Acutely Disturbed Intestinal Blood Flow in a 71-Year-Old Patient with COVID 19 Disease (SARS-Cov-2)
Elvin Bairamov1*, Schwab AC2*, Vogel S3, Gailani M1,
1Department of internal medicine and cardiology, Helios Frankenwaldklinik Kronach, Kronach, Germany
2Department of cardiology, electrophysiology, Helios Frankenwaldklinik Kronach, Kronach, Germany
3Department of anesthesiology and intensive care medicine, Helios Frankenwaldklinik Kronach, Kronach, Germany
*Corresponding author: Elvin Bairamov, Department of Internal Medicine and Cardiology, Helios Frankenwaldklinik Kronach, Kronach, Germany
AC Schwab, Department of Cardiology, Electrophysiology, Helios Frankenwaldklinik Kronach, Kronach, Germany
Received: 29 March 2022; Accepted: 07 April 2022; Published: 14 April 2022
Article Information
Citation:
Elvin Bairamov, Schwab AC, Vogel S, Gailani M. Acutely Disturbed Intestinal Blood Flow in a 71-Year-Old Patient with COVID 19 Disease (SARS-Cov-2). Archives of Internal Medicine Research 5 (2022): 146-152.
View / Download Pdf Share at FacebookAbstract
We present a case report describing both pulmonary embolism and acute mesenteric ischemia as a complication of COVID-19 infection, the later despite adequate anticoagulation as per guidelines. Coagulation disorders in the context of SARS-CoV-2 infection can lead to thromboembolic complications in patients such as cerebrovascular events (cerebral infarction), myocardial infarction, arterial occlusion of the lower extremities, splanchnic vein thrombosis, portomesenteric vein thrombosis and mesenteric ischemia. SARS-CoV-2 causes a hypercoagulable and prothrombotic state or vasculitis in patients as previously described. Mesenteric ischemia is a serious, life-threatening disease and can significantly increase mortality in COVID-19 patients.
Keywords
<p>Coagulopathy, Thrombosis, Embolism, Fibrinogen, Von Willebrand Factor, Intestinal Pneumatosis, Portal Venous Gas</p>
Article Details
1. Case Report
A 71-year-old patient presented himself to our emergency room due 5 days ongoin to diarrhea associated with dehydration. The patient was not vaccinated against coronavirus infection. A routine smear test for COVID 19 (SARS-CoV-2 PCR) was positive for the patient. Slightly elevated D-dimer (5.4 µgFEG/ml) and troponin (0.082 µg/l) values were detected in the laboratory and a CT thorax with intravenous contrast medium was carried out. It revealed an acute subsegmental pulmonary embolism (the 9th lung segment on the left). Signs of chronic obstructive pulmonary disease with pronounced panlobular bullous pulmonary emphysema were also present. With volume administration and mild oxygen administration, the patient was cardiopul-monary stable and symptom-free in the emergency room and could be admitted to the normal ward. Weight-adapted administration of endoxaparin and dexamethasone as well as symptomatic therapy with volume and inhalation with bronchodilators were carried out. Transthoracic echocardiography showed preserved left ventricular pump function (LVEF 55%) with mild septal and inferior hypokinesia and no evidence of acute right ventricular overload.
2. Clinical Findings
On the 8th day of inpatient treatment, the patient vomited in the morning and complained of severe abdominal pain. The initial clinical examination was unremarkable (no defensive tension, no signs of peritonitis, except for a slight periumbilical tender-ness). Therefore, abdominal sonography and an X-ray of the thorax and abdomen were performed, without any specific findings. The symptoms decree-sed under symptomatic treatment with antiemetics and pain relief measures. On the same day in the evening, the patient complained of renewed massive abdominal pain and vomited haematin-like stomach contents. The clinical examination revealed an abdo-men that was extremely tender to pressure (9-10/10 pain scale), massive flatulence, defensive tension, a significant inflammatory reaction with a sudden increase in the inflammatory values and the lactate level (C-reactive protein initially 0.74 to 10.65 mg/dl; procalcitonin from 3.81 to 13.17 ng/dl, lactate from 1.2 to 9.9 mmol/l), all compatible with the diagnoses of sepsis. The patient was admitted to our intensive care unit. After protective intubation due to massive vomiting, we performed a radiocontrast CT abdome. There were edematous changes in the mesentery of the proximal third of the jejunum and a diffuse enlargement in the distal jejunum. The cecum was filled with fluid, a paralytic ileus aspect with dilat-ation of the small bowel loops, little intraperitoneal free fluid (in the pelvis minor and the parietocolic recess on the left). A truncus coeliacus stenosis was clearly visible. After further review of the images, indirect signs of acute mesenteric ischemia (or superior mesenteric artery with involvement of the proximal third of the jejunum) could also be detected.
