to the control group. Importantly, both CMV infection and ox-LDL treatment resulted in a 4.5-fold increase compared to the Mock group, and a 1.6~1.2-fold higher increase when compared to either single factor (p<0.0001) (Figure 3B, D and E).
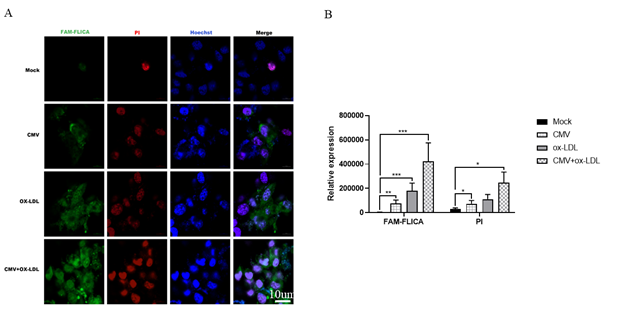
Figure 4: CMV infection or/and ox-LDL treatment induced caspase-1 activity by immunochemistry in bEnd.3 cells(n=3). (A) Representative microphotographs of immunochemistry staining of caspase-1 activity in bEnd.3 cells. (B) quantified evaluation of caspase-1 viability. *p<0.05, **p<0.01, *** p<0.001, and ****p<0.0001 compared with indicated group
CMV and/or ox-LDL augmented caspase-1 activity but not its expression
The decrease in cell viability and the increase IL-1β production may be attributed to caspase-1 and cell pyroptosis. Therefore, we used Western blot to detect caspase-1 and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) in bEnd.3 cells. Surprisingly, despite our expectations, we observed a decrease in the expression of caspase-1 proteins in bEnd.3 cells when using Western blot to detect caspase-1. However, we measured caspase-1 activity and found that CMV or ox-LDL significantly increased its activity. Moreover, the increase in activity was more pronounced when both factors were present compared to the Mock group (Figure 4). We also investigated NLRP3, an inflammasome sensor molecule upstream of caspase-1, and found that CMV or combined ox-LDL exposure could enhance NLRP3 protein expression as indicated by Western blot analysis (Figure 3 B and G). Our data revealed that the protein expression levels of NLRP3 increased 1.2 times in the CMV infection group compared to the normal control group (p=0.0009). Furthermore, the increase in NLRP3 induced by ox-LDL intervention was 1.7 times higher than the Mock group. Notably, when both factors were present, the expression of NLRP3 protein increased by approximately 2 times compared to the Mock group, surpassing the increase caused by any single-factor intervention. This suggests that CMV infection for 7 days and/or ox-LDL treatment for 48 hours induced an elevation in NLRP3 proteins and caspase-1 activity, but not caspase-1 protein expression.
The bEnd.3 cell activity and IL-1β levels induced by CMV or/and ox-LDL were corrected by the SIRT1 agonist SRT1720 or the NF-κB inhibitor helenalin.
Next, the effect of SRT1720 (SIRT1 agonist) and helenalin (NF-κB inhibitor) on cell activity and the expression of IL-1β levels in brain microvascular endothelial cells (bEnd.3 cells) was assessed. Interestingly, Helenalin intervention reversed the decrease in cell activity induced by CMV infection (p=0.00117) when compared to CMV infection alone. In addition, Helenalin intervention also corrected the cell activity in CMV-infected cells pretreated with ox-LDL (P =0.0032) compared to the CMV-ox-LDL group (Figure 2C). On the other hand, SRT1720 did not reduce cell activity (all P =0.2293) compared to the CMV group or the CMV-ox-LDL group, respectively (Figure 2C). These findings suggest that the inhibition of NF-κB activation can correct the mCMV-ox-LDL-induced decrease in cell activity, while SRT1720 cannot.
Furthermore, the effect of Helenalin and SRT1720 on the expression of IL-1β was investigated. It was found that Helenalin intervention reversed the increase in IL-1β induced by CMV infection (p=0.0005) compared to CMV infection alone. Helenalin intervention also reversed IL-1β levels in CMV-infected cells pretreated with ox-LDL compared to the CMV-ox-LDL group (P < 0.0001) (Figure 2D). In contrast, SRT1720 also reduced IL-1β levels compared to the CMV group (P=0.0006) or the CMV-ox-LDL group (P < 0.0001) (Figure 2D). These results indicate that the inhibition of NF-κB activation or simulation of SIRT1 can restore the mCMV-ox-LDL-induced decrease in IL-1β. Therefore, it can be speculated that CMV-induced reduction in SIRT1 or increase in NF-κB activation may further mediate the mCMV-ox-LDL-induced increase in IL-1β.
