Impact of Prebiotic Supplementation on the Incidence of Necrotizing Enterocolitis in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis
Ghazala S. Virk MD1, Sarah Hack MD2, Haifa Arain MBBS3, Asia Nasser kassem Alriashi MBBS4, Muhammad Sohail S. Mirza MBBS5*, Samra khalid MBBS6, Mohammad Saad Hassan MBBS7, Mahek Thorani MBBS8, Binish Essani MBBS9
1Avalon University School of Medicine, Willemstad, Curaçao
2St. George's University, Grenada, West Indies
3Dow Medical College, Karachi, Pakistan
4University of Science and Technology, University of Sana, Sanaa, Yemen
5Shandong University School of Medicine, Jinan, China
6Jinnah Medical and Dental College, Karachi, Pakistan
7Foundation University Medical College, Islamabad, Pakistan
8People's University of Medical and Health Sciences, Sindh, Pakistan
9Jinnah Medical and Dental College, Karachi, Pakistan
*Corresponding Authors: Muhammad Sohail S. Mirza MBBS, Shandong University School of Medicine, Jinan, China.
Received: 02 June 2025; Accepted: 11 June 2025; Published: 18 June 2025
Article Information
Citation: Ghazala S. Virk, Sarah Hack, Haifa Arain, Asia Nasser kassem Alriashi, Muhammad Sohail S. Mirza, Samra khalid, Mohammad Saad Hassan, Mahek Thorani, Binish Essani. Impact of Prebiotic Supplementation on the Incidence of Necrotizing Enterocolitis in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Journal of Pediatrics, Perinatology and Child Health. 9 (2025): 116-125.
View / Download Pdf Share at FacebookAbstract
Background: Necrotizing enterocolitis (NEC) is a tremendous cause of morbidity and mortality in neonates, especially in preterm infants. Probiotic supplementation has been proposed as a capability strategy to reduce NEC prevalence and enhance neonatal outcomes. Objectives: This systematic evaluation and meta-evaluation evaluated the efficacy of probiotics in lowering the prevalence of NEC, shortening the hospital period, and accelerating the fulfillment of complete enteral feeding in neonates.
Methods: A systematic search of databases recognized 2955 studies. After screening and high-quality assessment, eight randomized controlled trials (RCTs) met the inclusion standards. Data were analyzed using RevMan, with pooled effect sizes expressed as chance ratios (RRs) or standardized suggest differences (SMDs) with 95% confidence intervals (CIs). Heterogeneity was assessed the usage of I² statistics.
Results: The pooled analysis proved a huge discount in NEC incidence with probiotics (RR: 0.35, 95% CI: 0.24–0.50; p < 0.00001; I² = 0%), indicating a 65% lower risk compared to placebo. Probiotics additionally reduced the duration of clinic stay (SMD: –0.54, 95% CI: –0.95 to –0.14; p = 0.008; I² = 75%) and the time to attain complete enteral feeding (SMD: –0.54, 95% CI: –0.92 to –0.15; p = 0.006; I² = 81%). However, significant heterogeneity was found for the latter two results.
Conclusions: Probiotic supplementation appreciably reduces NEC risk and suggests the capacity for enhancing different neonatal outcomes, which include hospital stay period and feeding milestones. Variability in probiotic lines, dosing, and examination populations highlights the need for standardized protocols to optimize advantages.