3. Therapy and Progression
Despite the exhaustion of all intensive medical measures and therapy of the septic shock according to the guidelines with massive volume administra-tion, antibiotic therapy with piperacillin/tazobactam, steroid therapy, the general condition of the patient continued to rapidly deteriorate with cardiopulmo-nary instability and a high need for catecholamines. The progression was so fulminant that the patient was rapidly to unstable for the surgery to be carried out. The pulmonary condition also deteriorated with increasing gas exchange disorders and bipulmonary compressions in the sense of ARDS (adult respiratory distress syndrome), a combined acidosis with an increase in lactate led to multi-organ failure, from which he died the next day of intensive care. There was no autopsy as per family decision. The course of the disease with X-ray and CT images was shown in Figure 1.
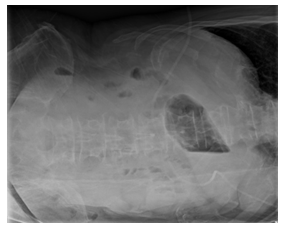
Figure 1a: X-ray abdomen in the morning. (in the initial phase of the AMI).
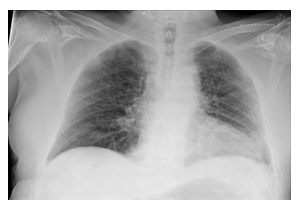
Figure 1b: Chest x-ray in the morning (in the initial phase of AMI).
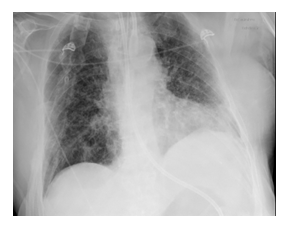
Figure 1c: X-ray chest in the evening (in the 3rd phase of AMI).
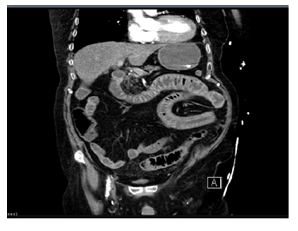
Figure 1d: CT abdomen in the evening (in phase 3 of AMI).
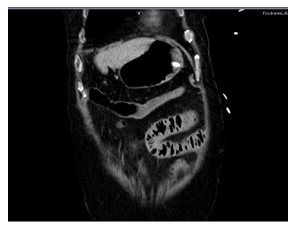
Figure 1e: CT abdomen in the evening (in phase 3 of AMI).
4. Discussion
Acute mesenteric ischemia is a rare abdominal emergency and is associated with high rates of morbidity and mortality that require prompt manag-ement. Analogously to heart attacks and strokes, this also applies to mesenteric heart attacks, since the ischemia tolerance of the intestine is only around 6 hours. The superior mesenteric artery supplies the distal duodenum, jejunum, ileum, and colon up to the middle of the transverse colon and up to the left flexure. The distal colon is supplied by the inferior mesenteric artery [1]. Bowel ischemia is commonly (75%) caused by an occlusion in a blood vessel, of which 75% is embolic and 25% thrombotic. The most frequently affected vessel is the superior mesenteric artery (85%), which is why the most frequently infarcted part of the bowel is the middle jejunum. Sometimes an adequate blood supply to the intestine is not sufficient due to other disease states, e.g. B. in the context of acute circulatory failure with hypot-ension, a severe injury with shock, or due to vascular spasms in pre-existing arteriosclerosis (non-occlusive mesenteric ischemia 25%). The heart is the source of the embolism in 90% of the cases. A venous occlusion occurs in only 5-10% of cases, causes can be coagulation defects (often common recently, due to COVID 19), myeloproliferative diseases, or portal hypertension.
