Congenital Infection by Sars Cov-2. About a Case
Drummond-Suinaga Tatiana1*, Benguigui Judith2, Ruiz Isabel2, Antequera Gloria2, Arias Yumaira2, Belmonte Magdalena2, Sparano Angelo3, Valery Francisco1
1Pediatric Infectologist, University Hospital of Caracas, Caracas, Venezuela
2Neonatologist, La Arboleda Polyclinic, Caracas, Venezuela
3Pediatric Cardiologist, La Arboleda Polyclinic, Caracas, Venezuela
*Corresponding Author: Drummond-Suinaga Tatiana, Infectious Medical Pediatric Service, University Hospital of Caracas, Venezuela: Orcid ID: https://orcid.org/0000-0002-5112-4738
Received: 15 June 2022; Accepted: 27 June 2022; Published: 30 August 2022
Article Information
Citation: SARS CoV2; Newborn; Congenital COVID-19; Vertical
TransmissionDrummond-Suinaga Tatiana, Benguigui Judith, Ruiz Isabel, Antequera Gloria, Arias Yumaira, Belmonte Magdalena, Sparano Angelo, Valery Francisco. Congenital Infection by Sars Cov-2. About a Case. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 390-396.
Abstract
SARS CoV-2 vertical transmission has been debated since the beginning of the epidemic. Clinical case: neonate born to a 24-yearold mother who was infected with SARS CoV-2 diagnosed by PCR is presented. The mother had respiratory deterioration; therefore, a segmental cesarean section was performed. A preterm male neonate at 36 weeks of gestation presented respiratory distress, admitted to the neonatal intensive care unit. A nasopharyngeal swab test was performed during the first 24 hours of life, resulting in a positive. During hospitalization, he presented clinical deterioration due to tachycardia, abdominal distension, and increased respiratory distress. Paraclinical exams reported anemia, leukopenia, thrombocytopenia, and increased D-dimer and ferritin levels. Therefore, he received immunoglobulin therapy for 5 days as well as supportive treatment. Conclusion: based on clinical and paraclinical evolution, congenital infection by SARS CoV-2 was concluded.
Keywords
SARS CoV2; Newborn; Congenital COVID-19; Vertical Transmission
SARS CoV2 articles; Newborn articles; Congenital COVID-19 articles; Vertical Transmission articles
Article Details
1. Introduction
Vertical transmission of an infectious pathogen is defined as the transmission of the microorganism from the mother to the fetus during the antepartum and intrapartum periods, or transmission to the newborn during the postpartum period through contact with body fluids during delivery, or by direct contact due to breastfeeding after childbirth. Although multiple infectious vectors are capable of vertical transmission, the possibility of vertical transmission of SARS CoV-2 from the infected mother to the fetus or newborn has been a point of debate [1]. When SARS CoV-2 was first detected, no evidence of vertical transmission from mother to fetus had been observed, however, it is not yet clear whether it occurs by transplacental or transcervical transmission or by environmental exposure. Once the representative authorization was obtained, this clinical case of a newborn diagnosed with SARS CoV-2 infection at the time of birth is presented.
2. Clinical Case
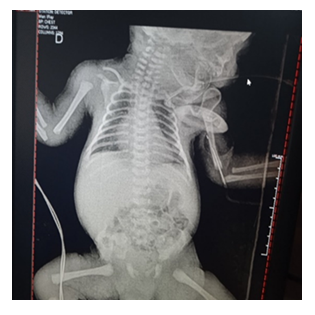
It is a male preterm newborn of 36 weeks of gestation, obtained from a 24-year-old mother, in her first gestation, with a controlled pregnancy which was complicated due to 1) Urinary infection in the 1st trimester and 3rd trimester of pregnancy (there were no urine cultures reports) 2) Pneumonia due to SARS CoV-2 (COVID-19) detected through a positive polymerase chain reaction (PCR) test for SARS CoV-2, that needed administration of lung maturation inducers. Maternal serology for HIV and VDRL were negative. Due to maternal respiratory deterioration, a segmental cesarean section under epidural anesthesia was performed at 36 weeks of gestation, showing clear amniotic fluid. The newborn had a cephalic presentation, cried and breathed spontaneously at birth, weighed 3080 grams, and height was 48 centimeters, the head circumference measured 34 centimeters, the chest circumference was 31 centimeters and the abdominal circumference was 29 centimeters. Apgar was 8-9 points in the first and fifth minutes. Several minutes after birth, he had respiratory distress evidenced by nasal flaring, and intercostal indrawing (2 points in Silverman Test). He had to be admitted to the neonatal intensive care unit with a diagnosis of high infectious risk for SARS CoV-2 and maternal urinary tract infection not bacteriologically corroborated. The newborn was admitted on an absolute diet, with parenteral hydration, antibiotic therapy based on ampicillin and amikacin (due to a history of maternal urinary tract infection), and oxygen supplied through a cephalic chamber. The performed laboratory tests were normal (Tables 1 and 2). Thorax X-ray shows bilateral marginal pneumomediastinum. (Figure 1). A sample for PCR SARS CoV-2 was taken by pharyngeal swab at 17 hours of life and was processed at the National Institute of Hygiene of Venezuela, resulting in a positive for SARS CoV-2 infection. It should be noted that the newborn had no contact with the mother before performing the nasal swab for the PCR (Figure 2).