Keywords
<p>Necrotizing enterocolitis (NEC); low birth weight (VLBW) toddlers; Bifidobacteria; Lactobacilli; Meta-analysis</p>
Article Details
1. Introduction
Necrotizing enterocolitis (NEC) is a crucial gastrointestinal disorder predominantly affecting preterm neonates. Characterized by means of irritation and bacterial invasion of the intestinal wall, NEC often progresses to sepsis, resulting in giant morbidity and mortality [1]. Gross rectal bleeding is regularly recognized as the maximum apparent symptom of NEC in preterm infants, making it a primary gastrointestinal emergency in neonatal extensive care gadgets (NICUs) and a primary contributor to perinatal deaths [2]. The overall incidence of NEC is approximately 1–3 in line with 1,000 live births inside the United States [3], with a better occurrence of 5–7% observed amongst very low beginning weight (VLBW) infants. Early detection and intervention are critical to prevent existence-threatening complications [4]. The etiology of NEC is complex and entails several factors, which includes prematurity, components feeding, intestinal ischemia, and disrupted microbial colonization of the gut [5]. Gut dysbiosis, or an imbalance in microbial populations, is a key hazard component for each NEC and past due-onset sepsis (LOS) in preterm infants [6]. These babies frequently show delayed development of a numerous gut microbiome, with pathogenic microorganism like Enterobacteriaceae predominating over useful strains consisting of Lactobacillus and Bifidobacterium. Probiotics have been proposed as a preventive approach for NEC and sepsis, primarily based on their ability to promote an intestine microbiota just like that of complete-term toddlers, beef up the intestinal barrier, and modulate immune responses [7].
Probiotics, defined as stay microorganisms that offer health benefits while fed on in good enough quantities, have shown capacity in decreasing the occurrence of NEC (level II or higher), LOS, and mortality in VLBW toddlers [8]. Meta-analyses of randomized controlled trials (RCTs) propose that probiotics paintings by means of improving intestine barrier integrity, regulating immune characteristic, and addressing microbial imbalances through direct inhibition of pathogens and aggressive exclusion [9]. Among various probiotics, Bifidobacterium bifidum G001 (BBG001) has emerged as a promising candidate for NEC prevention in neonates, specifically preterm toddlers. BBG001 contributes to intestine health by means of colonizing the intestinal tract, strengthening the mucosal barrier, and suppressing the growth of dangerous microorganism including Enterobacteriaceae. It achieves this through competing for important vitamins, inclusive of human milk oligosaccharides (HMOs) [10]. Additionally, BBG001 supports immune machine improvement by way of activating anti-inflammatory pathways, along with the production of indole-three-lactic acid (ILA) from tryptophan metabolism. These mechanisms collectively foster intestine health and immunological adulthood [11]. The inclusion of BBG001 in this analysis is nicely founded, given it set up position in gut colonization and immune regulation, making it a sturdy candidate for targeted NEC prevention strategies. Bifidobacterium bifidum has validated specific efficacy in promoting gut colonization, decreasing pathogenic microorganism, and growing defensive immune surroundings. However, notwithstanding growing evidence supporting probiotics in NEC prevention, the unique role of BBG001 stays inadequately explored [12]. Most existing RCTs and meta-analyses have centered on multi-strain probiotic formulations, regularly selected based on availability in place of robust preclinical proof of efficacy.
Rationale: Necrotizing enterocolitis (NEC) is a life-threatening gastrointestinal circumstance often affecting very low birth weight (VLBW) toddlers, with considerable morbidity and mortality notwithstanding advancements in neonatal care. Its multifactorial etiology, which include gut dysbiosis, inflammation, and prematurity, highlights the want for progressive preventive strategies. Prebiotics, which selectively enhance the boom of useful gut bacteria including Bifidobacteria and Lactobacilli, offer a promising method to mitigating the microbial imbalances associated with NEC. By selling gut health, strengthening the intestinal barrier, and decreasing the proliferation of dangerous bacteria, prebiotics can also play a critical role in NEC prevention, especially in synergy with human milk. However, present proof stays confined and fragmented, with most studies focusing on probiotics or blended interventions. This systematic evaluation and meta-analysis goal to evaluate the efficacy of prebiotic supplementation in reducing NEC occurrence amongst VLBW infants, presenting critical insights to guide scientific practice and improve neonatal consequences.
