Diagnosis and Management of Genetic Derivation 22 and 11 Chromosome-Emanuel Syndrome in 10-Year Old Boy
Juna Musa1*, Edlira Horjeti2, Ali Guy3, Kristi Saliaj1, Diamant Shtiza4, Elton Ceka5, Dorina Musa6, Loran Rakovica7
1University of Medicine, Tirane, Albania
2Department of Family Medicine, University of Medicine, Tirane, Albania
3Physical Medicine and Rehabilitation, University School of Medicine, NYU, Medical Center, New York, USA
4University Hospital Center of Tirane, Albania
5Department of Radiology, Regional Hospital of Durres, Albania
6Obstetrics and Gynecology Department, Kettering General Hospital, UK
7University of Prishtina, Prishtina, Kosova
*Corresponding Author: Juna Musa, Medical Doctor, University of Medicine, Tirane, Albania
Received: 15 June 2020; Accepted: 26 June 2020; Published: 08 July 2020
Article Information
Citation:
Juna Musa, Edlira Horjeti, Ali Guy, Kristi Saliaj, Diamant Shtiza, Elton Ceka, Dorina Musa, Loran Rakovica. Diagnosis and Management of Genetic Derivation 22 and 11 Chromosome-Emanuel Syndrome in 10-Year Old Boy. Journal of Pediatrics, Perinatology and Child Health 4 (2020): 052-057.
View / Download Pdf Share at FacebookAbstract
Emanuel syndrome (ES) is a very rare chromosomal disorder, also known as Supernumerary derivative (22)t(11;22) Syndrome. Although the chromosomal disorder exhibits classic clinical features to the post-natal child, parents usually may be phenotypically normal. In most of the cases, a carrier parent has inherited the t(11;22) from one of his parents. Usually it presents a wide range of clinical presentation, such as growth delay, failure to thrive, congenital heart defects, renal abnormalities, craniofacial dysmorphism and microcephaly. ES as a complex disorder that requires a multidisciplinary team of different specialists aims for improving the quality of life and obtaining a stationary condition. Early detection and follow-up of the development of milestones in these patients leads to rapid improvement of their clinical and laboratory findings. We here present an interesting case of a 10 year old male with ES managed step by step complications in years.
Keywords
<p>Emanuel Syndrome, Genetic syndrome, 22 and 11 chromosome disorder, Supernumerary derivative (22)t(11;22) Syndrome</p>
Article Details
1. Introduction
Emanuel syndrome (ES) is a chromosomal disorder caused by the presence of a supernumerary derivative chromosome (der[22]) that contains genetic material from chromosomes 11 and 22 [1]. This condition affects the offspring of individuals who carry a balanced t(11,22) translocation, the most common, recurrent, non-Robertsonian, and constitutional translocation in humans [2]. The balanced carriers are phenotypically normal and usually unaware of the mutation until they either have problems with conception, repeated miscarriages or have a child with ES. The affected progeny unlike their parent are genotypic ally unbalanced because they carry the der(22) as a supernumerary chromosome [3] therefore manifesting the distinctive phenotype of the disorder. It is a rare genetic condition with little over 400 identified individuals, an unknown prevalence and complete penetrance in carriers of der(22) [4]. Current literature suggests that the predominant mechanism leading to an unbalanced translocation is a 3:1 meiosis I malsegregation in the balanced t(11,22) carrier. Genetic mosaicism and alternate segregation at meiosis I may explain reported cases that do not fall in this category, however the same studies support the hypothesis that 3:1 meiosis I malsegregation is the decisive mechanism because it is the most likely one to result in a viable pregnancy [5]. Other segregations possibly result in spontaneous abortions because of the chromosomal abnormalities. Patients diagnosed with ES present with a wide range of clinical manifestations. The most common manifestations includepre and post-natal growth deficiency, failure to thrive, microcephaly, central hypotonia, craniofacial dysmorphism (preauricular ear pits and/or tag, micrognathia, cleft or high-arched palate) congenital heart defects, renal malformations (hypoplasia or complete renal agenesis) and genital anomalies (cryptorchidism, small scrotum and micropenis) in males [6]. Less frequently associated clinical findings include diaphragmatic hernia, anal atresia, gastroesophageal reflux with poor weight gain, musculoskeletal and central nervous system malformations.
Psychomotor development is severely impaired in all afflicted individuals, however, more than 70% eventually learns to walk with support. Global developmental delay, profound intellectual disability and significant communication disorders are evident from infancy all throughout adulthood. After being clinically suspected, definitive diagnosis of ES is established by detection of a duplication of 22q10-22q11 and duplication of 11q23-qter through genetic testing. Methods include karyotype, chromosomal microarray (CMA), or targeted duplication analysis by fluorescence in situ hybridization (FISH). Genetic counseling is always offered and risks for possible future pregnancies are discussed [7].
