Effect of the Third Dose of Astrazeneca Vaccine on the Ability to Generate Anti-Rbd Antibodies within Two at-Risk Populations, Geriatrics and Health Personnel in The Province of Tucumán
Medina Ruiz Luis1, Ferre Contreras Miguel2, Molina Eliana3, Aguilar Mónica4, Costas Dardo4, Aznar Patricia4, Alcorta María Elena4, Lara Laura5, Barrenechea Guillermo Gabriel6, Ortega Eugenia Silvana6 and Peral de Bruno María de los Ángeles7*
1Head of SI.PRO.SA (Province Health System), Ministry of Health, Tucumán, Argentina
2President of the Medical Executive Secretary, SI.PRO.SA Tucumán, Argentina
3Undersecretary of health, Ministry of Health, SI.PRO.SA, Tucumán, Argentina
4Public Health Laboratory, Ministry of Health, SI.PRO.SA, Tucumán, Argentina
5Maternity institute Ministry of Health, SI.PRO.SA, Tucumán, Argentina
6Health Research Institute, Ministry of Health, SI.PRO.SA, Tucumán, Argentina
7Health Research Institute, Ministry of Health, SI.PRO.SA, Tucumán, Argentina, Superior Institute of Biological Research (INSIBIO – CCT CONICET) Argentina
*Corresponding Author: Maria Peral de Bruno, Ph.D., Health Research Institute, Ministry of Health, Virgen de La Merced 189, (4000) San Miguel de Tucumán, Tucumán, Argentina.
Received: 10 March 2023; Accepted: 17 March 2023; Published: 27 March 2023
Article Information
Citation: Medina Ruiz Luis, Ferre Contreras Miguel, Molina Eliana, Aguilar Mónica, Costas Dardo, Aznar Patricia, Alcorta María Elena, Lara Laura, Barrenechea Guillermo Gabriel, Ortega Eugenia Silvana and Peral de Bruno María de los Ángeles. Effect of the Third Dose of Astrazeneca Vaccine on the Ability to Generate Anti-Rbd Antibodies within Two at-Risk Populations, Geriatrics and Health Personnel in The Province of Tucumán. Archives of Internal Medicine Research 6 (2023): 20-27.
View / Download Pdf Share at FacebookAbstract
This study aims to evaluate the effect on the ability to generate Anti- Anti RBD antibodies (Anti-SAR-CoV 2-Spike-RBD) to the third dose of the AstraZeneca vaccine in two at-risk populations in the Province of Tucumán. Humoral immune responses, assayed by anti-SARS-CoV- 2-Spike-RBD IgG ELISA, were measured in 223 participants, including geriatric patients and healthcare workers, at 0, 14 and 28 days after receiving third dose of the AstraZeneca between October and December 2021 in Tucuman, Argentina. The antibodies title in geriatric patients with and without previous COVID-19 diseases and complete vaccine schedule increased significantly 14 days after receiving the third dose of the AstraZeneca vaccine (p<0.001). When compared Antibody Concentration in geriatric patients with and without a history of COVID-19 14 days postvaccination, the increase in antibodies was higher in those who did not have a previous history of COVID-19 (p<0.05). The antibodies title in healthcare personnel with and without previous COVID-19 diseases and complete vaccination increased significantly 14 days post-vaccination with the third dose of AstraZeneca (p<0.01). There was a negative correlation between age and antibodies titer 14 days after the third dose of COVID vaccine in persons with no history of disease (rs= -0.36, p=0.0001). While persons with a history of COVID-19 did not have a significant correlation (rs= -0.03; p=0.8). The results obtained provide information on antibodies dynamics after the third dose with AstraZeneca in a context of vaccine heterogeneity, indicating the importance of baseline studies of antibodies and hybrid immunity when considering vaccination policies.
Keywords
<p style="text-align:justify">COVID-19; Anti-SARS-CoV-2-Spike-RBD; SPUTNIK V; AstraZeneca; Humoral immune responses</p>
Article Details
Introduction
In late December 2019, the incidence of atypical pneumonia cases of unknown cause was reported in the Chinese city of Wuhan. Since then, a successive series of spreads have been generated on a global scale what would be the COVID-19 pandemic, which represents the largest global public health crisis of this generation, and potentially, since the 1918 pandemic influenza outbreak [1].