Objectives: The objectives of this systematic overview and meta-analysis are: (1) to assess the effect of prebiotic supplementation at the prevalence of necrotizing enterocolitis (NEC) in very low beginning weight (VLBW) toddlers; (2) to assess its effects on secondary outcomes, which include late-onset sepsis (LOS), mortality, and normal neonatal fitness; (3) to take a look at versions in efficacy based totally on factors together with prebiotic type, dosage, length, and feeding practices; (four) to become aware of ability negative consequences or protection worries related to prebiotic use; and (5) to examine prebiotic supplementation with different preventive strategies, such as probiotics or widespread care, in lowering NEC occurrence.
2. Methodology
The method follows the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.1 Protocols and Registration:
No registration or ethical approval was required for this systematic review and meta-analysis, as it is based on previously published studies.
2.2 Eligibility Criteria:
The inclusion standards for this systematic evaluation are as follows: (1) research concerning very low start weight (VLBW) toddlers (<1500 g); (2) studies evaluating the usage of prebiotic supplementation as an intervention; (3) research reporting outcomes associated with necrotizing enterocolitis (NEC), late-onset sepsis (LOS), mortality, or overall neonatal fitness; (4) randomized managed trials (RCTs), cohort research, or case-control studies; and (5) studies published in English. The exclusion standards encompass: (1) studies concerning combined populations without separate records for VLBW infants; (2) studies assessing blended interventions, which include prebiotics with probiotics (symbiotic), except consequences for prebiotics alone are stated one after the other; (3) studies missing relevant final results measures; (4) reviews, editorials, convention abstracts, or unpublished statistics; and (5) non-English publications (Table 1).
|
Population |
Intervention |
Comparison |
Outcomes |
Study Design |
|
Very low birth weight (VLBW) infants (<1500 g) |
Probiotic supplementation |
Standard care, placebo, or other preventive strategies |
Primary: Incidence of necrotizing enterocolitis (NEC) |
Randomized controlled trials |
Table 1: PICOS framework.
2.3 Information Sources:
A complete search for studies on the efficacy of probiotics on necrotizing enterocolitis was conducted across more than one digital database, consisting of PubMed, Google Scholar, and Cochrane Library. Independent journals and other scholarly guides have also been covered. The seek method adhered to PRISMA tips to ensure comprehensive coverage.
2.4 Search Strategy:
The search method concerned the use of Boolean operators (AND/OR) to combine phrases related to the study title. Filters have been applied to attention in randomized controlled trials and human research. The search yielded sixteen studies (n=08) that met the inclusion criteria (Table 2).
|
Sr No. |
Databases |
Search String |
Number of studies |
|
1 |
PubMed |
(("very low birth weight" OR "VLBW" OR "low birth weight" OR "preterm" OR "premature infant*" OR "neonate*" OR "infant, low birth weight"[MeSH] OR "infant, premature"[MeSH] OR "infant, newborn"[MeSH]) AND ("prebiotic*" OR "fructooligosaccharide*" OR "galactooligosaccharide*" OR "oligosaccharide*" OR "inulin" OR "human milk AND ("necrotizing enterocolitis" OR "NEC" OR "necrotising enterocolitis" OR "enterocolitis, necrotizing"[MeSH])) oligosaccharide*" OR "prebiotics"[MeSH]) |
1527 |
|
2 |
Cochrane Library |
("necrotizing enterocolitis" OR NEC) AND ("prebiotic*" OR "oligosaccharide*" OR "inulin" OR "fructooligosaccharides" OR "galactooligosaccharides") AND ("very low birth weight" OR VLBW OR "preterm infant*" OR neonate*) |
213 |
|
3 |
Google Scholar |
"Necrotizing enterocolitis" AND "prebiotic supplementation" AND ("very low birth weight" OR VLBW) AND ("clinical outcomes" OR "late-onset sepsis" OR mortality) |
1215 |
Table 2: Search strategy on individual databases.