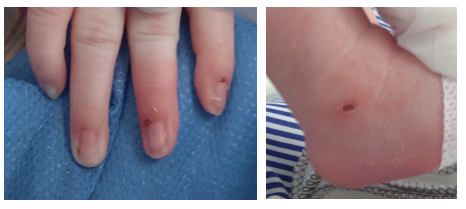
According to his respiratory signs, the patient remained stable. Oxygen was supplied through a cephalic chamber for 72 hours. Later, oxygen saturation remained between 96%-99% without supply. On the fourth day of life, he presented tachycardia, abdominal distention showing bloody gastric residual secretions, Paraclinical tests were performed showing a hematocrit and hemoglobin decrease, thrombocytopenia, hypoglycemia, elevated C-reactive protein, positive qualitative procalcitonin. Because of those findings, the patient required the administration of blood products (platelet and erythrocyte concentrate). A SARS CoV-2 positive PCR result was received. The results of the tests were LDH 210, Ferritin 306ng/ml (normal value up to 200 ng/ml), D Dimer 1796 ng/ml (normal value up to 500 ng/ml). Because of that, the newborn was thought to have a SARS CoV-2 infection. On the fifth day, clinical deterioration of the patient was seen due to exacerbation of the symptoms. Vasculitis-type lesions in the hands and feet (Figure 3) appeared, and a diagnosis of sepsis was associated. Antibiotic therapy was switched to vancomycin/meropenem, which was received for 10 days. Due to clinical and paraclinical deterioration of the patient, intravenous immunoglobulin treatment was started at a dose of 400mg/kg/day for 5 days. The newborn was then evaluated by a pediatric cardiologist on the seventh day of life. According to the specialist evaluation, the patient had:
- Myopericarditis with mild global pericardial effusion.
- Concentric and symmetric left ventricular hypertrophy without hemodynamic compromise.
- Patent ductus arteriosus with transient flow chart.
- Wide foramen oval of 7-8 mm and bidirectional shunt.
- Moderate pulmonary hypertension.
- Fetal pattern.
- Adequate biventricular systole-diastolic function.
After antimicrobials switching and intravenous immunoglobulin administration, the patient had a satisfactory evolution. On day 10, he was breastfed with no problems. At the age of 18 days, he was discharged after being diagnosed with a resolved SARS CoV-2 infection.
|
Days |
Hb |
Leuco |
Seg % |
Linf % |
Mono % |
Platel |
CPR mg/dl |
Procal ng/ml |
Glyc |
Creat |
|
1 |
12,5 |
10300 |
42 |
57 |
1 |
258000 |
0,2 |
47 |
0,77 |
|
|
4 |
10,7 |
7800 |
40 |
55 |
5 |
60000 |
7,8 |
|||
|
5 |
12,2 |
6400 |
40 |
48 |
8 |
2 |
0,35 |
|||
|
8 |
10,1 |
4500 |
37 |
49 |
11 |
34000 |
4,1 |
2 |
||
|
11 |
5700 |
51 |
42 |
4 |
34000 |
6,7 |
0,37 |
Hb: hemoglobin, Leuco: leukocytes, Seg: segmented, Linf: lymphocytes, Mono: monocytes, Platel: platelets, CRP: C Reactive Protein, Procal: procalcitonin, Glyc: glycemia, Creat: creatinine
Table 1: Paraclinical Exams 1.
|
Days |
D Dimer (ng/ ml) NV 500 |
LDH |
Ferritin ng/ml NV: 212 |
TB |
DB |
IB |
GOT UI/L |
GPT UI/L |
Urea mg/dl |
Albumin gr/dl |
|
1 |
||||||||||
|
2 |
7 |
0,84 |
7,16 |
|||||||
|
5 |
1796 |
210 |
306 |
23 |
12 |
4,1 |
2,6 |
|||
|
8 |
162 |
|||||||||
|
11 |
27 |
10 |
2,7 |
LDH: Lactate dehydrogenase, TB: Total bilirubin, DB: Direct bilirubin, BI: Indirect bilirubin, GOT: glutamate oxaloacetate transaminase, GPT: glutamate pyruvate transaminase
Table 2: Paraclinical Exams 2.
