Early vs Delayed Feeding after Endoscopic Esophageal Variceal Ligation: A Systematic Review and Meta-Analysis
Mona Hassan1, Joyce Badal2, Syeda Faiza Arif3, Mohamad Nawras2, Ahmad Nawaz4, Muhammad Aziz1, Anita Kottapalli2, Wade Lee Smith5, Umer Farooq6, Manesh Kumar Gangwani7, Abdallah Kobeissy1, Zohaib Ahmed7
1 Department of Gastroenterology and Hepatology, University of Toledo, Toledo, OH.
2 University of Toledo College of Medicine and Life Sciences, Toledo, OH.
3 Dow Medical University, Karachi, Pakistan.
4 Yale New Haven Hospital, New Haven, CT.
5 University of Toledo Libraries, University of Toledo, Toledo, OH.
6 Rochester General Hospital, Rochester, NY.
7 Department of Internal Medicine, University of Toledo Medical Center, Toledo, OH.
*Corresponding Author: Zohaib Ahmed, MD, MPH, CNSC, Department of Internal Medicine, University of Toledo Medical Center, USA
Received: 01 April 2023; Accepted: 10 April 2023; Published: 24 April 2023
Article Information
Citation: Mona Hassan, Joyce Badal, Syeda Faiza Arif, Mohamad Nawras, Ahmad Nawaz, Muhammad Aziz, Anita Kottapalli, Wade Lee Smith, Umer Farooq, Manesh Kumar Gangwani, Abdallah Kobeissy, Zohaib Ahmed. Early vs delayed feeding after endoscopic esophageal variceal ligation: a systematic review and metaanalysis. Archives of Internal Medicine Research 6 (2023): 28-34
View / Download Pdf Share at FacebookAbstract
Endoscopic esophageal variceal ligation (EVL) is the recommended endoscopic modality to achieve hemostasis of actively bleeding esophageal varices and is also utilized for primary and secondary prophylaxis. Physicians may delay feeding up to 48-72 hours after EVL due to concerns of precipitating rebleeding. We conducted a systematic review of the literature using Embase, Medline, the Cochrane Central Register of Controlled Trials, and the Web of Science Core Collection to identify all studies that compared outcomes of early vs delayed feeding in patients after undergoing EVL. All analyses were conducted using RevMan metaanalysis software. Four RCTs, including 271 patients (average age 49.8 years and 82.9% male) were included in the final meta-analysis. The average Child-Pugh scores in the early and delayed feeding groups were 8.5 and 8.4 respectively, and the average MELD scores were 13.7 and 13.6 respectively. The early refeeding group had a significantly shorter length of hospital stay, by an estimated 1.59 days, compared to the delayed feeding group (95% CI: -2.06, -1.11, p<0.00001, I2=0%). There was no significant difference between the groups regarding post-EVL rebleeding rates (RR: 0.65, 95% CI: 0.22-1.91, p=0.44, I2=0%), development of ascites (RR: 0.58, 95% CI: 0.25-1.34, p=0.2, I2=0%), infection (RR: 0.59, 95% CI: 0.15-2.28, p=0.44, I2=47%), or overall mortality (RR: 0.55, 95% CI: 0.15-2.03, p=0.37, I2=0%). In conclusion, early feeding is safe and associated with a decreased length of hospital stay in patients after undergoing EVL for esophageal varices.