2.5 Selection Process:
The article selection was accomplished in stages. First, titles and abstracts were screened for relevance. In the second level, the full texts of the selected articles were reviewed to verify eligibility. Data on the primary creator, year of guide, observation layout, use of a sample size, results, and methods were extracted using a standardized records extraction tool.
2.6 Data Items:
For every study, information on the sample size, study layout, effects, and statistical measures (means, standard deviations) was extracted. Data were synthesized and analyzed the usage of RevMan software for meta-analysis.
2.7 Study Risk of Bias Assessment:
The Cochrane Risk-of-Bias (version 2) tool was used to evaluate the threat of bias throughout seven domains: random series era, allocation concealment, blinding, incomplete outcome records, selective reporting, and other biases. The risk of bias for every look was assessed as low, unclear, or excessive.
2.8 Statistical Analysis:
Meta-analysis changed into completed usage of Review Manager (RevMan) software (version 5.4). A random-consequences model was used due to predicted heterogeneity throughout the research. Heterogeneity was assessed the usage of the I² statistic, and meta-regression turned into performed where applicable.
2.9 Reporting Bias Assessment:
Potential reporting biases have been minimized by means of selecting high-quality studies and undertaking a thorough search for all relevant publications. Funnel plots have been used to visually check for e-book bias.
3. Results
3.1 Study Selection and Screening
The initial search of the database yielded 2955 papers. After the removal of duplicates and applying the inclusion criteria total of 54 studies for selected for full-text analysis. Based on the methodological quality assessment and inclusion and exclusion criteria, a total of 08 articles finally met the criteria to be included in this systematic review and meta-analysis. Figure 1 presents the detailed PRISMA flow chart diagram of the selection process of the included studies.
3.2 Study Characteristics:
Study Characteristics of all the included studies are given in Table 3.
Table 3: Characteristics of included studies.
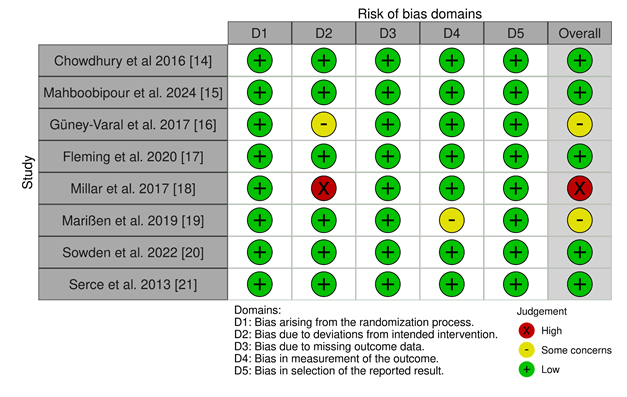
3.3 Risk of Bias: Risk of Bias [22] of the included studies was calculated using the Cochrane ROB 2 tool [23] since all of the included studies are Randomized Controlled Trials (Figure 2).
3.4 Meta-Analysis:
RevMan was used to perform the meta-analysis for this study.
(i) Incidence of Necrotizing Enterocolitis in Probiotic vs. Placebo Groups:
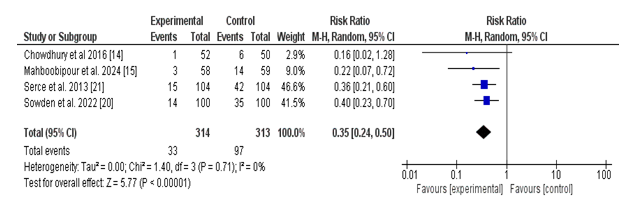
A total of four studies reported the occurrence of necrotizing enterocolitis (NEC) among neonates receiving probiotics as compared to placebo. The pooled analysis established a statistically significant reduction in the threat of NEC within the probiotic organization. The general risk ratio (RR) turned into 0.35 (95% CI: zero.24 to 0.50; p < 0.00001), indicating that probiotics reduced the risk of NEC by 65% compared to placebo.