The need to control the transmissibility and mortality associated with SARS-CoV-2 has made vaccines a critical resource to mitigate the devastating effects of the current pandemic. The emergence of new variants reinforces the need to accelerate vaccination rates in all countries. However, despite the speed of research, development and implementation of different vaccines worldwide remains limited as regards their availability in developing countries [2, 3].
Serological testing for SARS-CoV-2 employs enzyme-linked immunosorbent assay (ELISA), immunofluorescence and lateral flow techniques to detect antibodies directed against the nucleocapsid (N) and/or spike (S) proteins of the viral proteome. The S protein, which plays a key role in receptor recognition and in the cell membrane fusion process, is composed of two subunits, S1 and S2. The S1 subunit contains a receptor binding domain (RBD) which recognizes and binds to the host receptor (angiotensin-converting enzyme 2 [ACE2]). The N protein plays a vital role in transcription and replication and, because of its abundance, it has been suggested to be the ideal antigen for detecting early infections [4]. IgG antibodies directed to S have been shown to be more specific, whereas those directed against N may be more abundant at the earliest stage of infection. The sensitivity and specificity of most serological tests have been validated with specimens drawn from patients who endure the acute phase of infection. It has been assumed that determination of antibody responses against either protein would be equally adequate in population-based seroprevalence studies. However, anti-S and anti-RBD antibodies correlate better with virus neutralization [4]. This has been determined by virus neutralization testing (VNT) (the gold standard for measuring neutralization). Because this assay is very laborious and requires highly trained personnel working under BSL3 conditions, it is not suitable for high-throughput detections. In contrast, ELISA with recombinant S and/or RBD as substrate show a strong correlation with neutralization assay results. Furthermore, as most vaccines approved to date carry the genetic information of S or its RBD domain, only tests that determine anti-S or anti-RBD antibodies can assess individual and population seroconversions triggered by vaccination. The use of RBD not only streamlines the diagnostic kit production process, but also provides a more specific target for neutralizing antibodies. Therefore, determination of anti-S or anti-RBD antibody titers, rather than anti-N, provides more valuable information.
Tucumán is a province located in northwestern Argentina, and one of the most densely populated in the country. It has been one of the cities most affected by the pandemic, with more than 143,000 infections and 2,400 deaths due to COVID in a population of 1.5 million inhabitants. Currently, while the Government is moving forward with more relaxations and the announcement of a reinforcement of doses against Covid-19, Tucumán continues to add consecutive days without deaths. Although new cases continue to be confirmed in Tucumán, the number of infected people reached 199,694, 194,287 recovered and 3,430 patients died since the beginning of the pandemic.
During the year 2021, after the second wave that had a milder upward profile, incidence peaked between Epidemiological Weeks 20 and 24, and then declined. On the other hand, we are currently in a stage of community transmission of the Omicron strain. An example of this is the continuous epidemiological surveillance carried out by the Ministry of Public Health, through which it was observed that in the town of Famaillá, confirmed patients for Covid with the Delta variant were detected, which were processed in the Public Health Laboratory of Tucumán. In these cases, patients were isolated, followed up and medically treated by the Ministry (asymptomatic or with mild symptomatology patients).
Since the initial genomic characterization of SARSCoV-2, the virus has generated different genetic variants. They are classified into two major groups: Variants of Concern (VOC) and Variants of Interest (VOI). One of the objectives of genomic surveillance is to monitor the geographical and temporal trends of SARS-CoV-2 variants circulating in Argentina. Samples are processed at the National Reference Laboratory (ANLIS - Dr. Carlos C. Malbrán), and it is performed according to epidemiological surveillance criteria (traveler surveillance, unusual events, population sampling). So far (October 2021), out of a total of 484 samples analyzed by genomic sequencing, 81% corresponded to the VOC group.
In this context of new variants and the same already circulating in the community in our province, it became necessary to reinforce the vaccination schedule. Tucumán presents heterogeneity of vaccines applied, but this work took this situation into account and worked with relatively homogeneous groups. In this case, the anti-RBD antibody response was studied in two different population groups, a group of geriatric patients who had received two doses of AstraZeneca and received a third dose of the same vaccine, and on the other hand, a group of health personnel who had received two doses of Sputnik V and received AstraZeneca in the third dose. In both cases, the third dose corresponded to AstraZeneca.
Primary Objective
To evaluate the effect on the ability to generate Anti-Anti RBD antibodies (Anti-SAR-CoV 2-Spike-RBD) to the third dose of the AstraZeneca vaccine in two at-risk populations in the Province of Tucumán.