The lethality of the disease is high at around 80%. Atherosclerosis and atrial fibrillation are predisposing factors. Other risk factors are old age (more than 75% of the patients are >75 years), cardiac insufficiency, mechanical heart valves, and pulmonary artery disease). Intestinal ischemia also occasionally occurs in younger patients after cocaine consumption and major physical exertion (e.g. running a marathon). There are 3 stages of acute ischemia: The first stage (1-6 h) where nausea, vomiting, and possibly diarrhea occur suddenly. The pain is mostly located in the periumbilical region and is classically very severe (NRS 8-10). Initially, upon admission, we saw relatively bland examination findings, since no signs of peritonism had developed at this point. The second stage occurs after several hours at the earliest (usually between 6-12 hours). The pain often decreases somewhat ('lazy peace'). Bloating may occur due to gas formation, and blood may be present with vomiting or diarrhea. 1The third stage, final phase (12-24 h): Without treatment, the disease progresses to migratory peritonitis with a hemo-dynamically relevant state of shock. Those affected show a septic condition, the severely tense and painful abdomen is extremely sensitive to pressure. In this phase, there is peritonism (ileus, intestinal gangrene with migration) with sepsis (after bacteria from the intestinal contents have entered the blood) and ultimately multi-organ failure (e.g. kidney, liver, and heart failure). Laboratory tests usually only show leukocytosis initially. In the course, an increase in LDH, CRP, CK, lactate, D-dimer (normal D-dimer largely excludes AMI), amylase (in 50%), and phosphorus (80%), as well as metabolic acidosis, are typical. Diagnostic methods are ultrasound and X-ray examinations, but the gold standard is radiocontrast computed tomography.
In the case of acute intestinal ischemia, the patient should be admitted to the intensive care unit and stabilized with volume administration, antibiotics, and full anticoagulation using heparin. Adequate analgesia should also be ensured. An interventional procedure is only permitted and possible in individual cases (proximal embolism with mechanical thrombo-embolectomy, distal embolism with local lysis). Surgical intervention (emergency exploratory laparo-tomy) should always be performed without loss of time if there is evidence of occlusion of mesenteric or if there are signs of peritonitis. An embolectomy and, if necessary, resection of necrotic parts of the intestine (also, if necessary, second-look surgery after 8-12 h in the case of initially questionable vital parts of the intestine) is almost always necessary. A study in 67 patients with AMI (Nuzzo et al, Am J Gastroenterol 2017) showed that all of the patients presented were predictors of irreversible intestinal necrosis with the need for resection if lactate >2 mmol/l, another organ failure (e.g. renal failure), and showed dilated bowel loops on CT.
4.1 SARS-CoV-2 und mesenteric ischemia
SARS-CoV-2 has been associated with gastrointes-tinal symptoms and elevated liver enzymes. Intestinal abnormalities, including mesenteric ischemia, can be found in patients with coronavirus disease. The exact pathological mechanism leading to AMI in COVID-19 is currently not well understood. Possible causes of gastrointestinal symptoms are endothelial inflam-mation due to direct virus invasion and coagulation disorders: Direct invasion of the intestinal tissue by the virus when the angiotensin-converting enzyme 2 is expressed on enterocytes, which leads to diffuse endothelial inflammation or to increased procoa-gulant factors such as factor VIII, von Willebrand factor, fibrinogen or a virus-induced cytokine storm, which in turn leads to activation of coagulation and fibrinolysis. Angiotensin-converting enzyme 2 (ACE-2) shows the most frequent surface expression in lung alveolar epithelial cells as well as on small intestinal enterocytes and on the vascular endothelium, which may explain that abdominal symptoms (nausea, vomiting, diarrhea, including liver failure and cholestasis) can also occur frequently as a result of SARS-CoV2 [1]. Furthermore, increased values of the von Willebrand factor have been described in the literature in severe COVID-19. Von Willebrand factor is released from Weibel-Palade bodies in response to endothelial damage. The vascular endo-thelium expresses angiotensin-converting enzyme 2, the target receptor for severe acute respiratory syndrome 2 (SARS-CoV-2), possibly explaining the endothelial cell tropism of SARS-CoV-2 and subsequent endothelial dysfunction or damage with resultant vascular thrombosis [1].