Heterogeneity of most of the covered research becomes low (Tau² = 0.00; Chi² = 1.40, df = 3; p =0.71; I² = 0%), suggesting consistency within the direction and value of effect across research. Individual study RRs ranged from 0.16 to 0.40, with all research favoring the use of probiotics over placebo, although Chowdhury et al. (2016) had a wide self-belief interval crossing the road of no effect.
These findings offer strong proof helping the prophylactic use of probiotics in lowering the occurrence of necrotizing enterocolitis in neonates (Figure 3).
(ii) Effect of Probiotics on Length of Hospital Stay:
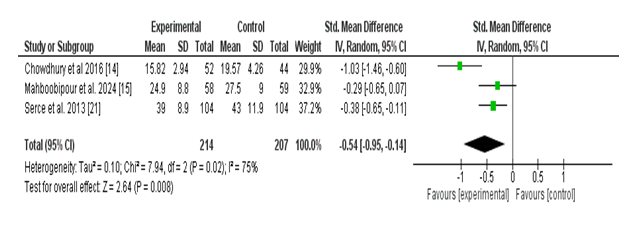
Three studies examined the effect of probiotic supplementation during the duration of health center life in neonates. The meta-evaluation found a statistically significant decrease during the period of hospitalization among neonates receiving probiotics in comparison to those receiving a placebo. The pooled standardized imply difference (SMD) was –0.54 (95% CI: –0.95 to –0.14; p = 0.008), indicating a slight effect favoring the probiotic organization.
However, there has been full-size heterogeneity some of the protected research (Tau² = 0.10; Chi² = 7. Ninety-four, df = 2; p = 0.02; I² = seventy-five%), suggesting variability in effect sizes that might be attributed to variations in study populations, probiotic strains, or baseline health center live intervals. Despite this, all included research confirmed a trend toward decreased hospital life with probiotic use, with Chowdhury et al. (2016) showing the most stated impact (SMD: –1.03; 95% CI: –1.46 to –zero.60).
These findings recommend that probiotic supplementation may also contribute to a modest, however clinically applicable, decrease in hospital stay duration in neonates (Figure 4).
(iii) Effect of Probiotics on Age of Achievement of Full Enteral Feeding:
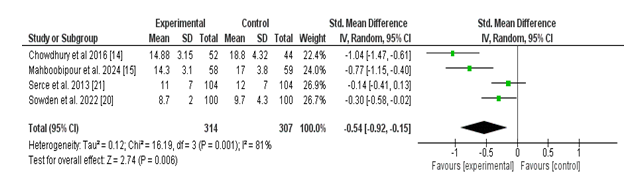
Four studies assessed the effect of probiotic supplementation on the time (in days) required to obtain full enteral feeding in neonates. The pooled evaluation demonstrated a statistically significant discount in the time to complete feeding inside the probiotic institution as compared to the manipulate organization. The standardized imply difference (SMD) was –0.54 (95% CI: –0.92 to –0.15; p = 0.006), indicating a slight beneficial effect of probiotics.
There is extensive heterogeneity in the various studies (Tau² = 0.12; Chi² = 16.19, df = 3; p = 0.001; I² = 81%), reflecting variability in all likelihood because of variations in probiotic lines, feeding protocols, or neonatal characteristics. Chowdhury et al. (2016) and Mahboobipour et al. (2024) confirmed large effect sizes, even as Serce et al. (2013) confirmed a small and non-large impact.
Overall, those results recommend that probiotic administration may additionally boost the establishment of complete enteral feeding in neonates, potentially contributing to improved dietary effects and earlier hospital discharge (Figure 5).