Keywords
<p>Esophageal Varices; Endoscopic Variceal Ligation; Early Feeding; Nutrition; Cirrhosis; Variceal Bleeding</p>
Article Details
Abbreviations
CI – Confidence Interval; EV – Esophageal Varices; EVL – Endoscopic Variceal Ligation; INR – International Normalized Ratio; MELD – Model For End-stage Liver Disease; MINORS – Methodological Index for Non-Randomized Studies; NPO – Nothing By Mouth; PT – Prothrombin Time; RR – Relative Risk; SD – Standard Deviation
1. Introduction
Esophageal varices occur as a complication of cirrhosis secondary to portal hypertension and variceal hemorrhage is a decompensating event associated with high mortality rates. [1] Endoscopic esophageal variceal ligation (EVL) is the recommended endoscopic modality to achieve hemostasis of actively bleeding esophageal varices, along with hemodynamic stabilization and medical management. EVL is also utilized for primary and secondary prophylaxis in cirrhotic patients with esophageal varices. [2] The most common complication following EVL is rebleeding, which in severe cases can lead to hemorrhagic shock and even death. [1,3,4]
Most patients who develop esophageal variceal hemorrhage are critically ill, with 6-week mortality rates of 15-25%. [3,5,6] Those with additional manifestations of decompensated liver cirrhosis such as ascites or hepatic encephalopathy have a 5-year mortality rate of 80%. [7] Patients with cirrhosis are also commonly sarcopenic and malnourished, making adequate nutrition imperative in their overall treatment. [8] Furthermore, withholding an oral diet may also lead to patient dissatisfaction due to hunger. [9] Early enteral nutrition may reduce the risk of mortality and pneumonia when compared to delayed enteral nutrition in critically ill patients. [10] However, the optimal time to feed patients presenting with esophageal variceal hemorrhage after EVL has been under investigation due to the risk of rebleeding. Theoretically, enteral feeding increases splanchnic blood flow, which could lead to increased portal pressures and increased risk of rebleeding, but the extent of this effect and its clinical relevance is uncertain.
Previous publications have suggested that clinicians wait 48-72 hours before feeding patients hospitalized with active variceal bleeding after endoscopic EVL. [11-13] Although early feeding after therapeutic endoscopy has previously been determined to be safe [14], some clinicians may still delay feeding after EVL in apprehension of rebleeding. Thus, we conducted a systematic review and meta-analysis to synthesize information from the currently available literature on patient outcomes after EVL in early vs delayed feeding groups.
2. Methods
2.1. Systematic Review
A comprehensive search strategy to identify reports of studies of effect of timing of food intake on postoperative variceal bleeding after endoscopic variceal ligation was constructed in Embase (Embase.com, Elsevier) by an experienced health sciences librarian (WLS) using truncated keywords, phrases, proximity searching and subject headings. This strategy was translated to MEDLINE (PubMed platform, National Center for Biotechnology Information, National Library of Medicine), Cochrane Central Register of Controlled Trials (CochraneLibrary.com, Wiley), and the Web of Science Core Collection (Web of Science platform, Clarivate) with all searches performed on 28 July 2022 (see Supplementary Information for detailed search strategies). No publication date or language limits were used. All results were exported to EndNote 20 citation management software (Clarivate, Philadelphia, PA, USA) and duplicates were removed by successive iterations of EndNote's duplicate detection algorithms and manual inspection.
2.2. Inclusion/Exclusion Criteria
We used the following parameters for study inclusion: (1) inclusion of patients undergoing EVL for bleeding esophageal varices, (2) studies that reported comparisons of clinical outcomes and adverse events between patients receiving early vs delayed feeding after undergoing EVL. Outcomes of interest included post-EVL length of hospital stay, bleeding, ascites, infection, and overall mortality.
2.3. Screening and Data Collection
The studies were screened by two independent reviewers (ZA and SFA). The initial screening was based on titles and abstracts, with the full-text screening of relevant publications following. Next, two independent reviewers extracted the data (ZA and JB). Discrepancy in study selection and data extraction was resolved through mutual discussion. Finally, data on demographics (age and male sex), definitions of early/delayed feeding, and outcomes (length of hospital stay, post-EVL bleeding, ascites, infection, and overall mortality) were collected and summarized using Microsoft Excel (Microsoft, Redmond, Washington, United States).
2.4. Data Synthesis and Statistical Analysis
All statistical analyses were conducted using RevMan meta-analysis software. Given the presumed heterogeneity in studies, a random-effects model was used as a priori to pool and compare outcomes. Risk ratios (RR) with 95% confidence intervals (CI) and p-values were determined for dichotomous outcomes. Mean difference (MD), 95% CI, and p-values were obtained for continuous outcomes. A p-value of ≤0.05 was considered statistically significant for all outcomes studied. An I2 test was used to evaluate the heterogeneity of the studies. An I2 of >50% was considered to represent significant heterogeneity.