Secondary Objectives
- To study the presence of hybrid humoral immunity, determining if the history of previous COVID disease can modify or potentiate the titers reached after the application of the third dose of Anti-Anti RBD antibodies.
- To study by follow-up, the permanence of Anti-Anti RBD antibodies by means of repeated measurements determining their titers at day zero or inoculation day, at day 14 and at day 28.
Materials and Methods
A follow-up study was performed after the administration of the third dose of the AstraZeneca vaccine. The time of vaccine administration was considered as time 0 of the follow-up time (day zero) and then the control was performed at 14 and 28 days.
The selected populations, although with different profiles Geriatric Population and Healthcare Personnel, have the characteristic of being higher risk populations. To evaluate the effect of the third dose of the vaccine, the concentration of Anti-RBD antibodies was measured by IgG dosage through the chemiluminescent microparticle immunoassay (CMIA; SARS-CoV-2 IgG [ARCHITECT System; Abbott Laboratories, Abbott Park, IL, USA]) at the time of administration of the third dose and 28 days later.
Population
The populations were made up of two groups, one corresponding to geriatric patients and the other group was made up of people belonging to the personnel working in health care centers of the health system of Tucumán Si.Pro.Sa (Ministry of Health of Tucumán). Both groups at the beginning of the study, met the corresponding inclusion/exclusion criteria. Both groups agreed to the administration of the third dose having at least 3 months after having received the primary scheme. In addition, the effect of the third dose was taken into account and compared between those who had a history of previous COVID-19 and those who did not in each group.
This study was approved by the Research Committee of the Public Health System of Tucumán. The Ethics Committee of the Ministry of Public Health of the Province of Tucumán evaluated and approved the research protocol of the present study (File: 9338/410-SEM-2021; Opinion: 47/2021) following the Declaration of Helsinki. All enrolled volunteers were duly informed about their choice to participate in the study and provided a signed informed consent. The personal data of all volunteers was encrypted.
Inclusion Criteria
Voluntary participants belonging to the health personnel of Si.Pro.Sa (Ministry of Health of Tucumán) and the geriatric population of the province of Tucumán in both cases with or without pre-existing pathologies of either sex who meet the following conditions:
- Who are older than 18 years old.
- Who have been vaccinated with both components of any vaccine against COVID and who have completed a minimum interval of not less than 3 months since the last dose administration. The maximum interval since the application of the second dose should be between 6 and 12 months.
- Whether or not they have had previous coronavirus disease recorded and diagnosed by PCR (+) or antigen test (+) with an evolution of more than 30 days.
- Who have consented to the study by signing an informed consent form.
Exclusion Criteria
Patients with:
- Hematologic diseases of vascular compromise or coagulopathies.
- Those who have reported serious adverse events to any vaccine (ESAVI: adverse event presumably attributable to vaccination and immunization).
- Patients who have not completed and/or signed the informed consent form.
Identity protection
Patient information will be encrypted. Analysts will use only anonymized data with no identifiable patient details in publications.
Antibody dosage
For the dosage of Anti-RBD IgG, a 5 ml blood sample was taken at each time point (day zero, 14 and 28 days). Antibody measurement was then performed by chemiluminescent microparticle immunoassay for the qualitative detection of IgG against the SARS-CoV-2 nucleoprotein (SARS-CoV-2 IgG for use with ARCHITECT; Abbott Laboratories, Abbott Park, IL, USA).
Technique
For the detection of antibodies, serum samples were used to develop immunoassay techniques by chemiluminescence or ELISA. Antibodies are generated against different parts of the virus; however, immunodominant antigens stand out for the early diagnosis of COVID-19: they are the N proteins of the nucleocapsid, since these proteins are the most abundant in the virus. However, other antibodies such as RBD-S ("Receptor Binding Domain of S"), which allow binding to host cells, could be more specific and disease neutralizing. Positive interpretation has been defined as IgM positive or convalescent serum with a fourfold increase in IgG titer compared to the acute phase. Although the presence of neutralizing antibodies can only be confirmed by a plaque reduction neutralization test, there is evidence of positive correlation of elevated IgG titers with the presence of neutralizing antibodies. In this method, time must be considered as an important variable when interpreting the results. Some recent ELISA studies have shown that both IgM and IgG rise simultaneously around the fourth day of symptom onset, but that elevated levels begin to occur in the second and third week. The difference between the two is that the IgM curve begins to decrease more rapidly from week 5 and disappears by week 7, while IgG levels remain elevated and persist beyond week 7. For the chemiluminescent microparticle immunoassay test for the qualitative detection of IgG against the SARS-CoV-2 nucleoprotein, the ARCHITECT system (ARCHITECT; Abbott Laboratories, Abbott Park, IL, USA; reference 06R8620) will be used. This immunoassay test was selected since numerous studies of various high-throughput serological kits at the National Center for Microbiology, including ELISA and chemiluminescent immunoassays.