2. Case Report
Ten-year-old male, presented with severe developmental delay, speech and language disability, reduced eye contact, hyperactivity, hand-flapping, flat feet, loose joints, deep hoarse voice.Moreover, on close inspection coarse features,normal palate, large ears, a prominent jaw and forehead, unusually flexible fingers, full lips, large rounded cheeks, a broad nose and macroglossia are evident. No presence of hepatosplenomegaly. During his hospital stay a complete blood count (CBC) and metabolic panel detected microcytic hypochromic anemia and an elevation of the BUN/Creatinineratio as a result of chronic renal failure. His past medical history included generalized tonic-clonic seizures that first started at the age of 36 months for which he receives pharmacological treatment with barbiturates.Based on his changing weight the dosing is optimized accordingly. Family history was not significant for any similar or inherited conditions. He is up to date with his immunizations.
Past medical history was positive for an atrial septal defect (ASD) and ostium secundum (OS). On auscultation a systolic murmur and fixed split of S2 were present. An ultrasound was performed, confirming the presence of a medium sized ASD and subsequent successful surgical repair was performed.
After the cardiac surgery as the patient’s health condition improved, genetic testing was performed. Del\Dup analyses identified a two large duplication, a 17.6-Mb duplication on chromosome chr11q23.3-q25 and a 1.8-MB duplication on chromosome 22q11.21. The genomic position of the 11q23.3-q25 duplication is 11:116691446-134257854 bp and the genomic position of the 22q11.21 duplication is 22:18642987-20456831. These represent an estimation of the genomic region that the duplications cover.
2.1 Genetic test results
|
Seq[GRCh37] dup(11)(q23.3q25) Chr8:g.116691446_134257854dup |
Seq[GRCh37] dup(22)(q11.242981) chr8:g,18642987_2045683dup |
Table 1: The patient has a 17.6-Mb duplication on chromosome 11q23.3-q25 and a 1.8-Mb duplication on chromosome 22q11.21. It is likely that these detected duplications would constitute a supernumerary derivate chromosome 22, der(22) and the observed duplications are classified as pathogenic. Thus, the findings suggest the diagnosis of Emanuel Syndrome. Sequence analysis did not detect any novel or rare variants that were considered deleterious.
Based on his clinical presentation, developmental milestone delay and intellectual disability, congenital malformations involving both cardiovascular and renal systems, his craniofacial dysmorphism and the results of the genetic test settled the diagnosis of ES or Supernumerary der(22) syndrome. It is likely, the patient inherited the unbalanced translocation from one of his parents who is a balanced carrier of a t(11:22)(q23:q11.2) and phenotypically normal.
Throughout the years he has been followed-up closely by specialized pediatricians, especially by the nephrologist in an attempt to keep his renal parameters within normal range limits at all times, through a few intermingling sessions of hemodialysis. One year ago, due to the aggravation of his clinical condition he has become a “revolving door” patient. During his frequent hospitalizations, he was supported by a multidisciplinary medical team with the aim of normalizing all altered parameters. As long as, the severity of his persisting symptoms (hypertension, vomiting, fatigue, fever, recurrent pneumonias, and toxic uremia) and the increased frequency of his seizures, an abdominal and head CT was performed.
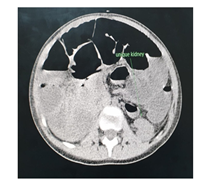
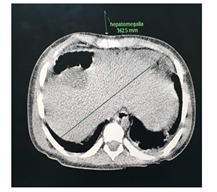
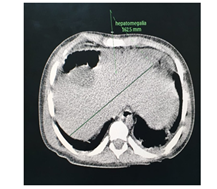
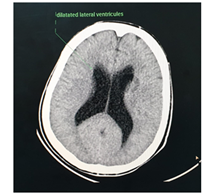
The abdominal CT (Figures 2-5) revealed a unique left kidney with normal parenchyma, enlarged pancreatic tail, hepatomegaly with a liver span up to 16 cm and dilatation of the intestinal ansa was present in all images. (Note accessory spleen). Furthermore the head CT (Figures 6-7) showed moderate hydrocephalus (Note cisterna magna present in both images 6 and 7). As a result of complexity of the condition the management was overseen by a multidisciplinary team of specialists with the main goal being improving the patient's quality of the life. The patient was started on peritoneal dialysis and immediate improvement was noticed. In addition to antihypertensive medications he was also prescribed a new scheme of anti-epileptic medications by the neurologist.
