Ex vivo Microsphiltration Profile of Plasmodium Falciparum Infected Red Blood Cells in Patients with Malaria in Kéniéroba, Mali: Exploring the Spleen Retention Function
Bourama Keita*, Agnes Guindo, Drissa Konaté, Karim Traoré, Saidou Balam, Bakaina Diarra, Sory Ibrahim Diawara, Ibrahim Sanogo, Modibo Sangaré, Mahamadou Diakité, Seidina Diakité
ICER-Mali, University of Sciences, Technics and Technologies of Bamako, Bamako, Mali
*Corresponding author: Bourama Keita, ICER-Mali, University of Sciences, Technics and Technologies of Bamako, Bamako, Mali
Received: 15 July 2022; Accepted: 29 July 2022; Published: 30 August 2022
Article Information
Citation: Bourama Keita, Agnes Guindo, Drissa Konaté, Karim Traoré, Saidou Balam, Bakaina Diarra, Sory Ibrahim Diawara, Ibrahim Sanogo, Modibo Sangaré, Mahamadou Diakité, Seidina Diakité. Ex vivo Microsphiltration Profile of Plasmodium Falciparum Infected Red Blood Cells in Patients with Malaria in Kéniéroba, Mali: Exploring the Spleen Retention Function. Archives of Internal Medicine Research 5 (2022): 431-435.
View / Download Pdf Share at FacebookAbstract
P. falciparum- Malaria pathophysiology is still not fully understood. The main mechanisms of the malaria physiopathology involve interactions between host and parasite. Although, the role of the spleen has been mentioned in various clinical forms, supportive clinical evidence is still needed. We conducted a pilot study to determine the impact of the spleen functional statuse in different clinical forms of malaria. Ex vivo microsphiltration was utilized to assess the splenic function in patients received during routine consultation with mild malaria at the Kéniéroba health center, a high malaria endemic area in Mali. A total of 25 patients were enrolled for ex vivo microsphiltration. The spleen was non-palpable (Hackett stage 0) in two patients, palpable with deep inspiration (Hackett stage 1) in 22 patients and without deep inspiration (Hackett stage 2) in one patient. The parasitaemia ranged from 5360 -to 342720 trophozoites/μl with a mean parasitemia of 50774 trophozoites/μl ± 65540 trophozoites/μl. The mean hemoglobin level was 11.2g/dl ± 1.2 [8.7-13.4]. The retention rate of the infected red blood cell ranged from 11.11% to 94.44% with an average of 65.4% ± 23.7%. A higher ex vivo retention rate of infected red blood cells was observed in patients with palpable spleen (p= 0.03). This pilot study proved the feasibility of exploring the spleen filtering function in malaria patients using the ex vivo microsphiltration exploreof the veinous collected red blood cell.
Keywords
Microsphiltration; Malaria; Plasmodium Falciparum; Splenic retention
Article Details
Abbreviations:
MRTC: Malaria Research and Training Center; PBS: Phosphate Buffer Saline; Pf: Plasmodium falciparum; RBCs: Red Blood Cells; RPMI: Roswell Park Memorial Institute Medium; RR: Retention Rate; USTTB: University of Sciences, Technics and Technologies of Bamako; WACCBIP: West African Centre of Cell Biology of Infectious Pathogens
1. Introduction
Malaria is the most important tropical parasitic disease with 241 million cases and 627,000 deaths in 2020 worldwide [1]. Africa bore in 2020 a disproportionate share of the global malaria burden with 95% morbidity and 96% mortality rates [1]. Despite of the ongoing basic and experimental research on malaria, the malaria pathophysiology is not yet fully understood due to its complex and multifactorial mechanisms. The main mechanisms involve synergistic host-parasite interactions [2]. The high frequency of splenomegaly in malaria endemic areas, the occurrence of splenic ruptures during or immediately after malaria disease, the more frequently marked severity of the first episodes of malaria in splenectomized patients are suggestive of a central role of the spleen in the malaria pathogenesis [3]. Pathogenic stages of P. falciparum develop in red blood cells (RBCs) and modify their physical properties [4, 5]. Understandably, the spleen, responsible of controlling the RBCs deformability, influences the fate of infected RBCs. In fact, clinical manifestations of P. falciparum infection result from the development of asexual stages within the RBCs [6]. Splenic microcirculatory beds filter altered RBCs, so the spleen can naturally eliminate subpopulations of infected or uninfected RBCs modified during P. falciparum malaria [7]. The spleen seems to be more protective against severe manifestations of malaria in naive patients [8]. It is involved in parasite clearance after certain antimalarial treatments, including artemisinin derivatives (artesunate and dihydroartemisinin) [9]. The loss of RBCs during malaria contributes to malaria anemia, a clinical form associated with a subacute progression, frequent splenomegaly and a chronic subclinical parasitaemia. This loss of RBCs results from the splenic clearance of either newly infected RBCs or uninfected, but parasite-modified RBCs [10].