The amount of IgG antibody against SARS-CoV-2 in each sample is determined by comparing its chemiluminescent relative light unit (RLU) to the calibrator RLU (S/C index). Using an S/C index threshold of 1-4, the manufacturer reported a sensitivity of 89-7% at 7 days after symptom onset and 100% at 14 days, and a specificity of 99-6%, using RT-PCR as the reference standard. IgG testing of serum samples was analyzed on the Abbott Architect instrument using the Abbott SARS-CoV-2 IgG assay after FDA notification following the manufacturer's instructions. The assay is a chemiluminescent microparticle immunoassay for the qualitative detection of IgG in human serum or plasma against the SARS-CoV-2 nucleoprotein. The Architect platform requires a minimum of 100 µL of serum or plasma.
Statistic analysis
Data were expressed as mean ± standard error. Values of p < 0.05 were considered statistically significant. Statistical 5.0 and Graph-Pad Prism 5.02 software were used for statistical analysis and graphics. The different statistical tests used are described in all cases in the legend of the figure.
Results
The concentration of Anti-Anti RBD antibodies (Anti-SAR-CoV 2-Spike-RBD) was evaluated in 223 volunteer participants, including geriatric patients and healthcare personnel, who received a third dose of SARS-CoV-2 vaccine (AstraZeneca) in Tucumán, Argentina. Demographic data of the study participants are shown in Table 1.
|
Characteristics |
Geriatric |
Health Personnel |
|
|
N° of study participants |
0 days 14 days 28 days |
150 97 91 |
73 47 46 |
|
Sex |
Men Women |
33 (34.02) 64 (65.98) |
15 (20.55) 58 (79.45) |
|
Age |
Median IQR25 IQR75 |
80 74 86 |
43 37 54 |
|
Health Background |
Hypertension Diabetes Hypothyroidism Obesity Asthma Alzheimer's Dementia |
47 (48.45) 9 (9.28) 3 (3.09) 0 (0) 0 (0) 19 (19.59) 15 (15.46) |
5 (6.85) 2 (2.74) 3 (4.11) 2 (2.74) 3 (4.11) 0 (0) 0 (0) |
|
Pre-detection of SARS-Cov-2 (PCR-RT or Rapid Ag Test) |
YES NO |
53 (35.33) 97 (64.66) |
36 (49.32) 37 (50.68) |
Table 1: Demographic data of the study participants. The numbers in parentheses correspond to the percentage in each case.
49% (37/73) of the healthcare personnel participants reported having had a previous COVID-19 diagnosis (either a PCR-RT or a Rapid Ag Test) (Table 1).
48% (47/97) of geriatric patients had hypertension or baseline cardiovascular disease (Table 1).
Geriatric population
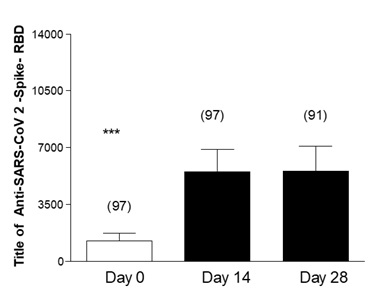
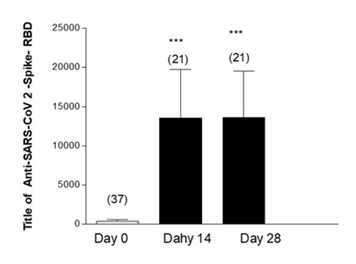
Figure 1 shows the measurement of Anti-Anti RBD antibodies in geriatric patients who had no history of COVID-19 prior to vaccination. The baseline antibody levels of the geriatric participants were measured, finding an average of 1267 Anti-Anti RBD. This concentration increased significantly 14 days after receiving the third dose of the AstraZeneca vaccine, where a second measurement of antibodies was performed, registering an average of 5529 Anti-Anti RBD. In the third measurement, taken 28 days after vaccination, the increase in the average antibody level was not significant (5561).
