Evaluating the Effectiveness of Vancomycin in Preventing Infections in Spinal Fixation Surgeries
Dr. Md. Mahamudul Hasan1, Prof. Dr. Md. Shah Alam2, Dr. Md Shahidul Islam Akon3, Dr. Sarwar Jahan4, Dr. Sharif Ahmed Junayed5, Dr. Md. Mahabubur Rahman Khan6, Dr. Mohammad Abdul Hannan7, Dr. Abdullah Al Mamun Chowdhury8, Dr. Md. Ziaul Hasan9, Dr. S. M. Zubaer Hasan10
1Assistant Professor, Department of Spine Surgery, Khulna Medical College Hospital Attached to Satkhira Medical College, Satkhira, Bangladesh.
2Chief Consultant, Bangladesh Spine & Orthopaedic Hospital (BSOH), Dhaka, Bangladesh.
3Associate professor (Spine surgery), Dhaka Medical College, Dhaka, Dr Md Shahidul Islam Akon, Associate professor (Spine surgery), Dhaka Medical College, Dhaka, Bangladesh
4Assistant Professor, Department of Spine Surgery, NITOR, Dhaka, Bangladesh.
5Assistant Professor, Department of Spine Surgery, NITOR, Dhaka, Bangladesh.
6Assistant Professor, Department of Orthopedics, NITOR, Dhaka, Bangladesh.
7Assistant Professor of spine surgery, National Institute of Traumatology & Orthopaedic Rehabilitation, Dhaka, Bangladesh
8Senior Consultant, Department of Spine Surgery, NITOR, Dhaka, Bangladesh.
9Indoor Medical Officer, NITOR, Dhaka, Bangladesh.
10Senior Consultant, Department of Anaesthesia, National Institute of Traumatology and Orthopedic Rehabilitation (NITOR), Dhaka, Bangladesh
*Corresponding Author: Dr. Md. Mahamudul Hasan, Assistant Professor, Department of Spine Surgery, Khulna Medical College Hospital Attached to Satkhira Medical College, Satkhira, Bangladesh.
Received: 23 June 2025; Accepted: 27 June 2025; Published: 08 July 2025
Article Information
Citation: Dr. Md. Mahamudul Hasan, Prof. Dr. Md. Shah Alam, Dr. Md Shahidul Islam Akon, Dr. Sarwar Jahan, Dr. Sharif Ahmed Junayed, Dr. Md. Mahabubur Rahman Khan, Dr. Mohammad Abdul Hannan, Dr. Abdullah Al Mamun Chowdhury, Dr. Md. Ziaul Hasan, Dr. S. M. Zubaer Hasan. Evaluating the Effectiveness of Vancomycin in Preventing Infections in Spinal Fixation Surgeries. Journal of Spine Research and Surgery. 7 (2025): 73-79.
View / Download Pdf Share at FacebookAbstract
Objective:
This study aimed to evaluate the efficacy of topical vancomycin in preventing surgical site infections (SSI) following spinal fixation surgeries.
Methodology:
This retrospective cohort study was conducted at the Bangladesh Spine & Orthopaedic Hospital, Dhaka, Bangladesh. Participants were divided into two groups: treatment and control. In the treatment group, topical vancomycin powder was applied immediately after spinal surgery. Two-thirds of the powder was applied directly to the bones and muscles at the end of the surgery, while the remaining powder was applied between the fascia and fat layer after fascia closure. The vancomycin dose ranged from 0.5 to 2 grams, depending on wound size and surgery type; 75% of patients received 1 gram of vancomycin.
Results:
A total of 456 patients underwent spinal surgeries in this study. Among them, 81 patients were treated with vancomycin powder, and 375 were assigned to the control group. In total, 28 cases of SSIs were observed across both groups: 8 cases in the vancomycin group and 20 in the control group. The SSI incidence rate was higher in the treatment group (9.9%) compared to the control group (5.3%), with infections typically occurring within 14 days post-surgery. Of the reported SSIs, 43% were superficial, while 57% were classified as deep infections.
Conclusion:
In conclusion, this study did not find evidence supporting the efficacy of topical vancomycin powder in preventing SSIs following spinal fixation surgeries. However, no adverse effects were observed during the study period.