However, the strengthen filtaring function of the spleen seems to be associated with a reduced risk of serious complications with and high parasitic loads, such as in cerebral malaria [11]. These hypotheses remain speculative despite their relevance. Exploring the role of the spleen in the pathophysiology of malaria remains overly complex due to the in vivo experimental challenges. Many technics have been described to explore the filtering function of the spleen. Microsphiltration is an experimental technic, which mimics the mechanical retention of particles with little deformation in the human spleen [12, 13]. This technic can be used either in vitro (plasmodium in culture) or, ex vivo (parasites harvested directly from patients). The ex vivo microsphiltration of RBCs from patients with malaria could be utilized to explore the splenic retention capability. Indeed, RBCs from malaria patients with have previously undergone splenic filtration in vivo could therefore provide informations on the splenic filtering functionality of the patient. Thus, the patient RBCs deformability, depending on the spleen filtering function will determine their ex vivo, microsphiltration retention rate. Such the ex vivo microsphiltration retention rate may reflect the ability of the spleen in vivo to filter out the least deformed RBCs. Here, we explored the ex vivo microsphiltration as tools to assess the splenic filtering function in patients with different clinical forms of malaria (supplementary Figure 1).
2. Methods
The patients were enrolled at the health center in the village of Kenioroba located along the Niger river in the Guinean-type forest savannah in Mali. In Kenioroba, malaria is endemic all year long with a stable and intense malaria transmission season (May to January).
We conducted a prospective cross-sectional study during the 2018 transmission season.
2.3 Study population and sampling
The study population consisted of all patients aged
six (6) months or older and who presented to health centers for malaria or signs of malaria. The sampling was exhaustive involving all the patients who presented themselves to the health centers during the study period, who agreed to participate in the study and from whom we could collect a venous blood sample for laboratory tests. Blood collection was performed in patients those had more than 8 dg/l hemoglobin rate, more than 5000 P.f trophozoites/μl parasitemia only when the laboratory was ready to perform microsphiltration. Data from samples with 5% parasitemia were excluded from the analysis to avoid the confounding effects due to the filter saturation.
Splenic retention of P. falciparum infected-RBCs in malaria patients has been studied using previous described tip microsphiltration method [14]. Briefely, calibrated metal microbeads composed of 96.5% tin, 3.0% silver and 0.5% copper of different diameters were used to make layers in tips of very narrow spaces to imitate the inter-endothelial clefts of the micro-veins of the red pulp of the human spleen (sinus). An equal-weight mixture (1 g) of microsphere powder (5-15 μm in diameter and 15-25 μm in diameter) was suspended in 5 mL 1% PBS/ AlbuMAX I solution (life technologies Cat#11020-021). A total of 800 μL of this suspension of microspheres were poured into an inverted anti-aerosol pipette tip of 1000 μL (Neptune, BarrierTips) and left to stand, leading to the formation of a layer of microspheres 5 mm thick at - above the aerosol filter. The microspheres were obtained from the Spherical Powder industry (24A, rue de la Résistance-BP 438, Annemasse 74108, France). P. falciparum-infected RBCs were collected from malaria patient and immediately kept at 4°C in a cooler until the sample are ready for microsphiltration (4 -5 hours). This procedure stop the parasite growth between the blood collection and the microsphiltration. A total of 600 μL of RBCs suspension (patientsRBCs washed with RPMI and suspended at 1% hematocrit in PBS/AlbuMAX II 1%) were introduced instantly upstream of the layer of microspheres and entrained through the layer of microspheres with 6 ml of PBS / AlbuMAX II 1% using an electric pump (Syramed μsp6000, Arcomed'Ag). The filtration rate was 60 ml / H. A downstream sample (6.6 mL) and an aliquot of the upstream sample were collected to determine parasitemia. Each sample was filtered in duplicate. The mean parasitaemias (% of infected RBCs in the RBC suspension) in the upstream (PAm) and downstream (Pav) samples were determined. The RBC retention rate (RR) for each sample was calculated using the following formula: TR = [(PAm - PAv) / PAm] × 100 [14]. The cells were counted using an Accuri C6 flow cytometer (Becton - Dickinson) after labeling with Syto 61.