3. Discussion
Since December 2019, SARS CoV-2 infection has shown clinical and epidemiological characteristics of a rapid spread and the ability to infect the general population. SARS CoV-2 belongs to the Coronaviridae family. Its main route of transmission is through respiratory droplets, and it can also be transmitted by direct contact. Furthermore, current studies have suggested that SARS CoV-2 may also be transmitted by the fecal-oral route [2]. The possibility of SARS CoV-2 transmission from mother to fetus, causing SARS CoV-2 disease, is currently a highly debated concept in perinatal medicine. Evidence of intrauterine transmission from mother to fetus or intrapartum transmission from mother to newborn is rare [3]. A woman could become infected at any time during pregnancy and the impact on the fetus would vary depending on the time of infection. The infection occurring earlier in the pregnancy may be different than the one occurring two weeks before delivery. When maternal infection occurs during the first or second trimester, it has the potential to cause miscarriage, preterm birth, birth defects, or it may cause other congenital problems. Whereas if infection occurs late in gestation; the newborn may have an active infection and consequently; he is at risk of an adverse outcome [3].
During pregnancy, there are crucial periods in which there is a greater impact of SARS CoV-2 infection on fetal development. Fetuses are most vulnerable to maternal stress during the fifth and sixth months of pregnancy; so, infection during this period results in higher rates of preterm birth, low birth weight, and newborns with delayed growth according to their gestational age; than when exposure occurs during different months of pregnancy [4]. Piekos [4] et al conducted a study in the United States between March 2020 and July 2021 where they evaluated 73,666 pregnant women, 18,335 of whom had at least one SARS CoV-2 test during pregnancy. Finally, 882 pregnant women who were infected with SARS CoV-2 during their pregnancy (first trimester n=85; second trimester n=226; and third trimester n=571) were included and compared with 19,769 women who never tested positive for SARS CoV-2 and received at least one SARS Negative CoV-2 test during their pregnancy. It was observed that SARS CoV-2 infection is linked to a higher risk of premature birth (p<0 05) and fetal death (p<0 05), mainly explained by SARS CoV-2 infection in the first and second trimester. Gestational age at the time of SARS CoV-2 infection matched gestational age at delivery (p<0·01). People in this study had mild or moderate SARS CoV-2 infections. There was an increase in preterm births due to infection in all trimesters of pregnancy (all pregnancies p<0.05), but the most pronounced increase occurred after infection during the first trimester of pregnancy.
Pregnant patients with SARS CoV-2 infection had children with low birth weight (first-trimester p<0.05, second trimester p=0.07, third-trimester p<0.05, all pregnancies p<0.01); there were higher rates of small-for-gestational-age infants among those born from mothers who had a positive SARS CoV-2 test during pregnancy (p<0 05), with the most pronounced increase after infection in the third trimester [4]. The neonate of the present clinical case was 36 weeks of gestation preterm newborn, coinciding with Piekos´ findings. Other researchers have evaluated SARS CoV-2 infection effects during pregnancy, obtaining the same results. In a review by Sankaran et al [5] regarding cases in the United States, they found neonatal infection in 1% to 3% of children born from American mothers with COVID-19, with lower chances of infection if the mother had tested positive more than 14 days before delivery. Premature births (12.9%, compared to the national average of 10.2% in 2019), low birth weight, cesarean section, and NICU admissions were commonly seen among children born with COVID-19 infection. Villar et al. observed that maternal SARS CoV-2 infection increased the risk of preterm birth (RR, 1.59; 95% CI, 1.30-0.94) in a study conducted in the United Kingdom [6].
Congenital SARS CoV-2 infection is considered when the newborn and the mother have clinical characteristics which are compatible with COVID 19 infection. Those are classified as [2, 3, 7-10]
- Confirmed: Detection of the virus by PCR in umbilical cord blood, neonatal blood collected within the first 24 hours of birth, or amniotic fluid collected before the rupture of the membranes.