2.1 Treatment
Emanuel Syndrome as a permanent genetic disorder is untreatable despite the early settled of diagnosis. In addition, there are studies under investigation for different genetic diseases for any probable gene therapy or enzyme replacement, but still is not reached any concrete result. This hereditary condition has a potential importance of the right management, that requires a multidisciplinary approach, especially in early infancy when correction of life-threatening congenital malformations is paramount [8]. Treatment of clinical manifestations and the appropriate follow-up are personalized according to the extent of systemic involvement in each affected individual. In our case, actually patient is being treated chronically particularly by nephrologist due to renal insufficiency, infecionist and pneumologist as a result of immunity weakened system leading to chronic pulmonary infections. Physical, occupational, speech therapies and alternative communication methods are highly recommended to improve development outcomes as well.
3. Conclusion
Considering current literature and the well-established role of duplication of chromosome 11q23 and 22q10-22q11 as the main cause of Emanuel Syndrome, we classify the identified combination of seq[GRCh37] dup(11) (q23.3q25),chr8:g.116691446_134257854dup and seq [GRCh37] dup (22) (q11.21), chr8: g.186429987_2045683 dup as pathognomonic. Apart from abnormal genetic findings, an observation of infant growth and development milestone by pediatrician is important to recognize any deficiencies and failure to thrive. Moreover, it is crucial to underline regular visits of the parent regarding to their toddler, while paying attention to physical exam, cognitive development, sensory, motor skills, emotional and being up to date with vaccination as well. However, the underlying genetic disorder remains permanent, an early established diagnosis make things easier for an accurate observation or necessary intervention which can improve the quality of life and increase the longevity of the child.
3.1 Prenatal diagnosis
It is worth bringing to attention the significance of prenatal diagnosis, given that this condition has huge impact on patient’s and parents’ life. Suspicions regarding chromosomal abnormality may rise from antenatal screening in early pregnancy or from anomaly ultrasound scan at 18-20+6 weeks, where congenital anomalies can be detected. These are easily accessible even in developing countries. Whenever indications present, amniocentesis should be offered for karyotyping and based on its results full counselling to the couple can be done and options of management offered. It is important also that genetic counselling and cytogenetic testing be offered in future pregnancies as there is risk of Emanuel Syndrome recurrence or of occurrence of other meiotic malsegregations. If there are unaffected siblings counselling to them and carrier testing should be offered when childhood reached so that they will be able to process and understand the information given and make fully informed decision.
This case report highlights the importance of the rigorous management of infrequent clinical manifestations such as renal agenesis to ameliorate the patient’s quality of life, to prevent secondary complications and to improve life expectancy. Patient and his care givers should regularly be in touch with a clinical geneticist and a team of specialists in physical, occupational and speech therapy to help facilitate communication. Finally, we would like to emphasize the value of periodic follow-ups to evaluate current treatments, asses for possible complications and inform parents of new developments and recommendations.
References
- Carter MT, St. Pierre SA, Zackai EH, et al. Phenotypic delineation of Emanuel syndrome (supernumerary derivative 22 syndrome): Clinical features of 63 individuals. Am J Med Genet 149 (2009): 1712-1721.
- Shaikh TH, Budarf ML, Celle L, et al. Clustered 11q23 and 22q11 breakpoints and 3:1 meiotic malsegregation in multiple unrelated t(11;22) families. Am J Hum Genet 65 (1999): 1595-1607.
- Zackai EH, Emanuel BS, Optiz JM. Site-specific reciprocal translocation, t(11;22) (q23;q11), in several unrelated families with 3:1 meiotic disjunction. Am J Med Genet 7 (1980): 507-521.
- Emanuel BS, Zackai EH, Medne L. Emanuel Syndrome. In Eds.: Adam MP, Ardinger HH, Pagon RA, et al. Gene Reviews®. Seattle (WA): University of Washington, Seattle (2007): 1993-2020.
- Zackai EH, Emanuel BS, Optiz JM. Site-specific reciprocal translocation, t(11;22) (q23;q11), in several unrelated families with 3:1 meiotic disjunction. Am J Med Genet 7 (1980): 507-521.
- Martin RH. Analysis of human sperm chromosome complements from a male heterozygous for a reciprocal translocation t(11;22)(q23;q11). Clin Genet 25 (1984): 357-361.
- Emanuel syndrome. OMIM 609029 (2004).
- Genetics home reference. How are genetic conditions treated or managed?. NIH (2020).




 Impact Factor: * 4.8
Impact Factor: * 4.8 Acceptance Rate: 69.70%
Acceptance Rate: 69.70%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 