A retrospective analysis (R. Bhayana et al, Radiology, May 11, 2020) has already shown that the proportion of patients who initially presented clinically with at least one gastrointestinal symptom of COVID 19 disease was 34% (n = 142 of 412) . Abdominal imaging was performed in 134 of the patients (33 percent). As the authors report, thickening of the intestinal walls was evident in 13 of 412 patients with COVID-19 (3.2%) in the CT abdomen. Gas formation in the intestinal wall (pneumatosis intestinalis) or the portal vein was also detected in 4 patients, which indicates ischemic damage. These 4 patients underwent immediate laparotomy. In two of these patients, at laparotomy, the surgeon noted a specific yellow discoloration of the necroses of the small bowel loops - in contrast to the usual purple or black color of the necrotic bowel. Microscopic findings revealed diffuse ischemic injury with multifocal necrosis, marked submucosal edema with void spaces consistent with pneumatosis, and occasional fibrin thrombi in submucosal arterioles beneath necrotic mucosa. The other 2 patients in the above analysis did not have bowel necrosis at laparotomy, however, yellow spots on the antimesenteric side of the transverse colon and yellowing of the stomach were found in these patients. The authors suspect that the yellow discoloration was caused by fibrin deposits [1].
On the one hand, the viruses could have infected the intestinal mucosa. However, the cause could also be thrombosis of the small mesenteric vessels, either as a result of infection directly or as a result of damage to the endothelia or as part of generally increased susceptibility to thrombosis of the blood due to systemic inflammation, endothelial activation, hypo-xia, and immobilization [2]. During the pandemic, we often saw increases in laboratory transaminases in our intensive care unit, or signs of severe paralytic ileus with intestinal ischemia, which were observed significantly more in COVID-19 patients than in non-COVID-19 patients [1]. The etiology of the observed intestinal paralysis is probably more complex, as already described above. Patients with COVID-19 on ventilation and patients with ARDS required more sedation and also higher doses of opiates, also because of the long coma and the need to lie prone [3]. Both also promote the development of intestinal paralysis. Furthermore, the AMI cases already presented in the literature were not solitary, as was the case in our case, which showed an acute, subseg-mental pulmonary embolism (9th lung segment on the left) in connection with the AMI. Our case distinguish itself for the bi-organic involvement in two separate phases (first pulmonary embolism, then AMI despite anticoagulation) and the rapdly progression of the second condition, without even time for the surgery. This case report brings attention to the possibility of fulminant progression and there-fore emphasizes the need for immediate recognition and treatment of such condition. The other tissues and sites of thrombosis of the COVID-19 cases published concurrently with AMI were splenic and renal infarctions, digital necrosis of both feet, portal vein thrombosis, right middle cerebral thrombosis.
5. Conclusion
In summary, it can be said that COVID-19 patients can also have pronounced gastrointestinal symptoms, even if their respiratory condition is stable. With the case report described above, we would like to point out that AMI is a serious, life-threatening disease and can significantly increase mortality in COVID-19 patients. A pulmonary embolism in COVID-19 disease is usually easy to diagnose using CT, while indirect signs of intestinal ischemia are more difficult to detect on abdominal CT. A thickened, edematous, and dilated bowel (> 3 cm) with a corresponding clinical condition and laboratory changes (e.g. metabolic acidosis, increase in lactate) should raise the suspicion of acute mesenteric ischemia in COVID 19 disease. Early intervention is life-saving in these patients. The presence of intestinal pneumatosis or portal venous gas indicates intestinal ischemia and laparotomy should be strongly considered. Like other authors, we emphasize that further studies are required to better understand the pathogenesis and to be able to treat intestinal findings in COVID-19 patients better and earlier.
References
- Herold V. Internistische Intensivmedizin (2022).
- Rajesh Bhayana, Avik Som, Matthew D Li, et al, Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology 297 (2020).
- Can Chen, Yi-Wei Li, Peng-Fei Shi, et al, Acute Mesenteric Ischemia in Patients with COVID-19: Review of the literature. Journal of the National Medical Association (2021).


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 