3.5 Publication Bias:
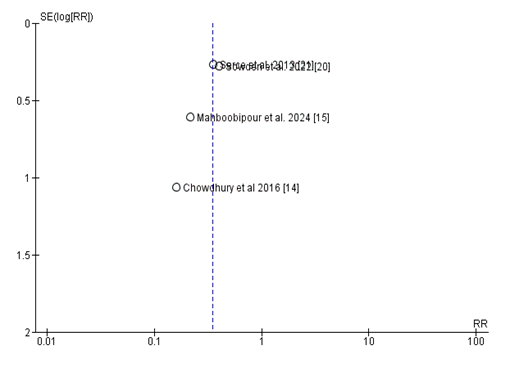
The funnel plot [24] assessing e-book bias for research evaluating the relative threat (RR) of probiotic supplementation on the incidence of NEC in VLBW toddlers appears largely symmetrical round the line of no impact (RR = 1), indicating a low probability of big publication bias. Most research cluster carefully around the important axis, with a mild dispersion in impact sizes consistent with predicted sampling variability. However, one has a look at emerges as an outlier, located similarly from the significant line with larger fashionable mistakes and a extra pronounced impact estimate. While this raises a few worries approximately capability small-look at outcomes, the general distribution does no longer exhibit the feature asymmetry normally associated with significant publication bias (Figure 6).
4. Discussion
This systematic review and meta-analysis provide evidence supporting the prophylactic use of probiotics in preterm neonates, especially for reducing the risk of necrotizing enterocolitis (NEC), enhancing feeding tolerance, and shortening the period of hospitalization. However, variability in effects across research highlights the significance of additional studies to refine the medical utility of probiotics in this population.
The pooled analysis discovered a large 65% reduction in the hazard of NEC with probiotic supplementation as compared to placebo (RR = 0.35; 95% CI: 0.24–0.50; p < 0.00001). The low heterogeneity (I² = 0%) suggests regular findings throughout studies, helping the efficacy of probiotics in stopping NEC. These results align with previous evaluations demonstrating the beneficial position of probiotics in improving intestinal fitness and stopping inflammatory situations in preterm neonates. Variations in NEC prevalence across research may be attributed to differences in probiotic strains, dosages, and neonatal characteristics, highlighting the need for standardized protocols in future research.
Probiotic supplementation drastically decreased feeding intolerance and the time to attain full enteral feeding (SMD = –0.54; 95% CI: –0.92 to –0.15; p = 0.006). Despite excessive heterogeneity (I² = 81%), the regular trend across research suggests a potential position for probiotics in improving gastrointestinal function and achieving early nutritional milestones. Faster success of full enteral feeding may additionally reduce the reliance on parenteral nutrition, subsequently lowering the risk of related headaches which including sepsis or cholestasis. The length of hospitalization changed appreciably shorter in neonates receiving probiotics (SMD = –0.54; 95% CI: –0.95 to –0.14; p = 0.008), even though there was widespread heterogeneity among studies (I² = 75%). This finding is clinically applicable, as shorter health facility stays lessen healthcare costs and the threat of medical institution-obtained infections. However, versions in probiotic lines, formulations, and management protocols across studies warrant a warning when generalizing those outcomes.
The protection profile of probiotics becomes consistent across studies, and not using a full-size negative result is pronounced. However, pressure-particular differences had been referred to. For instance, studies making use of multi-strain probiotics, together with those containing Lactobacillus rhamnosus and Bifidobacterium infantis, confirmed more efficacy in lowering NEC and feeding intolerance in comparison to single-strain formulations like Saccharomyces boulardii, which no longer show full-size benefits. These findings underscore the need to determine the most appropriate strains and dosages for specific neonatal populations.