2.5. Bias and Quality Assessment
The Cochrane risk-of-bias assessment tool was employed to assess the quality of the included randomized controlled trials (Table 2). [15] Two authors (ZA and UF) independently completed the assessment, and discordances were handled by a third reviewer (SFA). Publication bias assessment and sensitivity analysis were not performed due to the limited number of studies included in the final analysis.
3. Results
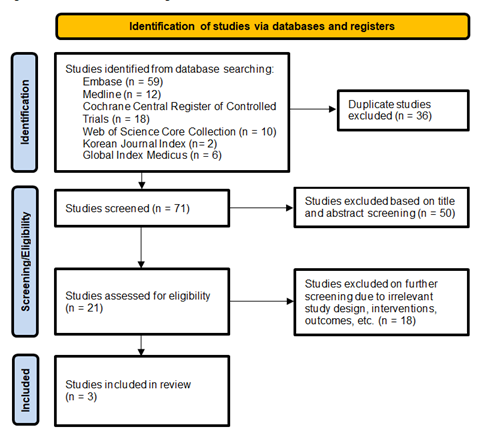
The initial search revealed a total of 107 studies. Three RCTs that included a total of 271 patients, met our inclusion criteria and were included in the final meta-analysis. [16-18] The average patient age was 49.8 years and 82.9% were male. The average Child-Pugh scores in the early and delayed feeding groups were 8.5 and 8.4 respectively, and the average MELD scores were 13.7 and 13.6 respectively. The PRISMA flow diagram in Figure 1 elaborates our systematic literature search process. Study characteristics, including patient demographics and definitions of early and delayed feeding, are reported in Table 1.
Table abbreviations: E – early feeding group, D – delayed feeding group, EV – esophageal varices, EVL – esophageal variceal ligation, MELD – Model for End-stage Liver Disease, PT – prothrombin time, INR – international normalized ratio, NPO – nothing by mouth, EN – enteral nutrition, NR – not reported, SD – standard deviation, *F1/F2/F3 (size and form of varices) – small (minimally elevated)/medium (enlarged and tortuous)/large (>33% of lumen)
Table 1: Study Characteristics
Table 2a: Quality Assessment via Cochrane Risk-of-Bias Tool
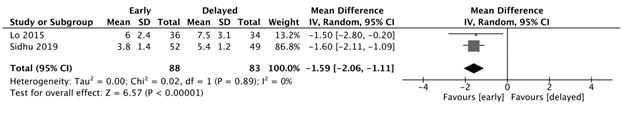
Length of hospital stay
The early feeding group had a significantly shorter length of hospital stay, with an estimated 1.59 fewer days, compared to the delayed feeding group (95% CI: -2.06, -1.11, p<0.00001, I2=0%). (Figure 2a)
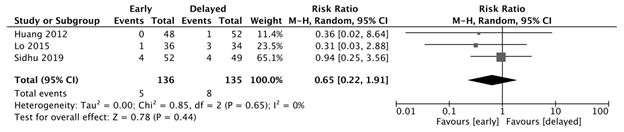
Post-EVL bleeding
There was no significant difference in post-EVL bleeding observed between the early and delayed feeding groups (RR: 0.65, 95% CI: 0.22-1.91, p=0.44, I2=0%). (Figure 2b)
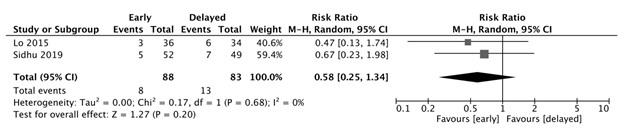
Ascites
There was no significant difference in post-EVL development of ascites between the early and delayed feeding groups (RR: 0.58, 95% CI: 0.25-1.34, p=0.2, I2=0%). (Figure 2c)
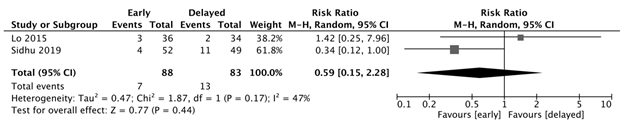
Infection
There was no significant difference in the development of infection between the early and delayed feeding groups (RR: 0.59, 95% CI: 0.15-2.28, p=0.44, I2=47%). (Figure 2d)
Overall mortality
There was no significant difference in overall mortality between the early and delayed feeding groups (RR: 0.55, 95% CI: 0.15-2.03, p=0.37, I2=0%). (Figure 2e)
Figure 2e: Overall mortality. There was no significant difference in overall mortality between the early and delayed feeding groups (RR: 0.55, 95% CI: 0.15-2.03, p=0.37, I2=0%).