Discussion
CMV infection is one of the risk factors for cerebrovascular diseases. Our study revealed that CMV infection alone increases IL-1β protein in brain microvascular cells. Additionally, we found that CMV further enhances IL-1β protein in these cells when synergized with ox-LDL intervention. Furthermore, CMV or/and ox-LDL induce the reduction of SIRT1 protein and the activation of the NF-κB pathway. We also observed that both an NF-κB inhibitor and a SIRT1 agonist reduce the IL-1β production induced by CMV infection or/and ox-LDL intervention. However, only the NF-κB inhibitor, not the SIRT1 agonist, corrects the cell viability. This suggests that SIRT1/NF-κB mediates the production of IL-1β protein. Our findings suggest an increase in NLRP3 and caspase-1 activity, but not caspase-1 expression. This indicates that CMV or/and ox-LDL may induce NLRP3-mediated pyroptosis in brain endothelial cells.
CMV infection-induced microvascular dysfunction has been extensively studied and supported by ample evidence [12]. Furthermore, it has been observed that CMV infection worsens the response to hypercholesterolemia [4]. In our current study, we observed a decrease in the activity of bEnd.3 cells when exposed to CMV infection or/and ox-LDL treatment, indicating that CMV infection plays a significant role in the brain endothelial cell injury, either alone or in conjunction with ox-LDL. Additionally, previous research has shown that IL-1β is linked to brain endothelial cell injury and influences the permeability of the BBB[6] . In our study, we found a substantial increase in the IL-1β protein levels in the supernatant of brain microvascular cells treated with CMV infection for 7 days or/and ox-LDL for 48 hours. These findings offer insight into the mechanism by which CMV infection and/or ox-LDL contribute to brain endothelial dysfunction.
Compelling evidence indicates that CMV infection promotes oxidative stress and reduces nitric oxide bioavailability, which are responsible for the reduction of SIRT1 protein [13,14]. SIRT1 is known to control key metabolic functions by deacetylating target proteins [15], but it is downregulated in diabetic kidneys, resulting in high acetylation levels of p65 NF-κB[15]. Additionally, the phosphorylation levels of p65 NF-κB are significantly elevated in SIRT1 knockout mice-derived kidney tissue following lipopolysaccharide challenge [16,17]. These findings highlight the important association between SIRT1 and the acetylation as well as phosphorylation of p65 NF-κB. Consistent with these findings, CMV or/and ox-LDL induce the reduction of SIRT1 expression and increase the acetylation and phosphorylation of p65 NF-κB. Hence, a hypothesis arises that CMV or/and ox-LDL may downregulate SIRT1 protein and upregulate the NF-κB pathway to induce an increase in IL-1β secretion. As predicted, the administration of the specific SIRT1 agonist SRT1720 or NF-κB inhibitor helenalin corrects the IL-1β elevation mediated by CMV infection or/and ox-LDL treatment. This evidence indicates that the SIRT1/NF-κB pathway partially accounts for the CMV or/and ox-LDL-mediated production of IL-1β protein in brain endothelial cells.
The above evidence suggests a hypothesis that SIRT1 protein may rectify the activity of brain microvascular cells due to its protective role. However, it was surprising to find that the activity of cells could not be corrected by the SIRT1 agonist SRT1720. One possible explanation could be that cell damage may not be reversible. Nevertheless, it was observed that the NF-κB inhibitor helenalin was able to restore cell viability. This leads to the speculation of two potential reasons for this observation. Firstly, helenalin directly inhibits inflammatory signaling, whereas SIRT1 agonists act indirectly. Thus, the limited action time might not be sufficient to protect the cells from damage. Secondly, it is possible that CMV or/with ox-LDL activates the NF-κB pathway through mechanisms other than the SIRT1 protein, making the comprehensive role of helenalin in inhibiting inflammatory signaling more effective. In contrast, SIRT1 agonists may not be capable of protecting against cell damage caused by NF-κB pathway activation due to other pathways such as oxidative stress.
In the present study, we observed that bEnd3.cells displayed increased NLRP3 expression and caspase-1 activity, accompanied by a decrease in caspase-1 expression induced by CMV infection or/and ox-LDL treatment. These findings suggest that pyroptosis, a form of programmed cell death triggered by infections, could be a potential mechanism responsible for the decreased cell activity. Furthermore, we plan to investigate the role of inflammasomes, other pyroptosis-related proteins, and the caspase family in this model, including examining pro-caspase-1 and cleaved caspase-1, in order to elucidate the cause of caspase-1 decline.