Keywords
<p>Surgical site infection; spinal surgeries; vancomycin powder</p>
Article Details
Introduction
Surgical site infections (SSIs) are a common complication following spinal surgeries, with a reported prevalence ranging from 3.1% to 13%. [1-3] These infections are defined as occurring within 30 to 90 days post-surgery, particularly in procedures requiring implants. [4] High rates of SSIs not only increase patient morbidity but also impose a significant economic burden on healthcare systems. [5] In the United States, an estimated $10 billion is spent annually on managing SSIs associated with spinal surgeries. Despite substantial financial investments, these infections still result in approximately 8,000 deaths each year. [6]
SSIs are more frequently observed in thoracic spinal surgeries (3.7%) compared to cervical and lumbar surgeries (3.4% and 2.7%, respectively). [1] These infections are associated with poor postoperative recovery and significantly impact patient satisfaction. [7,8] Instrumented surgeries have higher SSI rates compared to decompression surgeries. Several patient-related factors, such as socio-economic status, advanced age, diabetes, hypertension, increased blood loss, prolonged surgical duration, smoking, obesity, and revision surgeries, are linked to an increased risk of SSIs. Patients in developing countries face a higher prevalence of SSIs due to limited access to advanced medical care and lower socio-economic conditions. [9–11]
Prophylactic antibiotics, such as cefazolin, have been effective in reducing SSIs; however, there is a growing concern about the emergence of methicillin-resistant Staphylococcus aureus (MRSA) and coagulase-negative staphylococci (CoNS) following antibiotic use. [12] To address SSI incidents, efforts have been made to improve postoperative care, adopt minimally invasive surgical techniques, and develop new antimicrobial agents. Despite these measures, SSI rates remain concerning. [13] In clinical settings, preventive strategies such as blood glucose control, treatment of urinary tract infections, and nutritional support are implemented. Nevertheless, SSIs often lead to prolonged hospital stays, increased re-operation rates, and higher medical costs. [12-16]
Reducing SSI rates is necessary to decreasing morbidity and mortality associated with spinal surgeries. Recently, the use of vancomycin powder applied directly to spinal incisions has shown promise in lowering SSI rates without significant side effects. Previous studies [15] have highlighted vancomycin’s antibacterial effectiveness, with its concentration peaking within 2 hours post-surgery and remaining detectable for up to 7 days. These studies reported positive outcomes, with no associated deaths or severe complications. Although numerous studies have evaluated vancomycin’s efficacy in preventing SSIs across various spinal surgeries, significant variability in findings has been noted. Hence, this study aimed to evaluate the efficacy of topical vancomycin in preventing surgical site infections (SSI) following spinal fixation surgeries.
Methodology:
Study Design
This retrospective cohort study evaluated patients who underwent spinal surgeries between 2019 and 2024. A total of 456 patients were included, and data were extracted from the Bangladesh Spine & Orthopaedic Hospital, Dhaka, Bangladesh, Dhaka, Bangladesh, which provided comprehensive information on patients’ socio-demographic characteristics, laboratory investigation results, types of surgeries, readmission timings, follow-up durations, and outcomes. Laboratory results, including culture sensitivity findings, were reviewed. Additional data, such as surgery duration, intraoperative drug usage, and vital signs, were retrieved from patients’ original medical charts.
Population and Sample Selection
The study included 456 patients who underwent spinal surgeries during the study period. The inclusion criteria encompassed patients receiving standard prophylactic intravenous cefazolin treatment. Patients who had received prolonged post-surgical antibiotic treatment were excluded. A power analysis was conducted to determine the appropriate sample size. Based on an expected infection rate of 15% in the control group and a desired 80% power to detect a 10% difference in infection rates between the treatment and control groups, a sample size of 450 patients was deemed sufficient. A total of 456 patients met the inclusion criteria and were included in the study.
Data Collection
The treatment group received topical Vancomycin immediately after surgery. At the end of the procedure, two-thirds of the Vancomycin powder (ranging from 0.5 to 2 grams depending on wound size and surgery type) was applied directly to bones and muscles. The remaining powder was applied between the fascia and the fat layer following fascia closure. The control group did not receive topical Vancomycin but received standard prophylactic intravenous cefazolin administration. Baseline data, including patient demographics (age, sex, BMI, comorbidities such as diabetes and hypertension), surgery type, and intraoperative details, were extracted from the hospital records. Data on preoperative methicillin-resistant Staphylococcus aureus (MRSA) screening were also collected, including the identification of colonization in two patients. The primary outcome was the occurrence of surgical site infection (SSI), defined based on CDC criteria, which classifies SSIs as superficial or deep depending on the level of tissue involvement. Secondary outcomes included the duration of hospital stay, need for reoperation, and readmission rates.