4. Discussion
To our knowledge, our study is the first comprehensive systematic review and meta-analysis performed specifically looking at outcomes of early vs delayed feeding in patients after post-EVL for esophageal varices. In this study, patients in the early feeding group spent an estimated 1.489 fewer days in the hospital than those in the delayed feeding group (95% CI: -2.166, -0.812, p<0.01, I2=16.64%). We found no significant difference between rates of adverse events including post-EVL bleeding, blood transfusion requirements, development of ascites, infection, and overall mortality, between the early and delayed feeding groups. Our findings mirror the findings of Kan et al., who reported in their 2022 meta-analysis that early feeding is safe and associated with a decreased length of hospital stay in patients after undergoing therapeutic endoscopic procedures. [14] Their team studied a broader patient population than us, including patients undergoing endoscopic sclerotherapy for upper gastrointestinal bleeding, sclerotherapy/banding/ligation for bleeding esophageal varices, and endoscopic submucosal dissection for gastric neoplasms.
We found that early feeding post-EVL was associated with a decreased length of hospital stay, although differences in other outcomes including post-EVL bleeding and mortality did not reach statistical significance. A potential explanation could be that delayed feeding delays the presentation of the complication, but does not prevent its occurrence. For patients who develop rebleeding or other complications with feeding, an early feeding timeline would allow for earlier identification, intervention, and resolution of the complication compared to delayed feeding, which lead to decreased length of hospital stay. It is also possible that the time difference between the early feeding and delayed feeding groups included in our study was not long enough to allow for enough healing to reduce the risk of rebleeding. The delayed feeding groups included in our study resumed a diet between 1 and 4 days, but the process of strangulation, sloughing, and ulcer formation usually takes 4-10 days, while complete healing can take up to 14-21 days. [19] No significant difference in the rate of rebleeding was found in our meta-analysis.
Our meta-analysis found no difference in the development of ascites after EVL between the early and delayed feeding groups. While previous studies have reported significant increases in the incidence and severity of portal hypertensive ascites after EVL, [20, 21] whether EVL leads to an increase in portal pressures that leads to the development of ascites is unknown. Current AASLD guidelines support the use of splanchnic vasoconstrictors, such as somatostatin and its analogues, in patients with acute variceal hemorrhage. [2] These vasoactive agents are typically given for 2-5 days, which could mitigate the increase in splanchnic blood flow caused by early feeding. It is also possible that early nutrition increases serum protein and oncotic pressure enough to balance the hydrostatic pressure behind ascites development. [22] In addition, the AASLD guidelines recommend antibiotic prophylaxis in acute variceal hemorrhage, usually with ceftriaxone for 5-7 days, [2] which could affect the lack of significant difference in rates of infection after EVL between the early and delayed feeding groups.
This meta-analysis has the following strengths: performance of a systematic literature search with inclusion of all RCTs that met well-defined inclusion criteria, careful exclusion of redundant studies, and low levels of heterogeneity in our study. However, our meta-analysis had several limitations. First, the sample size was small, due to the limited data available in published literature. Second, the studies in our meta-analysis defined early and delayed feeding differently (Table 1) in terms of timing, which could have impacted our results. Third, we were unable to perform a subgroup analysis based on severity of esophageal varices due to lack of specific data available. Two of the three included studies reported the severity of esophageal varices in their patients, with the majority classified as size F2 varices (medium and tortuous). (Table 1) However, data outcomes in patients with F2 and F3 varices (large and occupying >33% of the esophageal lumen) were not reported. [16,18] Fourth, our study is susceptible to selection bias, which is inherent to meta-analysis, although every effort was made to conduct a comprehensive review of the literature.