- Probable: Detection of the virus by PCR in nasopharyngeal swab at birth (collected after cleaning the baby), placental smear from the fetal side of the placenta in a newborn obtained by cesarean section before rupturing of the membrane or placental tissue.
- Possible: The virus is not detected by PCR in nasopharyngeal swabs at birth (collected after cleaning the baby) BUT anti-SARS CoV-2 IgM antibodies may be detected in umbilical cord blood or neonatal blood collected within the first 24 hours after birth or from placental tissue.
- Unlikely: The virus was not detected by PCR in nasopharyngeal swab at birth (collected after cleaning the baby) or umbilical cord blood, or neonatal blood collected within the first 24 hours after birth or amniotic fluid AND antibodies tests were not performed.
- Not infected: No virus detection by PCR in nasopharyngeal swab at birth (collected after cleaning the baby) or umbilical cord blood, or neonatal blood collected within the first 24 hours after birth or amniotic fluid AND no anti-SARS CoV-2 IgM was detected in umbilical cord blood or neonatal blood collected within the first 24 hours after birth [2, 3, 7-9].
Based on this classification, the present clinical case would be defined as a probable cause of congenital infection by the SARS CoV-2 virus, since the isolation by PCR of the virus by nasopharyngeal swab was obtained before 24 hours of life. Clinical manifestations in SARS CoV-2 infected neonates vary according to the days of life, being them mostly asymptomatic or presenting mild clinical symptoms [2, 5, 8, 11-14]. Symptoms will vary depending on days of life [2, 5, 8, 12-16]
The first week of life:
- Temperature: hypothermia, hyperthermia, normothermia
- Respiratory: signs of respiratory distress that may range from mild to severe.
- Cardiovascular: tachycardia, bradycardia, arterial hypotension, shock
- Food and gastrointestinal: hyperoxia, vomiting, abdominal distension, diarrhea.
- Others: lethargy, hypoactivity.
1 to 3 weeks of life:
- Rash, gastrointestinal symptoms, late neonatal sepsis, septic shock, and myocarditis.
- Respiratory distress or pneumonia, low birth weight, disseminated intravascular coagulation, asphyxia, and perinatal death have been seen as neonatal complications of SARS CoV-2 infection [17].
In a meta-analysis carried out by García [18] et al., including 72 studies, when evaluating the signs and symptoms associated with SARS COV-2 infection, it was observed that the primary clinical manifestation was fever in 43.2%, followed by respiratory signs and symptoms in 46.6% (including respiratory distress, tachypnea, and cough). Gastrointestinal manifestations were reported in 35.2% of the patients, including hyporexia and diarrhea. Neurological alterations were found in 23.7% of the neonates, mainly lethargy. Cutaneous in 6.8% and cardiovascular in 3.8% (including tachycardia, hypotension, acidosis, and/or delayed capillary refill time) were less frequently found. Neef et al. performed a meta-analysis including 32 studies and 261 newborns. Most of the newborns whose mothers were infected did not have clinical abnormalities (80.4%). In the group with symptoms; dyspnea was observed in 11 neonates (42.3%) and fever in 9 (19.1%) [11].
According to the severity of COVID-19, 142 (60.2%) were classified as mild to moderate, 63 as severe (26.7%), 4 as critical (1.7%), and 27 (11.4%) neonates as asymptomatic. Our patient had respiratory distress symptoms at birth but does not require mechanical ventilation. On the fourth day, abdominal distention, tachycardia, and subsequent deterioration were seen. Additionally, there was evidence of myopericarditis and vasculitis-type lesions in the limbs on the seventh day, so he had some of the several clinical manifestations found in that meta-analysis. Regarding laboratory findings, they may be nonspecific, most commonly showing normal or decreased leukocyte counts or decreased lymphocyte counts on early examination. Other findings may include mild thrombocytopenia and elevated levels of creatine kinase, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase [2]. In a study carried out in the United Kingdom by Gale et al., 66 neonates diagnosed with SARS CoV-2 were evaluated, showing that the most frequent biochemical and hematological abnormalities were elevated lactate (>2 mmol/L; 17 (55%) of 31 babies analyzed) and elevated C-reactive protein (>5 mg/L; 14 (29%) of 49 infants) levels. Five (9%) of the 54 evaluated infants had a low lymphocyte count (<1 × 109/L) [12]. In this clinical case, elevated C-reactive protein, thrombocytopenia, and anemia were found on the fourth day of life, and leukopenia after day 8. We also found a significant elevation of D-dimer and ferritin levels on the fifth day of life, which was not described in the neonates of the reviewed literature.