Our article has the following limitations: Firstly, the relationship between SIRT1 protein and the NF-kB pathway in CMV-ox-LDL-bEnd.3 cells is not clear. It is important to note that a decrease in SIRT1 protein can activate the NF-kB pathway. Additionally, the activation of NF-kB can also be triggered by CMV or/ox-LDL induced oxidative stress. There is evidence of cross-talk between the SIRT1 protein and the NF-kB pathway [15]. To clarify this relationship, we plan to conduct a separate study specifically addressing this issue. Secondly, the pyroptosis-related pathway is not fully understood. Pyroptosis can be divided into the caspase-1-mediated classical pyroptosis pathway and various other caspase-mediated non-classical pyroptosis pathways. Therefore, we intend to discuss this topic in detail in a forthcoming article (currently in submission) due to the involvement of numerous molecules in this process. Lastly, there is a correlation between the SIRT1 protein and cell pyroptosis [18]. Our future research endeavors will aim to enhance our understanding in this area.
In conclusion, our findings demonstrate that both SRT1720, a SIRT1 agonist, and helenalin, a NF-κB inhibitor, have the ability to mitigate the heightened levels of IL-1β induced by CMV or/and ox-LDL in brain endothelial cells. Additionally, we observed that helenalin can effectively reverse the diminished cell activity caused by CMV and ox-LDL, whereas SRT1720 does not possess this restorative capability. Furthermore, the decline in cell viability may be linked to the occurrence of NLRP3-associated pyroptosis.
Funding:
This project was supported by the National Natural Science Foundation of China (Grant No. 81801384). Chongqing Science and Health Joint Medical Research Project (Grant No. 2023MSXM089).
References
- Gupta M SM. Cytomegalovirus. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls.
- Nikolich-Zugich J, Cicin-Sain L, Collins-McMillen D, et al. Advances in cytomegalovirus (CMV) biology and its relationship to health, diseases, and aging. Geroscience 42 (2020): 495-504.
- Carlson JR, Chang WLW, Zhou SS, et al. Rhesus brain microvascular endothelial cells are permissive for rhesus cytomegalovirus infection. J Gen Virol 86 (2005): 545-549.
- Khoretonenko MV, Leskov IL, Jennings SR, et al. Cytomegalovirus infection leads to microvascular dysfunction and exacerbates hypercholesterolemia-induced responses. Am J Pathol 177 (2010): 2134-2144.
- Zhu H, Hu S, Li Y, et al. Interleukins and Ischemic Stroke. Front Immunol 13 (2022): 828447.
- Versele R, Sevin E, Gosselet F, et al. TNF-alpha and IL-1beta Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-beta Peptide Efflux in a Human Blood-Brain Barrier Model. International journal of molecular sciences 23 (2022): 10235.
- Ministrini S, Puspitasari YM, Beer G, et al. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Frontiers in physiology 12 (2021): 733696.
- Yang Y, Liu Y, Wang Y, et al. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol 13 (2022): 831168.
- Blazek D and Peterlin BM. Tat-SIRT1 tango. Mol Cell. 29 (2008): 539-540.
- Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23 (2004): 2369-2380.
- Zhang S, Liu L, Wang R, et al. MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS One 8 (2013): e83620.
- Kawasaki H, Kosugi I, Meguro S, et al. Pathogenesis of developmental anomalies of the central nervous system induced by congenital cytomegalovirus infection. Pathology international 67 (2017): 72-82.
- Leskov IL, Whitsett J, Vasquez-Vivar J et al. NAD(P)H oxidase and eNOS play differential roles in cytomegalovirus infection-induced microvascular dysfunction. Free Radic Biol Med 51 (2011): 2300-2308.
- Alam F, Syed H, Amjad S, et al. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr Res Physiol 4 (2021): 119-124.
- Kauppinen A, Suuronen T, Ojala J, et al. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25 (2013): 1939-1948.
- Sun HJ, Xiong SP, Cao X, et al. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-kappaB and STAT3. Redox Biol 38 (2021): 101813.
- Gao R, Chen J, Hu Y, et al. Sirt1 deletion leads to enhanced inflammation and aggravates endotoxin-induced acute kidney injury. PLoS One 9 (2014): e98909.
- Zhang Y, Zhang H, Li S, et al. Metformin Alleviates LPS-Induced Acute Lung Injury by Regulating the SIRT1/NF-kappaB/NLRP3 Pathway and Inhibiting Endothelial Cell Pyroptosis. Front Pharmacol 13 (2022): 801337.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 