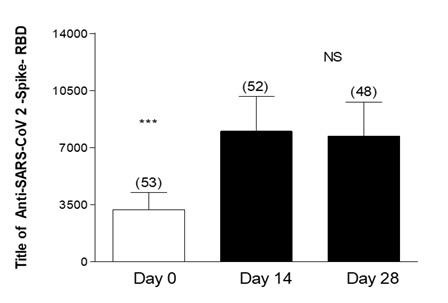
Figure 2 shows the measurement of Anti-Anti RBD antibodies in geriatric patients with a history of COVID-19 prior to vaccination. These patients presented an average antibody titer of 3202 in the baseline measurement. In the evaluation performed 14 days after vaccination, a significant increase in the antibody titer was observed, 8004 (p<0.001), this increase was maintained without significant differences in the third measurement performed at 28 days.
Figure 2: Effectiveness of third dose of ASTRAZENECA vaccine and presence of antibodies in Geriatric patients with previous COVID-19 and complete vaccine schedule. Title of Anti-SARS-CoV 2-Spike-RBD in geriatric patients with previous COVID-19 disease. Day 0, 14 and 28 post vaccine inoculation. Test: ANOVA one way; Newman-Keuls Multiple Comparison Post-Test. ***p<0.001 Day 0 Vs14 and 28; Day 14 Vs 28 Not Significant (NS).
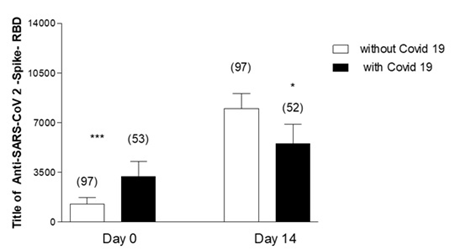
Figure 3 shows the Comparison of Anti-Anti RBD Antibody Concentration in geriatric patients with and without a history of COVID-19 post-vaccination. In the baseline measurement, these levels are higher in those with a previous history of the disease, however, in the 14-day post-vaccination measurement the increase in antibodies is higher in those who did not have a previous history of COVID-19.
Figure 3: Effectiveness of third dose of ASTRAZENCA vaccine and presence of antibodies in Geriatric patients with Vs without previous COVID-19 and with complete vaccine schedule. Anti-SARS-CoV 2- Spike-RBD in geriatrics patients without and with previous COVID-19 disease. Day 0: Vaccine inoculation. Unpaired Test t. ***p<0.001 Day 0 without COVID-19 Vs with COVID-19 *p<0.05 Day 14 without Vs with COVID-19.
Healthcare personnel
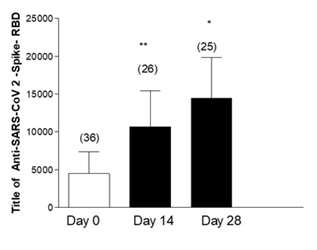
Figure 4 shows the Anti-Anti RBD antibodies in healthcare personnel who had a history of COVID-19 prior to vaccination. They were also found to have a significant increase in Anti-Anti RBD antibody levels at 14 days post-vaccination with the third dose of AstraZeneca, which was maintained at 28 days.
Figure 4: Effectiveness of the third dose of ASTRAZENECA vaccine and presence of antibodies in health personnel with previous COVID-19 and with a complete SPUTNIK scheme. Title of Anti-SARS-CoV 2-Spike-RBD in health personnel with previous COVID-19 disease. Day 0 vaccine inoculation Test ANOVA one way. Post Test Newman-Keuls. **p<0.01 Day 0 Vs 14; p<0.05 Day 0 Vs 28; Not Significant (NS) Day 14 Vs 28.
Figure 5 shows the Anti-Anti RBD antibodies in healthcare personnel with no history of Covid prior to vaccination. These presented a significant increase at day 14 measurement, maintaining these elevated Anti-Anti RBD titers at similar values at days 28 relative to those at days 14 (P: NS).
Figure 5: Effectiveness of third dose of ASTRAZENECA vaccine and presence of antibodies in healthcare personnel without previous COVID-19 disease and with complete vaccine scheme. Title of Anti-SARS-CoV 2-Spike-RBD in healthcare personnel without previous COVID-19 disease. Day 0: Vaccine inoculation, Test ANOVA one way and Post-test Newman-Keuls. ***p<0.001 Day 0 Vs 14; p<0.001 Day 0 Vs 28; NS (not significant) Day 14 Vs 28.