Statistical Analysis
The primary hypothesis of this study was that topical vancomycin reduces the incidence of SSI following spinal surgeries. To test this, we utilized a logistic regression model, calculating odds ratios (ORs) to estimate relative risk. Sensitivity analysis, including propensity score adjustment, was performed to address potential bias due to the preferential use of topical vancomycin in high-risk patients. Descriptive statistics, including frequency distributions, were used to summarize infection prevalence and patient demographics. A p-value of <0.05 was considered statistically significant. All analyses were conducted using SPSS version 23.0.
Results:
Table 1 provides a detailed demographic analysis of both groups. In this study, a total of 456 patients underwent spinal surgeries, of which 81 were treated with vancomycin powder, and 375 were placed in the control group. The mean age of the treatment group was 56.02 ± 19.58 years with a body mass index (BMI) of 24.97 ± 4.08, while the control group had a mean age of 57.08 ± 8.29 years and a BMI of 25.69 ± 1.95. Among the study participants, 343 (75.2%) underwent lumbar surgeries, 58 (12.7%) had cervical surgeries, 45 (9.8%) had thoracolumbar surgeries, and 10 (2.19%) underwent thoracic surgeries. The majority of patients had never smoked, and normal renal function was observed in 98.6% of cases (450 out of 456). Additionally, 127 (27.8%) patients had hypertension, 84 (18.4%) had diabetes mellitus, 73 (16%) had a history of spinal surgery, 41 (8.9%) had used antibiotics within 90 days prior to the study, and 7 (1.5%) had a history of infections. Prolonged surgical duration was identified as a risk factor for surgical site infections (SSIs) in the treatment group.
Table 1: Demographic Characteristics of patients.
|
Characteristics |
Treatment group |
Control group |
p-value |
|
Age in years |
56.02 ± 19.58 |
57.08 ± 8.29 |
0.06 |
|
Sex |
0.19 |
||
|
Female |
34 (42%) |
126 (33.5%) |
|
|
Male |
47 (58%) |
249 (66.4%) |
|
|
Body mass index |
24.97 ± 4.08 |
25.69 ± 1.95 |
0.52 |
|
Surgery type |
< 0.01 |
||
|
Cervical |
3 (4%) |
55 (15%) |
|
|
Lumbar |
58 (72%) |
285 (76%) |
|
|
Thoracolumbar |
15 (18%) |
30 (8%) |
|
|
Thoracic |
5 (6%) |
5 (1%) |
|
|
Smoking status |
0.12 |
||
|
Former smokers |
2 (3%) |
18 (5%) |
|
|
Never smoke |
60 (74%) |
232 (62%) |
|
|
Current smokers |
19 (23%) |
125 (33.3%) |
|
|
Renal function |
0.28 |
||
|
Abnormal |
2 (2%) |
4 (1%) |
|
|
Normal |
79 (98%) |
371 (99%) |
|
|
Hypertension |
29 (36%) |
98 (26%) |
0.1 |
|
Diabetes mellitus |
20 (25%) |
64 (17%) |
0.14 |
|
History of antibiotic |
13 (16%) |
28 (8%) |
0.06 |
|
History of spinal surgeries |
19 (23%) |
54 (14%) |
0.03 |
|
History of spinal infection |
4 (5%) |
3 (1%) |
0.02 |
|
Prolonged surgical duration |
71 (88%) |
29 (8%) |
< 0.01 |
The majority of surgeries were performed using a posterior approach, with 72 (89%) of the treatment group receiving implants. Two-thirds of the study population were administered a prophylactic gentamicin solution for irrigation (Table 2).
Table 2: Clinical presentation of both groups
|
Treatment group (N=81) |
Control group (N=375) |
p-value |
|
|
Surgical approach |
< 0.01 |
||
|
Posterior |
81(100%) |
344 (92%) |
|
|
Anterior |
0 |
31 (8%) |
|
|
Number of implants |
72 (89%) |
184 (49%) |
< 0.01 |
|
Prophylactic gentamicin solution for irrigation |
68 |
281 |
|
|
Disinfectant by Alchol |
70 (86%) |
303 (81%) |
|
|
Pre-surgical white blood cells count |
0.08 |
||
|
Median |
7.5 |
8.2 |
|
|
IQR |
3.8 |
3.1 |
|
|
Pre-surgical neutrophil counts (%) |
0.13 |
||
|
Median |
54.7 |
57.4 |
|
|
IQR |
13.9 |
13.9 |
|
A total of 28 cases of SSIs were observed across both groups: 8 in the vancomycin group and 20 in the control group. The SSI incident rate was higher in the treatment group (9.9%) compared to the control group (5.3%), with infections occurring within 14 days. Of the SSI cases, 43% were superficial, and 57% were deep infections (Table 3).