We used descriptive statistics to summarize the data. Quantitative variables were represented as means and standard deviations and categorical variables as frequencies. We used t-tests to assess statistical differences between the means. The box and whisker plot were used to visualize the distribution of data by quartiles. Simple linear regression was applied to determine the correlation between rates of spleen retention and parasitaemia, hemoglobin levels and age. For each variable, missing data was defined as no record of cases and unknown data, in this case the variable was not tested. A difference was considered significant at p <0.05. All reported p values were bilateral. Statistical analyzes were performed on the complete data using statat version 14 software, the figures using prism version 8 software.
3. Results
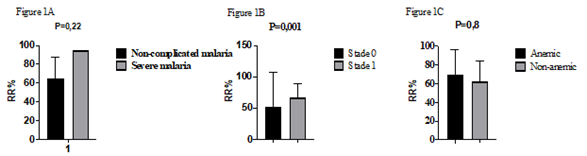
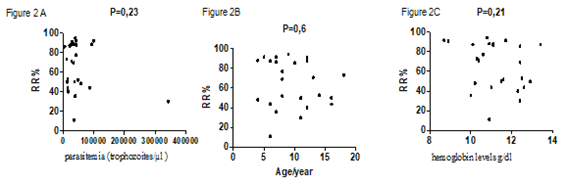
A total 25 blood samples from P. f-malaria patients were analyzed by microsphiltration. The sex ratio was 1.08. Only 4% (1/25) presented a severe malaria with prostration and vomiting. Two patients (8%) of patients had a no palpable spleen (hackette stage 0), 22 patients (88%) had a palpable deep inspiration spleen (stage 1 of Hackett) and one patient (4%) had a palpable spleen (Hackett stage 2) (Table 1). Patients were 9.68 ± 3.881 years old on average with the extremes of 4 and 18 years old. Parasitemia ranged from 5,360 to 342,720 trophozoites/µl (mean: 50,774.40 ± 65,540,854 ), the mean hemoglobin level was 11.23 ± 1.20 g/dl with the extremes of 8.70 and 13.40 g/dl. Retention rates ranged from 11.11% to 94.44% with a mean of 65.40 ± 23.77% (Table 2). We did not find significant difference between the average retention rates according to the clinical phenotype of malaria (p = 0.2) (Figure 1A) and the anemia status (p = 0.8) (Figure 1C) . No correlation was observed between retention rates and parasitemia (p = 0.23) (Figure 2A), the age (p = 0.6) (Figure 2B) or hemoglobin levels (p = 0.21) (Figure 2C). However, we observed a statistically significant difference between the average rate of splenic retention of RBCs infected and the Hackett stages (P = 0.001) (Figure 1B).
|
Hackett stages |
Frequency |
Percentage |
|
Stade 0 |
2 |
8.0 |
|
Stade 1 |
22 |
88.0 |
|
Stade 2 |
1 |
4.0 |
|
Total |
25 |
100.0 |
|
Clinical phenotype |
||
|
Non-complicated malaria |
24 |
96.0 |
|
Severe malaria |
1 |
4.0 |
|
Total |
25 |
100.0 |
|
Malaria severity criteria |
||
|
Hyperparasitemia |
0 |
0 |
|
Prostration et hyperpasitemia |
0 |
0.0 |
|
Vomiting and Prostration |
1 |
100.0 |
|
Convulsions |
0 |
0.0 |
|
Total |
1 |
100.0 |
|
Statut anemia |
||
|
Anemia |
11 |
44.0 |
|
Non-anemia |
14 |
56.0 |
|
Total |
25 |
100.0 |
Table 1: Clinical characteristics of the study participants.
|
Characteristics |
Average |
Standar deviation |
Minimum |
Maximum |
|
Parasitemia |
50774.40 |
65540.85 |
5360 |
342720 |
|
Hemoglobin levels |
11.22 |
1.20 |
8.7 |
13.4 |
|
Retention rate |
65.40 |
23.77 |
11.11 |
94.44 |
Table 2: biological characteristics of study participants.
Figure 2: Correlations between the spleen retention rates and parasitemia, host ages and hemoglobin level. 2A) correlation between spleen retention rates and the parasitemia. 2B) correlation between spleen retention rates and the ages. 2C) correlation between spleen retention rates and the hemoglobin levels.