3.1 Radiographic findings in SARS CoV-2 infected newborn
- Chest radiological findings: chest x-ray or ultrasound may show pneumonia [2].
- Abdominal radiological findings: intestinal ileus like images may be found in the abdominal x-ray [2].
In Gale's study, 25 of the 66 neonates underwent chest radiography, of which 14 (56%) had abnormal findings. Ground-glass opacities images were seen in seven (28%) babies (of which four were premature) [12]. The radiological tests performed on our patient revealed a marginal pneumomediastinum.
3.2 Diagnosis of SARS CoV-2 infection
Molecular detection of SARS CoV-2 is considered the standard test for the diagnosis of COVID-19. The sample for performing the polymerase chain reaction test is obtained by nasopharyngeal swab through the nostril and/or pharynx. All newborns of mothers with suspected or confirmed COVID-19 infection should undergo a PCR diagnostic test on respiratory tract exudate (nasopharynx, oropharynx, nose) at 24 hours of life, regardless of the presence of symptoms both in the newborn and in the mother [10, 19]. A test is done too early maybe not be useful and results may be indeterminate. The best time to test the newborn is still unclear. A negative or indeterminate test should be repeated at 48 hours of life. Asymptomatic infants who may be discharged earlier than 48 hours after birth should be tested before discharge. The newborn may be discharged without the result of the test if it appears to be clinically stable [10]. SARS CoV-2 serological test looking for specific serum antibodies should not be used for diagnostic purposes and do not replace the identification of viral genetic material from the nasopharyngeal swab. Serological tests are useful to assess the progress of the infection in the patient and the community. These tests should not be indicated in the newborn as their interpretation may be very difficult. In fact, during the second and third trimesters of pregnancy, maternal immunoglobulin G (IgG) is transferred to the fetus through the placenta. The presence of IgG antibodies in the newborn at birth may simply reflect the transfer of maternal antibodies and does not allow the diagnosis of intrauterine infection [10]. Our patient was diagnosed by a PCR performed in the first 24 hours of life, resulting in a positive for SARS CoV-2.
To date, there is no definitive recommendation for the specific treatment of children with COVID-19. The approach to SARS CoV-2 infection depends on the patient's symptoms. If respiratory support is required, the newborn of a mother with suspected or confirmed SARS CoV-2 infection should be handled carefully, limiting the generation of aerosols during oxygen supply and ventilation strategies [10]. Garcia and colleagues [18] evaluated 236 neonates in a meta-analysis, and they found that almost half of the neonates (48.2%) were admitted to a neonatal intensive care unit (NICU), while 26.6% were admitted to the pediatric ward, and 6% to the neonatal ward. Twenty-four infants required invasive mechanical ventilation, 32 received noninvasive ventilation (nCPAP), and 61 received supplemental oxygen via nasal cannula. One hundred four (46%) neonates received empiric antibiotics and 45 (19.9%) received antiviral therapy (oseltamivir, acyclovir, remdesivir, lopinavir/ritonavir), hydroxychloroquine and azithromycin. The use of immunomodulators (intravenous immunoglobulin, corticosteroids, and alpha-interferon) was not frequent (5.3%). Only 54 (23.9%) newborns were breastfed [18]. In severe cases (clinical worsening, respiratory distress, sepsis, shock), intravenous remdesivir can be started at a loading dose of 5 mg/kg/day intravenously on the first day, and 2.5 mg/kg/day on the subsequent nine days [10, 20] Empirical antibiotic therapy with amikacin/ampicillin was started for our patient due to a history of maternal urinary tract infection, which was then switched to vancomycin/meropenem on day 5 due to the patient's clinical deterioration. Antivirals were not used and immunomodulators (intravenous immunoglobulin) were started once the clinical deterioration began and the SARS CoV-2 diagnosis was confirmed.