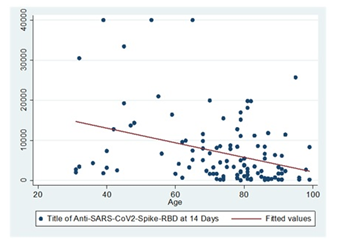
Age and the title of Anti-Anti RBD antibodies: There was a negative correlation between age and the title of antibodies 14 days after the third dose of COVID vaccine in persons with no history of disease (rs=-0.36, p=0.0001). As age increases, the titer of Anti-Anti RBD antibodies produced after a third dose of COVID vaccine in persons with no history of disease decreases.
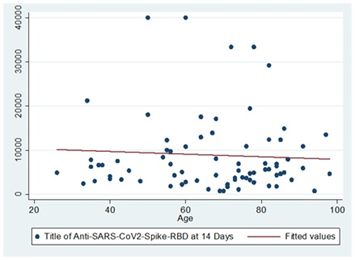
While persons with a history of COVID-19 who received a third dose of the vaccine had a negative correlation between age and Anti-Anti RBD antibody number 14 days after receiving the vaccine, but this was not significant (rs= -0.03; p=0.8).
Discussion
The mass immunization campaign against COVID-19 resulted in a change in the course of the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). [6]
In this study, we worked with two populations: Geriatric and Health Personnel, which have the characteristic of being high-risk populations due to their environment or context in which they are located. The main finding was that the third dose of ASTRAZENECA vaccine was effective in the ability to generate anti-RBD antibodies regardless of having had or not previous COVID in these two at-risk populations, geriatrics and health personnel in the province of Tucumán.
Our work focused on studying these two populations considered at risk: health personnel of the Public Health System of Tucumán and elderly people residing in nursing homes in the province, during the months of October - November, the period in which the application of the third dose was implemented in the province. The choice of these groups was due to the fact that, in most countries, health personnel were among the first groups to receive vaccines against COVID-19 because of their greater exposure to the virus and because of the essential tasks performed during the pandemic. [7, 8] While the choice of the elderly group was due to the fact that they present alterations in the immune system that make them more susceptible to infections and the development of chronic inflammatory states, which could reduce the efficacy of the response to vaccination. [9, 10].
With the use of COVID-19 vaccines from different platforms around the world, the question arose as to what type of humoral immune response individuals recovered from previous SARS-CoV-2 infection would have, compared to those without previous contact, which is called hybrid. [11]
According to work performed with mRNA vaccines, it was shown that, in previously infected individuals, the first doses essentially act as boosters. [12-14] This phenomenon was also reported for adenovirus-based platforms. [15, 16] Rossi et al. conducted a study with health care workers vaccinated with SPUTNIK V in Buenos Aires, which allowed them to suggest that those individuals who received one dose and were seropositive at baseline obtained higher titers than those volunteers who received two doses of vaccine and were seronegative at baseline. Thus, a second dose in previously infected individuals would provide no apparent benefit. [17] Similar behavior was demonstrated for the SPUTNIK V vaccine in the work performed by Chahla et al., 2021 [18] in our province, since individuals who had a previous SARS-CoV-2 infection obtained higher antibody titers than those who did not report having had the disease. In this study, which we conducted with the ASTRA ZENECA vaccine, a similar immune response was observed in geriatric patients, but not in health personnel, where individuals with no previous history of COVID-19 but with a complete schedule with SPUTNIK V had a higher antibody titer.
Although individuals who were vaccinated with SPUTNIK V and had a previous infection with SARS-Cov-2 had higher anti-RBD titers, not all responded equally. Only those with baseline anti-RBD IgG levels above 400 consistently achieved elevated titers after the first dose (median 2000), which increased further after the second dose (median 3600) [18].
It should be noted that our province is characterized by a high adherence of both health personnel and senior citizens to the vaccination campaign promoted by multiple mass media. Press and television media strongly supported the Ministry of Health of the Province, which made it possible for them to participate (close to 100%).