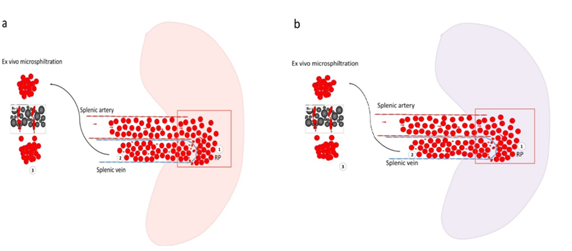
Figure 3: Illustration of the hypothetic retention behaviors of Plasmodium falciparum infected-RBCs in functional and altered spleen and the implication on ex-vivo microsphiltration retention rate. Experimental hypothesis to predict the splenic filtration function using ex-vivo microsphiltration of parasite-infected red blood cells from patients with mild malaria. a) In a fully functional spleen, the splenic artery brings infected RBCs into the splenic red pulp. Deformable infected RBCs arrive - through the inter- endothelial slit of the wall of the splenic veins in the lumen of the splenic vein while stiff infected RBC are retained in the splenic red pulp (1) resulting in a low venous parasitemia (2). The ex vivo microsphiltration of venous deformable infected RBCs may yield a lower retention rate (3).b) In an abnormally functional spleen, stiff infected RBC are not maximally retained in the splenic red pulp. Therefore, infected RBCs accumulate less in the red pulp (1) and veinous parasitemia is higher (2). The ex vivo microsphiltration of venous stiff infected RBC may result in a higher retention rate (3).
4. Discussion
The role of the spleen in clinical malaria symptoms occurrence has long time been discussed [3]. However, direct evidence for these claims has still not been reported due to difficulties to explore in vivo the splenic function. In this study, we proposed to explore the spleen function remotely in patients with malaria using ex vivo microsphiltration of RBCs infected. We carried out a cross-sectional study from May to December 2018 in Kéniéroba village. In this pilot study, clinical and biological data as well as ex vivo microphiltration retention rates were collected from 25 malaria patients. Microsphiltration is an experimental exploratory technique of RBCs deformability. It mimics the splenic retention of less deformable RBCs. This technique has been validated on suspensions of parasitized RBCs at different stages in the development of P. falciparum in vitro [13]. In this study, we assessed the deformability of naturally infected RBCs using ex vivo microsphiltration. We hypothesized that the deformability of venous Pf-infected RBCs may be informative of the spleen filtering function status. Thus, high ex vivo microsphiltration retention rates of P.falciparum-infected RBCs would be associated with a dysfunction of the splenic filtering function in patients and vice versa (supplementary figure1). The mean splenic retention rate of the infected-RBCs was much higher (65.40 ± 23.77%, as compared to that reported by Diakite et al. 2016 (54. 5 ± 4,7% in Hb AA subjects and 44.5 ± 3.3% in Hb AS subjects) [15]. This high rate could be explained by the experimental conditions, in particular the time elapsed between the sampling and the microsphiltration. Although anemia is associated with high splenic retention rate of infected-RBCs in vivo [11, 16], which may result into low ex vivo microsphiltration retention rates, we did not find a statistically significant correlation between the ex vivo retention rates of parasitized RBCs and the hemoglobin level (p = 0.21). Our hypothesis assumed that low ex vivo retention rates would reflect high splenic retention in vivo that would be associated with a low parasitemia.
We found no correlation between ages and ex vivo microsphiltration retention rate of infected-RBCs (p = 0.7). Age could also influence the filtering function of the spleen in patients living in malaria endemic areas. In fact, in malaria endemic areas, patients are more likely to develop splenic insufficiency due to multiple challenges the spleen face during successive infections [3, 17]. Analysis of parasitemia according to the ex vivo microsphiltration retention rate of infected-RBCs did not reveal any correlation between these two parameters (p = 0.23). High parasite density has been associated with serious manifestations of the disease such as cerebral malaria. This clinical phenotype is in favors of low splenic retention in vivo [7]. No correlation between the parasitemia and the ex vivo retention rates was found in this study. Ended due to technical challenges we only worked on parasitemia more than 5000 parasites per microliter, also only data from samples with real parasitemia (flow cytometry) less than 5% to avoid saturation of the filter. As expected, we observed high ex vivo retention of RBCs infected from patients with clinical Hacktt stages greater than or equal to 1 (p = 0.03). In fact, the Hackett clinical stage defines the degrees of splenomegaly that may be associated spleen function impairment.