Table 3: Surgical site infection microbiological analysis
|
Isolated microbe |
Superficial infections (N=15) |
Deep infection (N= 21) |
|
Acinetobacter baumannii |
0 |
1 (4.76%) |
|
Methicillin-susceptible Staphylococcus aureus |
5 (33.3%) |
3 (14.28%) |
|
Mycobacterium tuberculosis |
0 |
2 (9.5%) |
|
MRSA |
0 |
4 (19%) |
|
Pseudomonas aeruginosa |
2 (13.3%) |
4 (19%) |
|
Coagulase-negative Staphylococci |
4 (26.6%) |
0 |
|
Morganella morganii |
0 |
2 (9.5%) |
|
Klebsiella pneumoniae |
0 |
1 (4.76%) |
|
Enterobacter cloacae |
0 |
1 (4.76%) |
|
Escherichia coli |
2 (13.3%) |
0 |
|
Escherichia coli -ESBL |
1 (6.66%) |
1 (4.76%) |
|
Klebsiella pneumoniae –ESBL |
1 (6.66%) |
2 (9.5%) |
The microbiological analysis of surgical site infections (SSIs) reveals the distribution of isolated microbes in both the treatment and control groups. In the treatment group (n=11), the most commonly isolated organisms were Methicillin-resistant Staphylococcus aureus (MRSA) and coagulase-negative Staphylococci, each accounting for 18% of isolates, followed by Escherichia coli (18%) and other microbes such as Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae (each 9%). In contrast, the control group (n=25) showed a higher prevalence of Methicillin-susceptible Staphylococcus aureus (28%), Pseudomonas aeruginosa (20%), and Klebsiella pneumoniae (12%), with fewer cases of MRSA (8%) and coagulase-negative Staphylococci (8%). Overall, the total number of isolates (n=36) includes a diverse range of pathogens, with Methicillin-susceptible Staphylococcus aureus (22.2%) being the most prevalent, followed by Pseudomonas aeruginosa (16.6%) and MRSA (11.1%). Other microbes, such as Mycobacterium tuberculosis and Enterobacter cloacae, were isolated less frequently (Table 4).
Table 4: Isolated microbes of SSIs
|
Isolated microbe |
Treatment group (n=11) % |
Control group (n=25) % |
Total numbet of isolates (n=36) % |
|
Acinetobacter baumannii |
1 (9%) |
0 |
1 (2.77%) |
|
Methicillin-susceptible Staphylococcus aureus |
1 (9%) |
7 (28%) |
8 (22.2%) |
|
Mycobacterium tuberculosis |
0 |
2 (8%) |
2 (5.55%) |
|
MRSA |
2 (18%) |
2 (8%) |
4 (11.1%) |
|
Pseudomonas aeruginosa |
1 (9%) |
5 (20%) |
6 (16.6%) |
|
Coagulase-negative Staphylococci |
2 (18%) |
2 (8%) |
4 (11.1%) |
|
Morganella morganii |
1 (9%) |
1 (4%) |
2 (5.55%) |
|
Klebsiella pneumoniae |
1 (9%) |
3 (12%) |
4 (11.1%) |
|
Enterobacter cloacae |
0 |
1 (4%) |
1 (2.77%) |
|
Escherichia coli |
2 (18%) |
2 (8%) |
4 (11.1%) |
The logistic regression model identified significant predictors of vancomycin use during spinal fixation surgeries. Among the covariates, surgery type (coefficient: 5.008, p < 0.001) and renal function (coefficient: 3.685, p = 0.016) demonstrated statistically significant associations with the likelihood of vancomycin administration. Patients undergoing specific types of surgeries and those with normal renal function were more likely to receive vancomycin. Although other variables, such as age, BMI, and prolonged surgical duration, exhibited large coefficient estimates, their corresponding p-values were not statistically significant, indicating a lack of strong evidence for their association with vancomycin use. Notably, prolonged surgical duration showed a high coefficient (81.746), but its lack of significance (p = 1.000) suggests the influence of multicollinearity or limited subgroup representation. These findings highlight the need to account for surgery type and renal function as critical confounders when evaluating the relationship between vancomycin use and surgical site infections. The weighted logistic regression analysis for the outcome of surgical site infections demonstrated a statistically significant association between vancomycin use and infection risk. The coefficient for vancomycin use was 0.145 (p < 0.001), suggesting that vancomycin use was associated with a reduced likelihood of surgical site infections. The constant term was also significant (coefficient: 0.070, p < 0.001), reflecting the baseline risk of infection after adjusting for propensity scores. These findings underscore the potential efficacy of vancomycin in reducing infection rates in spinal fixation surgeries (Table 5).
Table 5: Logistic regression for propensity score estimation.
|
Covariate |
Coefficient |
p-value |
|
const |
-43.091 |
1.000 |
|
Age |
0.012 |
0.638 |
|
Sex |
-24.300 |
1.000 |
|
BMI |
0.029 |
0.830 |
|
Surgery type |
5.008 |
0.000 |
|
Smoking_Status |
25.134 |
1.000 |
|
Renal Function |
3.685 |
0.016 |
|
Hypertension |
8.463 |
1.000 |
|
Diabetes Mellitus |
12.092 |
1.000 |
|
History of antibiotics |
-0.655 |
0.735 |
|
History of spinal surgeries |
-65.744 |
1.000 |
|
Prolonged surgical duration |
81.746 |
1.000 |
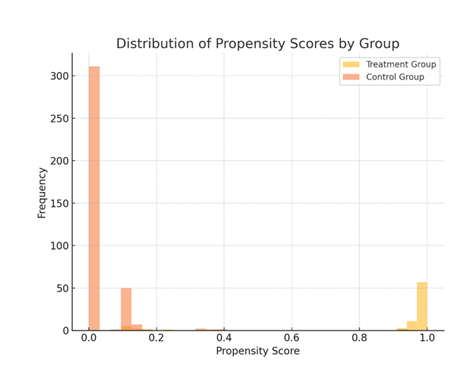
Patients in the treatment group predominantly had propensity scores close to 1, indicating a high likelihood of vancomycin use. In contrast, those in the control group had scores closer to 0, reflecting a low likelihood of vancomycin use. This distinct separation supports the robustness of the logistic regression model in distinguishing between the two groups based on observed covariates.
Discussion:
This study aimed to evaluate the effectiveness of topical vancomycin in preventing surgical site infections (SSI) following spinal surgeries in India. Our sensitivity analysis supports the use of topical vancomycin and underscores the need for validation through multicenter, large-scale studies. We observed 28 cases of SSI, yielding a prevalence rate of 6.9%. In the treatment group, 8 cases of SSI were reported, resulting in a higher infection rate compared to the control group (9.9% vs. 5.3%). These findings align with approximately two-thirds of the previous literature [5–15]. Oktay et al. [18] reported a 6.54% prevalence of SSI in their cohort, with only 1.96% in the treatment group. They identified advanced age and prolonged surgical duration as significant risk factors. Variations in outcomes between studies may be attributed to differences in adjustments for confounders. Few previous studies [19–23] accounted for confounding variables, while some large-scale studies lacked control groups. Our study utilized logistic regression analysis to identify factors influencing the use of vancomycin during spinal fixation surgeries. Among the covariates examined, surgery type (coefficient: 5.008, p < 0.001) and renal function (coefficient: 3.685, p = 0.016) emerged as significant predictors, suggesting that these factors play a crucial role in determining vancomycin administration. While other variables, such as age, BMI, and prolonged surgical duration, demonstrated varying degrees of association, their p-values were not statistically significant, indicating limited evidence of a meaningful relationship. The high coefficients observed for variables like prolonged surgical duration (81.746) and smoking status (25.134) likely reflect multicollinearity or small subgroup effects that limit statistical power. Overall, this model underscores the importance of controlling for surgery type and renal function when assessing vancomycin’s impact on surgical site infections. At the same time, the non-significant variables may require further investigation in larger, more diverse datasets. Our findings are consistent with prior studies that employed propensity score analysis to assess confounding risk factors [1, 23].
Topical vancomycin may influence the surgical site microbiota. In our study, the prevalence of Gram-positive and polymicrobial SSIs was similar across groups, contrasting with earlier studies reporting variations in Gram-positive pathogens [26, 27]. We observed two cases of Escherichia coli, one case of Organelle morganii, and two cases of coagulase-negative staphylococci across both groups. Wang et al. [28] similarly noted an increased presence of Escherichia coli (3/4 cases) in the vancomycin group. Another study suggested that while vancomycin doses of 1.0–2.0 g may not reduce SSI risk, they can alter the predominant bacterial species [29].
No adverse effects of vancomycin were observed in our study. However, prior research reported a 0.3% incidence of adverse events with vancomycin use [29], including complications such as lack of fusion. High doses of vancomycin have been shown to impair bone healing by exerting cytotoxic effects on osteoblasts, as demonstrated in three in vitro studies [9, 33, 34]. Conversely, Rathbone et al. [34] argued that vancomycin is less cytotoxic to osteoblasts than other antibiotics. Mendoza et al. [35] examined the development of pseudoarthrosis following vancomycin use and found no reduction in fusion rates. Similarly, two clinical trials reported no significant changes in pseudoarthrosis rates after vancomycin application [36, 14].
Limitations of the study:
Our study has several limitations, primarily the retrospective cohort design, which may introduce biases in treatment selection and data collection. The absence of randomization and the potential for confounding factors limit the ability to establish causal relationships. Additionally, the reliance on historical data may have allowed for the influence of unmeasured or unknown variables on the outcomes. To address these limitations, future prospective studies with randomized controlled designs are necessary to confirm these findings and further investigate the role of vancomycin, along with other factors, in reducing surgical site infections.
Conclusion:
Despite the use of vancomycin powder, the treatment group experienced a higher rate of surgical site infections (SSIs) compared to the control group. Prolonged surgical duration emerged as a key risk factor for SSIs, particularly within the treatment group. Although vancomycin use appeared to reduce the likelihood of infections in the logistic regression analysis, the results underscore the complexity of infection risk, with factors such as surgery type and renal function playing a significant role. The microbiological analysis revealed a similar bacterial profile in both groups, suggesting that other variables may also contribute to the incidence of SSIs. These findings highlight the need for further research to understand better the multifaceted nature of infection risks and the effectiveness of vancomycin in preventing SSIs in spinal surgeries.
References
- Jiaming Z, Wang R. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. SPINE 45 (2019): 208-16.
- The French Orthopaedic Surgery Traumatology Society. Early surgical site infections in adult spinal trauma: a prospective, multi-center study of infection rates and risk factors. Orthop Traumatol Surg Res 98 (2012): 788-794.
- Saeedinia S, Nouri M, Azarhomayoun A, et al. The incidence and risk factors for surgical site infection after clean spinal operations: a prospective cohort study and review of the literature. Surg Neurol Int 6 (2015): 154.
- World Health Organization WHO. Protocol for surgical site infection surveillance with a focus on settings with limited resources (2018).
- Anderson PA, Savage JW, Vaccaro AR, et al. Prevention of surgical site infection in spine surgery. Neurosurgery 80 (2017): S114-123.
- Hidron AI, Edwards JR, Patel J, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention. Infect Control Hosp Epidemiol 29 (2008): 996-1011.
- Farshad M, Bauer DE, Wechsler C, et al. Risk factors for perioperative morbidity in spine surgeries of different complexities: A multivariate analysis of 1,009 consecutive patients. Spine J 18 (2018): 1625-1631.
- Khan NR, Thompson CJ, DeCuypere M, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine 21 (2014): 974-983.
- Hey HW, Thiam DW, Koh ZS, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine 42 (2017): 267-274.
- Marc A, Weinstein JPM, Frank P, et al. Postoperative spinal wound infection: a review of 2391 consecutive index procedures. J Spinal Disord 13 (2000): 422-426.
- Devin CJ, Chotai S, McGirt MJ, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: a multicenter analysis. Spine 43 (2018): 65-71.
- de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 37 (2009): 387-397.
- Manoukian S, Stewart S, Graves N, et al. Bed-days and costs associated with the inpatient burden of healthcare-associated infection in the UK. J Hosp Infect 114 (2021): 43-50.
- Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine 36 (2011): 2084-2088.