In conclusion, early feeding is safe and associated with a decreased length of hospital stay in patients after undergoing EVL for esophageal varices. However, larger randomized controlled trials are needed to better evaluate the risks and benefits of early enteral feeding after EVL, as well as in specific patient populations, such as those with large F3 varices.
Declaration
The authors declare no conflicts of interest. No human subjects or animals were involved in this systematic review and meta-analysis. No funding was received for the preparation of this manuscript.
References
- Augustin, S., et al., Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol, 2009. 7(12): p. 1347-54.
- Garcia-Tsao, G., et al., Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology, 2017. 65(1): p. 310-335.
- Amitrano, L., et al., The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol, 2012. 107(12): p. 1872-8.
- Augustin, S., et al., Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol, 2011. 106(10): p. 1787-95.
- Reverter, E., et al., A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology, 2014. 146(2): p. 412-19 e3.
- Fortune, B.E., et al., Child-Turcotte-Pugh Class is Best at Stratifying Risk in Variceal Hemorrhage: Analysis of a US Multicenter Prospective Study. J Clin Gastroenterol, 2017. 51(5): p. 446-453.
- D'Amico, G., et al., Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther, 2014. 39(10): p. 1180-93.
- Lai, J.C., et al., Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology, 2021. 74(3): p. 1611-1644.
- Naithani, S., et al., Hospital inpatients' experiences of access to food: a qualitative interview and observational study. Health Expect, 2008. 11(3): p. 294-303.
- Tian, F., et al., Early Enteral Nutrition Provided Within 24 Hours of ICU Admission: A Meta-Analysis of Randomized Controlled Trials. Crit Care Med, 2018. 46(7): p. 1049-1056.
- European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol, 2019. 70(1): p. 172-193.
- McClave, S.A. and W.K. Chang, When to feed the patient with gastrointestinal bleeding. Nutr Clin Pract, 2005. 20(5): p. 544-50.
- Hebuterne, X. and G. Vanbiervliet, Feeding the patients with upper gastrointestinal bleeding. Curr Opin Clin Nutr Metab Care, 2011. 14(2): p. 197-201.
- Kan, S.W., et al., Early versus delayed feeding after therapeutic endoscopic procedures: Meta-analysis of randomized controlled trials. Dig Endosc, 2022. 34(3): p. 451-458.
- Higgins, J.P., et al., The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ, 2011. 343: p. d5928.
- Lo, G.H., C.W. Lin, and Y.C. Hsu, A controlled trial of early versus delayed feeding following ligation in the control of acute esophageal variceal bleeding. J Chin Med Assoc, 2015. 78(11): p. 642-7.
- Huang, S.Y. and H.Y. Wang, Post esophageal variceal ligation feeding time survey (abstract). Hepatol Int, 2012. 6:67–309: p. PP37-022.
- Sidhu, S.S., et al., Early feeding after esophageal variceal band ligation in cirrhotics is safe: Randomized controlled trial. Dig Endosc, 2019. 31(6): p. 646-652.
- Van Stiegmann, G. and J.S. Goff, Endoscopic esophageal varix ligation: preliminary clinical experience. Gastrointest Endosc, 1988. 34(2): p. 113-7.
- Yuksel, O., et al., Effects of esophageal varice eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci, 2006. 51(1): p. 27-30.
- Elwakil, R., A.M. Al Breedy, and H.H. Gabal, Effect of endoscopic variceal obliteration by band ligation on portal hypertensive gastro-duodenopathy: endoscopic and pathological study. Hepatol Int, 2016. 10(6): p. 965-973.
- Charlin de, G.V., et al., [Changes in protein repletion markers in undernourished patients receiving enteral nutrition and its relation with energy and nitrogen balance]. Rev Med Chil, 1995. 123(9): p. 1091-7.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 