5. Conclusion
This pilot study allowed to explore ex vivo filtering
function of the spleen in patients with malaria. Infected-RBCs from patients with higher clinical Hacktt stages chowed greater retention rates. Methodological corrections, especially in terms of planning and sample size, would help generating data to draw reliable conclusions.
What is Known about this Topic
Functional spleen retains stiff red blood cells. Splenomegaly is associated with stiff red blood cells circulation. Microbeads retains stiff red blood cells
What this Study Adds
Infected-RBCs from patients with higher clinical Hacktt stages showed greater retention rates in microsphiltration
Acknowledgements
We thank the parents, guardians and children who participated into this study, and the technical, clinical and nursing staff for assistance. We are grateful to many colleagues at MRTC for providing critical reviews of the manuscript which helped improve it. We are grateful to Dr Alioune Papa NDour and Prof Pierre Buffet for their technical support on microsphiltration.
Authors’ Contributions
Study setup: SD. Sample collection: BK, MD. Data collection: BK, AG, SD. Data analysis: BK, SD. Manuscript writing: SD, BK. Manuscript review: SD, BK. All authors read and approved the final manuscript.
Funding
This study is supported by a DELTAS Africa grant (DEL-15-007: Awandare). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107755/Z/15/Z: Awandare) and West Africa.
Ethics Approval and Consent to Participate
Before starting this study, we obtained a community consent from traditional and customary chiefs prior to the study. The study was approved by the ethics committee of the faculty of medicine and Pharmacy of the University of Sciences, Technics and Technologies of Bamako (USTTB), Mali. Written informed consent was obtained from a parent or guardian of each enrolled child. All methods were performed in accordance with the good laboratory and clinical practices guidelines and were respectful of the Helsinki declaration.
Consent for Publications
All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
References
- World malaria report 2021. World malaria report 2021 (who.int) (2022).
- Dalko E, Das B, Herbert F, et al. Multifaceted Role of Heme during Severe Plasmodium falciparum Infections in India. Infect Immun 83 (2015): 3793-3799.
- Gomez-Perez GP, van Bruggen R, Grobusch MP, et al. Plasmodium falciparum malaria and invasive bacterial co-infection in young African children: the dysfunctional spleen hypothesis. Malar J 13 (2014): 335.
- Cooke BM, Mohandas N, Coppel RL. The malaria-infected red blood cell: structural and functional changes. Adv Parasitol 50 (2001): 1-86.
- Schwartz RS, Olson JA, Raventos-Suarez C, et al. Altered plasma membrane phospholipid organization in Plasmodium falciparum-infected human erythrocytes. Blood 69 (1987): 401-407.
- Scherf A, Pouvelle B, Buffet PA, et al. Molecular mechanisms of Plasmodium falciparum placental adhesion. Cell Microbiol 3 (2001): 125-131.
- Huang S, Amaladoss A, Liu M, et al. In vivo splenic clearance correlates with in vitro deformability of red blood cells from Plasmodium yoelii-infected mice. Infect Immun 82 (2014): 2532-2541.
- Groom AC, Schmidt EE, MacDonald IC. Microcirculatory pathways and blood flow in spleen: new insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc 5 (1991): 159-173.
- Chotivanich K, Udomsangpetch R, McGready R, et al. Central role of the spleen in malaria parasite clearance. J Infect Dis 185 (2002): 1538-1541.
- Jakeman GN, Saul A, Hogarth WL, et al. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 119 (1999): 127-133.
- Buffet PA, Safeukui I, Deplaine G, et al. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117 (2011): 381-392.
- Buffet PA, Milon G, Brousse V, et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood 107 (2006): 3745-3752.
- Deplaine G, Safeukui I, Jeddi F, et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood 117 (2011): e88-e95.
- Lavazec C, Deplaine G, Safeukui I, et al. Microsphiltration: a microsphere matrix to explore erythrocyte deformability. Methods Mol Biol 923 (2013): 291-297.
- Diakite SA, Ndour PA, Brousse V, et al. Stage-dependent fate of Plasmodium falciparum-infected red blood cells in the spleen and sickle-cell trait-related protection against malaria. Malar J 15 (2016): 482.
- Buffet PA, Safeukui I, Milon G, et al. Retention of erythrocytes in the spleen: a double-edged process in human malaria. Curr Opin Hematol 16 (2009): 157-164.
- Hommel B, Galloula A, Simon A, et al. Hyposplenism revealed by Plasmodium malariae infection. Malar J 12 (2013): 271.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 