A current systematic overview and meta-evaluation (SRMA) highlights the efficacy of Bifidobacterium breve BBG001 (BBG001) in improving scientific effects amongst preterm babies. The findings indicate that probiotic supplementation with BBG001 extensively reduces the prevalence of necrotizing enterocolitis (NEC), bloodstream infections, and infection-related mortality. These effects are particularly critical given the vulnerability of preterm toddlers to intense infections and inflammatory situations. The defensive results of BBG001 can be attributed to its role in enhancing gut barrier function, modulating immune responses, and promoting a balanced intestinal microbiome. These findings strengthen the potential of BBG001 as a targeted probiotic intervention to mitigate existence-threatening complications on this high-threat population. However, further studies are warranted to optimize dosing, administration protocols, and to assess long-time period safety and efficacy [25].
Current trial facts offer low-reality proof regarding the effect of prebiotics on lowering the threat of necrotizing enterocolitis (NEC), all-cause mortality prior to discharge, and invasive infections in very preterm or very low birth weight (VLBW) toddlers. Additionally, the proof on the results of prebiotics on lengthy-time period neurodevelopmental impairment is of very low truth. As a end result, self-belief in these effect estimates stays limited, and its miles potential that the real results may additionally fluctuate drastically from the ones suggested. To guide scientific exercise and coverage efficaciously, there's a pressing need for massive-scale, excellent randomized controlled trials that can offer robust and reliable proof [26]. The mixture of **Bifidobacterium, Lactobacillus, and Enterococcus** verified the highest efficacy in decreasing both mortality and the incidence of necrotizing enterocolitis (NEC, Bell degree II or better) in preterm infants. Additionally, the usage of prebiotics and Lactobacillus on my own confirmed sizable effectiveness in shortening the length of hospitalization and accelerating the time required to gain full enteral feeding. However, there may be no conclusive proof to indicate that probiotics have an impact on lowering the threat of sepsis in this population [27].
This evaluation isn't always without barriers. High heterogeneity in consequences, consisting of feeding tolerance and sanatorium stay period, indicates variability in examination protocols, probiotic strains, and affected person populations. Furthermore, although the funnel plot did not imply widespread guide bias, the presence of an outlier raises the possibility of small-sample consequences. Methodological differences, together with sample size and a look at satisfactory, may also have additionally motivated the results.
Probiotic supplementation seems to be a safe, cost-effective intervention with extensive benefits for preterm neonates. The discount in NEC incidence, advanced feeding consequences, and shorter health facility stays advocate that probiotics will be included in habitual neonatal care, in particular in high-risk populations along with very low birth weight (VLBW) babies. However, similar research is required to set up standardized pointers regarding probiotic lines, dosages, and period of management.
Future studies should raise awareness on addressing the heterogeneity determined on this evaluation with the aid of engaging in large-scale, properly-designed randomized controlled trials with standardized protocols. Comparative research evaluating the efficacy of various probiotic lines and formulations is also needed to optimize remedy techniques. Additionally, long-term follow-up research is vital to evaluate the ability effects of probiotics on increasing neurodevelopmental outcomes and long-term gastrointestinal health in preterm neonates.
5. Conclusion
This meta-analysis highlights the sizable benefits of probiotics in lowering NEC prevalence, improving feeding tolerance, and shortening the length of stay in preterm neonates. While those findings guide the use of probiotics as a prophylactic intervention, in addition, studies are vital to refine their utility and deal with the observed heterogeneity in outcomes.
References
- Nair J, Longendyke R, Lakshminrusimha S. Necrotizing enterocolitis in moderate preterm infants. BioMed Research International (2018): 1-6.
- Muller MJ, Paul T, Seeliger S. Necrotizing enterocolitis in premature infants and newborns. Journal of Neonatal-Perinatal Medicine 9 (2016): 233-42.
- Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nature Reviews Gastroenterology & Hepatology 13 (2016): 590-600.
- Lin PW, Stoll BJ. Necrotising enterocolitis. The Lancet 368 (2006): 1271-83.
- Hodzic Z, Bolock AM, Good M. The role of mucosal immunity in the pathogenesis of necrotizing enterocolitis. Frontiers in Pediatrics 5 (2017).
