Correlation between Serum Testosterone Level and Erythrocytosis in Adolescent Males- A Cross-Sectional Study
Ariel Ben, Abu Arisha Rana*, Bechor Ariel Tal, Yana Moshe, Leibzon Maor, Ben-Acon Michael and Sharon Nechama
Department of Pediatrics, Laniado Hospital, Netanya, Israel
*Corresponding Author: Sharon Nechama, Department of Pediatrics, Laniado Hospital, Netanya, Israel
Received: 06 November 2022; Accepted: 14 November 2022; Published: 01 February 2023
Article Information
Citation:
Ariel Ben, Abu Arisha Rana, Bechor Ariel Tal, Yana Moshe, Leibzon Maor, Ben-Acon Michael and Sharon Nechama. Correlation between Serum Testosterone Level and Erythrocytosis in Adolescent Males- A Cross-Sectional Study. Journal of Pediatrics, Perinatology and Child Health 7 (2023): 01 - 05.
View / Download Pdf Share at FacebookAbstract
Background: Androgens and testosterone can induce erythrocytosis in several mechanisms. Adolescent males often present to primary clinics with an elevated Red Blood Cell values without Hemoglobin abnormalities. We investigated the correlation between serum testosterone levels and red blood cell values.
Methods: 91 adolescents who were referred to the Emergency department were included in the trial, for which a complete blood count and serum testosterone were obtained, along with assessing Tanner score. Patients were divided into two groups with high and low red blood cell count. Mann -Whitney U test was performed to compare Serum testosterone level and Tanner score between the groups. Pearson’s correlation coefficient was calculated to determine the correlation between red blood cell count and Testosterone levels.
Results: Testosterone levels was higher in the erythrocytosis group 14.05 (SD 5.53) vs non erythrocytosis group 11.94 (SD 7.38) although it did not meet statistical significance (p-value = 0.168). There is a weak positive correlation (Pearson’s R = 0.146) between testosterone and erythrocyte levels.
Conclusion: In healthy adolescent males, whose serum testosterone levels are within normal range we observed erythrocytosis in 22% of patients. Testosterone levels was positively correlated with the Red blood cell count.
Trial Registration: Trial was registrated in the Israeli ministery of health database – date 27/10/2020 – MOH_2020-10-27-009444
Article Details
Introduction
Polycythemia exists when red blood cell (RBC) count, hemoglobin level and total RBC volume all exceed the upper limits of normal. In post pubertal individuals, RBC mass greater than 25% above mean normal value or a hemoglobin level greater than 18.5 g/dl in males indicates absolute erythrocytosis [1,2].
Erythrocytosis is usually classified either as primary or secondary, which can be of either congenital or acquired. Primary erythrocytosis is a condition in which the erythropoietic compartment is expanded independently of extrinsic influences or responding inadequately to them due to a primary abnormality of the erythroid precursor cells within the bone marrow. So far, the only molecularly characterized congenital primary erythrocytosis is caused by erythropoietin receptor (EPOR) gene mutations (familial erythrocytosis type 1, ECYT1). The only known acquired form of a primary erythrocytosis occurs in polycythemia vera (PV), a clonal stem cell which primarily affect elderly patients [3]. Secondary polycythemia most often develops as a response to chronic hypoxemia, which triggers increased production of erythropoietin by the kidneys. The most common causes of secondary polycythemia include obstructive sleep apnea, obesity hypoventilation syndrome, obstructive pulmonary disease, asthma, heavy cigarette smoking and testosterone therapy [4].
Reference values for RBCs variables are lower in children in comparison with adults. Several studies which investigated hematologic parameters have been done in different populations, racial, ethnic and gender subgroups, even in different seasons [5-9]. Adolescents are a specific population whose normal levels are closer to adults then other pediatric population.
Clinical data suggests androgen replacement therapy, mainly testosterone can cause erythrocytosis among young man [10], and lack of testosterone causes anemia in older adults [11].
Androgens could stimulate erythropoiesis by several mechanisms. [12] Androgens can result in an increase in erythropoietin from renal tissue, by binding to cytoplasmic receptors, increasing RNA polymerase activity within a few hours. [13,14] Another mechanism is directly on bone marrow tissue – after entering the bone marrow, androgens are reduced to 17-keto-steroids, which then bind to the nucleus and increase the synthesis of mRNA. This generation of mRNA in uncommitted bone marrow cells results in their conversion from Epo-non-reponsive to Epo-responsive cells [15].
Testosterone is known to stimulate iron (Fe) incorporation into erythrocytes [16] and increasing the red blood cell glycolysis [17].
Teenage boys often get referred to the hematology clinic for a solitary finding of elevated Red Blood Cells levels, with or without an absolute increase in hemoglobin levels.
That notion made us suspect there is a correlation between high levels of serum testosterone and high levels of RBC’s, which was tested in this study.
Methods
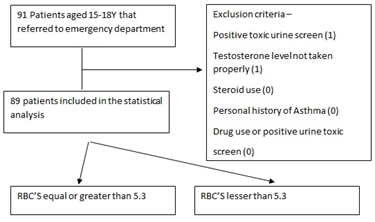
We conducted a Single center cross- sectional study on the population of pediatric patients aged 15y to 18y that referred to the Laniado Hospital Pediatric emergency department for various reasons. For each patient, after receiving informed consent from both parents, we obtained a Complete Blood Count, Serum Testosterone levels, and a urine sample for Toxicology. Each patient was asked about smoking habits, personal history of asthma, corticosteroid or drug use and ethnicity. Tanner score was assessed by the emergency department physician.
Patients with a positive toxic urine screen, sample disqualified by the lab or steroid use, and personal history of asthma were excluded from the trial.
Patients grouping:
Patients were assigned to two groups by Red Blood Cell count below 5.3 and equal or greater than 5.3. The latter defined as the Erythrocytosis group. RBC level cutoff was defined as 5.3 based on adolescent population studies [5,6].
Flowchart presenting exclusion criteria and patient grouping. For the exclusion criteria the number of patients excluded is presented within ().
Statistical Analysis
We performed a Two Way Mann-Whitney U test to compare the levels of testosterone between the Erythrocytosis and non-Erythrocytosis groups
We calculated the Pearson’s correlation coefficient to determine the correlation between RBC levels and testosterone levels.
|
Erythrocytosis (N=20) |
non- Erythrocytosis (N=69) |
P- value |
|
|
Mean Age (SD) |
16.62 (1.13) |
16.3 (0.94) |
0.261 |
|
Ethnicity |
|||
|
Sephardic jew |
5 |
39 |
0.011 |
|
Ashkenazi jew |
10 |
22 |
0.152 |
|
Ethiopian jew |
3 |
3 |
0.101 |
|
Other |
2 |
5 |
0.345 |
|
Smoker |
2 |
11 |
0.469 |
|
Table showing the demographic charasteristics of patients in both groups. For age mean and SD are shown. For each ethnicity and also for smoking the number of patients is presented. P-value was calculated using the Mann-Whitney U test |
|||
Table 1: Characteristics of Patients.
Results
Parameters of Testosterone levels, MCV, Hemoglobin and Tanner score were compared among the two groups.
There were higher levels of testosterone levels in the erythrocytosis group 14.05 (SD 5.53) vs non erythrocytosis group 11.94 (SD 7.38) although it did not meet statistical significance (p-value = 0.168). Hemoglobin levels were higher among erythrocytosis group as predicted with statistical significance (p-value = 0.001). MCV levels were slightly lower in erythrocytosis group (81.04 vs 84.13) with statistical significance (P-value =0.01). Tanner score was quite similar between the two groups with no statistical significance (P-value= 0.34).
|
Erythrocytosis (N=20) |
non- Erythrocytosis (N=69) |
P- Value |
|
|
Testosterone (nmol/L) |
14.05 (5.53) |
11.94 (7.38) |
0.168 |
|
MCV (μm3) |
81.04 (4.8) |
84.13 (4.0) |
0.01 |
|
Hemoglobin (g/dl) |
15.19 (1.19) |
13.89 (1.12) |
0.001 |
|
Tanner |
4.85 (0.34) |
4.57 (0.53) |
0.31 |
|
Table showing results in erythrocytosis versus non erythrocytosis groups. For each parameter mean (SD) is presented. P- Value was calculated by using the Mann-Whitney U test |
|||
Table 2: Results.
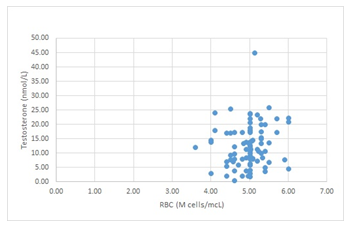
There is a weak positive correlation (Pearson’s R = 0.146) between testosterone and erythrocyte levels, scatterplot of RBC and Testosterone cases presented in Figure 2.
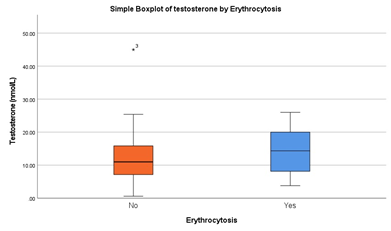
levels of testosterone by erythrocytosis and non-erythrocytosis subgroup presented in Boxplot in figure 3. Of all cases one individual in the non-erythrocytosis group exceeded normal age-adjusted levels of testosterone (45 nmol/L, normal level 10-35 nmol/L).
Conclusion
In this study we tried to figure out the correlation between high RBC’s and serum testosterone level at a given time. This study included a small cohort of adolescents who all except one had normal levels of serum testosterone. Hemoglobin and Hematocrit levels were also normal; hence no individual met the definition of polycythemia.
As demonstrated by several publishers there are several proposed mechanisms in which testosterone can induce erythrocytosis, including through increased erythropoietin secretion, inducing responsiveness of stem cells to erythropoietin and increase iron intake and glycolysis in the mature red blood cell.
Although testosterone levels were slightly higher among Erythrocytosis group no statistical significance was achieved. Pearson’s correlation coefficient showed a weak correlation between RBC’s and testosterone levels. Even though, this could show there is a basis to our theory that higher testosterone levels can induce erythrocytosis in healthy individuals with testosterone not exceeding normal levels.
Regarding the Tanner score there was no significant difference between the two groups, and all patients were ranked Tanner 4 or 5 which was age appropriate. This is predicted regarding the fact all except one individual had normal levels of testosterone and have all gone through normal puberty.
Further research including patients with high testosterone levels or who take external testosterone supplementation can be done to further investigate this theory.
Abbreviations:
RBC’s – Red Blood Cells
MCV- Mean Corpuscular Volume
EPOR – Erythropoietin Receptor
PV -Polycythemia Vera
ECYT1 -familial erythrocytosis type 1
Declarations
Ethics Approval and consent to participate:
Data collection and analysis was approved by the Institution’s Helsinki committee (request number -0133-19-LND).
Consent for Publication:
Written informed consent for the publication was obtained from both parents of each individual participating in the trial.
Availability of Data and Materials:
N/A
Competing Interests:
I declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Funding:
I declare no funding for the conduction of this trial.
Authors contributions:
B.A and R.A.A - main authors, writers and editors, prepared and created the manuscript.
T.B.A, M.B.A, M. L, M.Y - data curation and creators of database
N.S and B.A- conceptualization and formatting the main research idea, aims and goals.
B.A - statistical analysis, creation of figures and tables
Acknowledgements
This paper is part of resident’s duties.
Author’s information
For any questions about this research please address Ben Ariel by Email: benariel@gmail.com or Nechama Sharon by email: Nsharon@laniado.org.il
References
- Kliegman R. Nelson textbook of pediatrics (Edition 21.). Philadelphia, PA: Elsevier. Kaestner L, Bogdanova A. 2020, P: 2566.
- Cario H. Childhood polycythemias/erythrocytoses: classification, diagnosis, clinical presentation, and treatment. Annals of hematology, 2005; 84: 137-145.
- Cario H, Frances McMullin M, Bento C, Pospisilova D, Percy MJ, Hussein K, Hermouet S (2013). Erythrocytosis in children and adolescents-classification, characterization, and consensus recommendations for the diagnostic approach. Pediatric blood & cancer, 2013; 60: 1734-1738.
- Lee FS. Genetic causes of erythrocytosis and the oxygen-sensing pathway. Blood reviews, 2008; 22: 321-332.
- Kaestner L, Bogdanova A (Eds.). Regulation of red cell life-span, erythropoiesis, senescence and clearance. Frontiers E-books, 2014.
- Evans DM, Frazer IH, Martin NG. Genetic and environmental causes of variation in basal levels of blood cells. Twin Research and Human Genetics, 1999; 2: 250-257.
- El-Hazmi MA, Warsy AS. Normal reference values for hematological parameters, red cell indices, HB A2 and HB F from early childhood through adolescence in Saudis. Annals of Saudi Medicine, 2001; 21: 165-169.
- Taylor MRH, Holland CV, Spencer R, Jackson JF, O'connor GI, O'donnell JR. Haematological reference ranges for schoolchildren. Clinical & Laboratory Haematology, 1997; 19: 1-15.
- Gligoroska JP, Gontarev S, Dejanova B, Todorovska L, Stojmanova DS, Manchevska S. Red blood cell variables in children and adolescents regarding the age and sex. Iranian journal of public health, 2019; 48: 704.
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sexual Medicine Reviews, 2018; 6: 77-85.
- Roy CN, Snyder PJ, Stephens-Shields AJ, Artz AS, Bhasin S, Cohen HJ, Ellenberg SS. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA internal medicine, 2017; 177: 480-490.
- Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. Journal of endocrinological investigation, 2009; 32: 704-716.
- Nathan DG, Gardner FH, Kan AL. Effects of large doses of androgen on rodent erythropoiesis and body composition. Blood, 1965; 26: 411-420.
- Paulo LG, Fink GD, Roh BL, Fisher JW. Effects of several androgens and steroid metabolites on erythropoietin production in the isolated perfused dog kidney. Blood, 1974; 43: 39-47.
- Moriyama Y, Fisher JW. Effects of testosterone and erythropoietin on erythroid colony formation in human bone marrow cultures. Blood, 1975; 45: 665-670.
- Molinari PF, Rosenkrantz Erythropoietic activity and androgenic implications of 29 testosterone derivatives in orchiectomized rats. The Journal of Laboratory and Clinical Medicine, 1971; 78: 399-410.
- Valladares L, Minguell J. Characterization of a nuclear receptor for testosterone in rat bone marrow. Steroids, 1975; 25: 13-21.


 Impact Factor: * 4.8
Impact Factor: * 4.8 Acceptance Rate: 69.70%
Acceptance Rate: 69.70%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 