- Sono T, Fujibayashi S, Izeki M, et al. Decreased rate of surgical site infection after spinal surgery with instrumentation using bundled approach including surveillance and intrawound vancomycin application. Medicine 97 (2018): e12010.
- Han J, Yang Y, Lu J, et al. Sustained release vancomycin-coated titanium alloy using a novel electrostatic dry powder coating technique may be a potential strategy to reduce implant-related infection. Biosci Trends 11 (2017): 346-354.
- Center for Disease Control and Prevention. Surgical site infections. 2010. https://www.cdc.gov/hai/ssi/ssi.html. Accessed Aug (2021).
- Oktay K, Özsoy KM, Çetinalp NE, et al. Efficacy of prophylactic application of vancomycin powder in preventing surgical site infections after instrumented spinal surgery: A retrospective analysis of patients with high-risk conditions. Acta Orthop Traumatol Turc 55 (2021): 48-52.
- Martin JR, Adogwa O, Brown CR, et al. Experience with intra-wound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976). 39 (2014): 177-184.
- Heller A, McIff TE, Lai SM, et al. Intrawound vancomycin powder decreases Staphylococcal surgical site infections after posterior instrumented spinal arthrodesis. J Spinal Disord Tech 28 (2015): E584-589.
- Tomov M, Mitsunaga L, Durbin-Johnson B, et al. Reducing surgical site infection in spinal surgery with betadine irrigation and intra-wound vancomycin powder. Spine (Phila Pa 1976). 40 (7): 491-499.
- Gaviola A, McMillian WD, Ames SE, et al. A retrospective study on the protective effects of topical vancomycin in patients undergoing multilevel spinal fusion. Pharmacotherapy 36 (2016): 19-25.
- Mirzashahi B, Chehrassan M, Mortazavi SMJ. Intrawound application of vancomycin changes the responsible germ in elective spine surgery without significant effect on the rate of infection: a randomized prospective study. Musculoskelet Surg 102 (2018): 35-39.
- Hida T, Ando K, Kobayashiet K, et al. Intrawound vancomycin powder as the prophylaxis of surgical site infection after invasive spine surgery with a high risk of infection. Nagoya J Med Sci. 79 (2017): 545-550.
- Haimoto S, Schär RT, Nishimura Y, et al. Reduction in surgical site infection with supra-fascial intra-wound application of vancomycin powder in instrumented posterior spinal fusion: a retrospective case-control study. J Neurosurg Spine. 29 (2018): 193-198.
- Dodson V, Majmundar N, Swantic V, et al. The effect of prophylactic vancomycin powder on infections following spinal surgeries: a systematic review. Neurosurg Focus 1 (2019): E11.
- Gande A, Rosinski A, Cunningham T, et al. Selection pressures of vancomycin powder use in spine surgery: a meta-analysis. Spine J. 19 (2019): 1076-1084.
- Wang S, Yao R, Li Z, Xiangdong Gong, et al. Vancomycin Use in Posterior Lumbar Interbody Fusion of Deep Surgical Site Infection, Infection and Drug Resistance 15 (2022): 3103-3109
- Ghobrial GM, Cadotte DW, Williams K, Jr, et al. Complications from the use of intrawound vancomycin in lumbar spinal surgery: a systematic review. Neurosurg Focus. 39 (2015): E11.
- Bakhsheshian J, Dahdaleh NS, Lam SK, et al. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg 83 (2015): 816-823.
- Godil SS, Parker SL, O’Neill KR, et al. Comparative effectiveness and cost-benefit analysis of local application of vancomycin powder in posterior spinal fusion for spine trauma: Clinical article. J Neurosurg Spine 19 (2013): 331-335.
- Emohare O, Ledonio CG, Hill BW, et al. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 14 (2014): 2710-2715.
- Edin ML, Miclau T, Lester GE, et al. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res 333 (1996): 245-251.
- Rathbone CR, Cross JD, Brown KV, et al. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 29 (2011): 1070-1074.
- Mendoza MC, Sonn KA, Kannan AS, et al. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine. 25 (2016): 147–53.
- Strom RG, Pacione D, Kalhorn SP, et al. Lumbar laminectomy and fusion with routine local application of vancomycin powder: Decreased infection rate in instrumented and non-instrumented cases. Clin Neurol Neurosurg. 115 (2013): 1766–1769.


 Impact Factor: * 3.123
Impact Factor: * 3.123 Acceptance Rate: 75.30%
Acceptance Rate: 75.30%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 