Regarding newborn feeding, García's meta-analysis [18] reported breastfeeding only in 23.9% of them. At the beginning of the pandemic, some guidelines recommended stopping breastfeeding for babies born to mothers with suspected or confirmed COVID-19. Currently, this recommendation has changed and all babies should be breastfed, as there is no evidence of infectious transmission from mother to child through breast milk. If the mother is confirmed as infected, she should wear a face mask during lactation and fulfill all the preventive measures such as hand hygiene. If the newborn also has a positive SARS CoV-2 PCR test, healthcare personnel should wear personal protective equipment. At home, the recommendations are similar when handling a patient with COVID-19 is necessary. Quin et al. carried out a study where they followed up 23 women in the puerperium who had been infected with SARS CoV-2 in the third trimester or had breastfed their newborns during the puerperium. They were followed up and no positive detection of SARS CoV-2 was found in the newborns. All breast milk samples were negative for SARS CoV-2 detection. The presence of IgM for SARS CoV-2 in breast milk correlated with the presence of IgM in maternal blood samples. SARS CoV-2 IgG results were negative in all breast milk samples. All infants were healthy at follow-up and antibody tests for SARS CoV-2 were negative [21]. Our patient was breastfed after the tenth day of life due to the clinical conditions at the beginning, which did not allow breastfeeding.
3.3 Mortality
The meta-analysis carried out by García et al. showed a mortality rate of 1.7% for SARS CoV-2 infected newborns. These deaths occurred in patients with underlying pathology. Down Syndrome and atrioventricular shunt in two of them and the other two were preterm infants [18].
4. Conclusion
We think it is a case related to an intrauterine transmission of SARS CoV-2. The nasopharyngeal swab performed on the mother at the time of delivery tested positive for SARS CoV-2 and the newborn also tested positive in a nasopharyngeal swab performed previous to the 24 hours of life without having had contact with the mother.
Funding
The authors have no funding.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Authors Contribution
DT: planning, patient follow-up, research, and writing of the final work
BJ, RI, AG, AY, BM: research, patient follow-up
VF: writing of the final work
SA: paraclinical studies and patient follow-up
References
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol 224 (2021): 35.e3-53.e3.
- Wang L, Shi Y, Xiao T, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med 8 (2020): 47-47.
- Shah P, Diambomba Y, Acharya G, et al. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obs Gynecol Scand (2020): 565-568.
- Piekos SN, Roper RT, Hwang YM, et al. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Heal 7500 (2022): 1-10.
- Sankaran D, Nakra N, Cheema R. Perinatal SARS-COV-2 Infection and neonatal COVID 19. Neoreviews 22 (2021): e284-e295.
- Villar J, Ariff S, Gunier RB, et al. Maternal and Neonatal Morbidity and Mortality among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 175 (2021): 817-826.
- World Health Organisation (WHO). Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2 (2021): 1-14.
- Macías Avilés HA. Manejo del neonato sospechoso e infectado de COVID-19 en la UCIN. Acta Pediatr Mex 41 (2020): 101.
- Blumberg DA, Underwood MA, Hedriana HL, et al. Vertical Transmission of SARS-CoV-2: What is the Optimal Definition? Am J Perinatol 37 (2020): 769-772.
- Auriti C, De Rose DU, Mondì V, et al. Neonatal sars-cov-2 infection: Practical tips. Pathogens 10 (2021).
- Neef V, Buxmann H, Rabenau HF, et al. Characterization of neonates born to mothers with SARS-CoV-2 infection: Review and meta-analysis. Pediatr Neonatol 62 (2021): 11-20.
- Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Heal 5 (2021): 113-121.
- Raschetti R, Vivanti AJ, Vauloup-Fellous C, et al. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 11 (2020).
- Ciapponi A, Bardach A, Comandé D, et al. COVID-19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS One (2021).
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 9 (2020): 51-60.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol 40 (2020): 987-996.
- Zimmermann P, Curtis N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr Infect Dis J 39 (2020): 469-477.
- García H, Allende-López A, Morales-Ruíz P, et al. COVID-19 in Neonates with Positive RT–PCR Test. Systematic Review. Arch Med Res (2022).
- Hernández M, Carvajal A, Rísquez A, et al. Consenso de la COVID-19 en el embarazo. Bol Venez Infectol 32 (2021): 1-26.
- Saikia B, Tang J, Robinson S, et al. Neonates with SARS-CoV-2 infection and pulmonary disease safely treated with remdesivir. Pediatr Infect Dis J 40 (2021): E194-E196.
- Luo QQ, Xia L, Yao DJ, et al. Breastfeeding in Mothers with COVID-19: Insights from Laboratory Tests and Follow-Up from Early Outbreak of the Pandemic in China. J Women’s Heal 30 (2021): 1546-1555.



 Impact Factor: * 4.8
Impact Factor: * 4.8 Acceptance Rate: 69.70%
Acceptance Rate: 69.70%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 