In this work it was observed that, as age increases, the titer of antibodies produced after a third dose of COVID vaccine decreases in people with no previous history of the disease. Whereas, in persons with a history of COVID-19 disease who received a booster vaccine, the age-related decrease in antibody titer was not significant. This could follow along lines with the work of Pilz et al., 2022 [19], where based on the review of studies conducted on efficacy and duration of immunity it is concluded that hybrid immunity, combination of a previous SARS-CoV-2 infection and respective vaccination, seems to confer the highest protection against SARS-CoV-2 infections, but several knowledge gaps remain in this regard.
Conclusion
The results obtained provide information on antibody dynamics after the third dose with AstraZeneca in a context of vaccine heterogeneity, indicating the importance of baseline studies of antibodies and hybrid immunity when considering vaccination policies.
References
- https://www.who.int/es/news/item/29-06-2020-covidtimeline.
- Boehm, E., Kronig, I., Neher, R.A., et al. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect 2021.
- Fontanet, A., Autran, B., Lina, B., Kieny, M.P., Karim, S.S.A. and Sridhar, D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 2021; 397(10278): 952-4.
- Ministerio de Salud de la Nación, Argentina. Consenso sobre el uso de pruebas diagnósticas para SARS-CoV-2. https://bancos.salud.gob.ar/sites/default/files/2020-09/covid-19-consenso-sobre-uso-de-pruebas-diagnosticas-para-sars-cov-2.pdf
- Ojeda, D.S. Detección y titulación de anticuerpos anti-spike y neutralizantes para la infección con sars-cov-2. Fundación Instituto Leloir Instituto de Investigaciones Bioquímicas de Buenos Aires (IIBBA-CONICET).https://www.argentina.gob.ar/sites/default/files/informe_covidar_titulaciones_igg_2021.01.20.pdf
- Petras, M., 2021. Highly effective naturally acquired protection against COVID-19 persists for at least 1 Year: a meta-analysis. Am. Med. Dir. Assoc. 22, 2263–2265.
- Bandyopadhyay, S., Baticulon, R.E., Kadhum, M., et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health 2020; 5(12): e003097. https://doi.org/10.1136/bmjgh-2020-003097.
- Khunti, K., Kamal, A., Pareek, M. and Griffiths, A. Should vaccination for healthcare workers be mandatory? J R Soc Med 2021;114(5):235–6.
- Pawelec, G., Akbar, A., Caruso, C., Effros, R., Grubeck-Loebenstein,B. and Wikby, A. Is immunosenescence infectious? Trends in Immunology 2004 25: 406-410.
- Fulop, T., Larbi, A., Hirokawa, K., Mocchegiani, E., Lesourds,B., Castle, S., Wikby, A., Franceschi, C. and Pawelec, G.. Immunosupportive therapies in aging. Clinical Intervention Aging 2007- 2: 33-54.
- Dolgin, E. Is one vaccine dose enough if you've had COVID? What the science says. Nature 2021; 595(7866):161–2.
- Krammer, F., Srivastava, K., Alshammary, H., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021;384(14):1372–4.
- Prendecki, M., Clarke, C., Brown, J., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021;397(10280):1178–81.
- Saadat, S., Rikhtegaran Tehrani, Z., Logue, J., et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA 2021;325 (14):1467–9.
- Claro, F., Silva, D., Rodriguez, M., Rangel, R., de Waard, J.H. IgG antibody response to the SPUTNIK V vaccine: previous SARS-CoV-2 seropositive individuals might need just one vaccine dose. Int J Infect Dis 2021. https://doi.org/10.1016/j.ijid.2021.07.070.
- Eyre, D.W., Lumley, S.F., Wei, J., et al. Quantitative SARS-CoV-2 antispike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect 2021. https://doi.org/10.1016/j.cmi.2021.05.041.
- Rossi, A.H., Ojeda, D.S., Varese, A., et al. SPUTNIK V vaccine elicits seroconversion and neutralizing capacity to SARS CoV-2 after a single dose. Cell Rep Med 2021:100359.
- Chahla, R.E., Tomas-Grau, R.H., Cazorla, S.I., et al. Long-term analysis of antibodies elicited by SPUTNIK V: A prospective cohort study in Tucuman, Argentina. The Lancet Regional Health – Americas 2021;6: 100-123. https://doi.org/10.1016/j.lana.2021.100123
- Pilz, S., Theiler-Schwetz V., Trummer C., Krause R. and Ioannidis, J. 2022. SARS-CoV-2 re-infection: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 229: 112911. https://doi.org/10.1016/j.envres.2022.112911


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 