- Hu X, Liang H, Li F, et al. Necrotizing enterocolitis: current understanding of the prevention and management. Pediatric Surgery International 40 (2024).
- Van Belkum M, Alvarez LM, Neu J. Preterm neonatal immunology at the intestinal interface. Cellular and Molecular Life Sciences 77 (2019): 1209-27.
- Aceti A, Beghetti I, Maggio L, et al. Filling the Gaps: Current research directions for a rational use of probiotics in preterm infants. Nutrients 10 (2018): 1472.
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Library (2014).
- Calvo LN, Greenberg RG, Gray KD. Safety and Effectiveness of Probiotics in Preterm Infants with Necrotizing Enterocolitis. NeoReviews 25 (2024): e193-206.
- Chi C, Li C, Buys N, et al. Effects of probiotics in preterm infants: a network Meta-analysis. Pediatrics 147 (2020).
- Zhu X-L, Tang X-G, Qu F, et al. Bifidobacterium may benefit the prevention of necrotizing enterocolitis in preterm infants: A systematic review and meta-analysis. International Journal of Surgery 61 (2018): 17-25.
- Haddaway NR, Page MJ, Pritchard CC, et al. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Systematic Reviews 18 (2022).
- Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a Double-Blind Randomized controlled trial. PubMed. (2016).
- Mahboobipour AA, Bitaraf A, Mohammadi P, et al. Effects of synbiotics on necrotizing enterocolitis and full enteral feeding in very low birth weight infants: A double-blind, randomized controlled trial. Medicine 103 (2024): e39647.
- Güney-Varal I, Köksal N, Özkan H, et al. The effect of early administration of combined multi-strain and multi-species probiotics on gastrointestinal morbidities and mortality in preterm infants: A randomized controlled trial in a tertiary care unit. The Turkish Journal of Pediatrics 59 (2017): 13-9.
- Fleming P, Wilks M, Eaton S, et al. Bifidobacterium breve BBG-001 and intestinal barrier function in preterm babies: Exploratory Studies from the PiPS Trial. Pediatric Research 89 (2020): 1818-24.
- Millar M, Seale J, Greenland M, et al. The microbiome of infants recruited to a randomised placebo-controlled probiotic trial (PIPS trial). EBioMedicine 20 (2017): 255-62.
- Marißen J, Haiß A, Meyer C, et al. Efficacy of Bifidobacterium longum, B. infantis and Lactobacillus acidophilus probiotics to prevent gut dysbiosis in preterm infants of 28+0–32+6 weeks of gestation: a randomised, placebo-controlled, double-blind, multicentre trial: the PRIMAL Clinical Study protocol. BMJ Open 9 (2019): e032617.
- Sowden M, Van Weissenbruch MM, Bulabula ANH, et al. Effect of a Multi-Strain probiotic on the incidence and severity of necrotizing enterocolitis and feeding intolerances in preterm neonates. Nutrients 14 (2022): 3305.
- Serce O, Benzer D, Gursoy T, et al. Efficacy of saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Human Development 89 (2013): 1033-6.
- Ma L-L, Wang Y-Y, Yang Z-H, et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Medical Research 7 (2020).
- Sterne JC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019): l4898.
- Page MJ, Sterne JC, Higgins JPT, et al. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: A review. Research Synthesis Methods 12 (2020): 248-59.
- Abdullahi AM, Zhao S, Xu Y. Efficacy of probiotic supplementation in preventing necrotizing enterocolitis in preterm infants: a systematic review and meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine 38 (2025).
- Sharif S, Oddie SJ, Heath PT, et al. Prebiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Library (2023).
- Dai Y, Yu Q, Zhang F, et al. Effect of probiotics on necrotizing enterocolitis in preterm infants: a network meta-analysis of randomized controlled trials. BMC Pediatrics 25 (2025).
Article Views: 994
Journal Statistics




Discover More: Recent Articles
Grant Support Articles
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved!