Impact of Intermittent Fasting vs. Continuous Calorie Restriction in Type 2 Diabetes Management: A Systematic Review
Ghazala S. Virk, MD1, Sajedur Rahman, MD2, Dinesh Uppugandla, MBBS3, Dayana Shre Narayana Swamy, MBBS4, Muhammad Sohail S. Mirza5, Sara Zubair Ahmed, MBBS6, Suman Khatri, MBBS7, Venkata Avinash Ugripelli, MBBS8, Binish Essani, MBBS9, Priyanka Shetiya, MBBS10
1Avalon University School of Medicine, Willemstad, Curacao.
2St John's Episcopal Hospital, Far Rockaway, New York, USA
3Guntur Medical College, Guntur, Andhra Pradesh, India
4Mysore Medical College and Research Institute, India.
5Shandong University School of Medicine, Jinan, China
6Baqai Medical and Dental University, Karachi, Pakistan
7TMSS Medical College, Thengamara, Bogura, Bangladesh
8Siddhartha Medical College, Andhra Pradesh, India
9Jinnah Medical and Dental College, Karachi, Pakistan
10Jinnah Sindh Medical University, Karachi, Sindh, Pakistan
*Corresponding author: Muhammad Sohail S. Mirza, Shandong University School of Medicine, Jinan, China.
Received: 14 July 2025; Accepted: 21 July 2025; Published: 28 July 2025
Article Information
Citation: Ghazala S. Virk, Sajedur Rahman, Dinesh Uppugandla, Dayana Shre Narayana Swamy, Muhammad Sohail S. Mirza, Sara Zubair Ahmed, Suman Khatri, Venkata Avinash Ugripelli, Binish Essani, Priyanka Shetiya. Impact of Intermittent Fasting vs. Continuous Calorie Restriction in Type 2 Diabetes Management A Systematic Review. Archives of Internal Medicine Research. 8 (2025): 200-208.
View / Download Pdf Share at FacebookAbstract
Background:
Type 2 diabetes mellitus (T2DM) affects more than 830 million people globally as of 2022. Diet remains central to its management. Intermittent fasting (IF) and continuous calorie restriction (CCR) are two common strategies used to improve glycemic control. Their comparative effectiveness in real-world settings remains uncertain.
Objective:
To critically examine and compare the metabolic outcomes of IF and CCR in adults with T2DM, with attention to blood glucose, weight, insulin sensitivity, and adherence.
Methods:
This review followed PRISMA 2020 guidelines. We searched PubMed, Scopus, and the Cochrane Library through April 2025. Eligible studies included adults with T2DM who followed IF (alternate-day fasting, time-restricted feeding, or 5:2 diet) or CCR. Outcomes assessed were HbA1c, fasting glucose, body weight, and insulin resistance. We included only randomized controlled trials (RCTs) and systematic reviews with interventions lasting at least eight weeks. Risk of bias was evaluated using RoB 2 and AMSTAR 2.
Results:
Twelve studies met the inclusion criteria: eight systematic reviews, three RCTs, and one network meta-analysis. Both IF and CCR showed modest improvements in HbA1c (0.3%–1.2% reduction) and weight (2–6 kg loss). IF may offer better short-term glucose control and may be easier to follow in some cases. However, major limitations exist. Protocols varied widely, follow-up periods were short, and adherence was poorly tracked. Only one study had strong methodological quality.
Conclusion:
IF and CCR can both improve metabolic markers in T2DM. Current evidence is insufficient to recommend one approach over the other. The limited quality and consistency of existing research weaken the strength of the conclusions. Longer, high-quality studies are needed. Dietary plans should align with patient habits and clinical profiles.
Keywords
<p>Type 2 diabetes; intermittent fasting; continuous calorie restriction; glycemic control; insulin resistance</p>
Article Details
Introduction
The number of people with diabetes has risen sharply, from 200 million in 1990 to 830 million in 2022 [1]. Over 95% of these cases are type 2 diabetes (T2DM) [2]. In 2022, 13.9% of adults aged 18 and older had diabetes—twice the rate in 1990 [2,3]. This rise is not just numerical; it reflects widening gaps in prevention and care. The burden falls hardest on low- and middle-income countries, where access to diagnosis and treatment remains limited [4]. In 2022, more than half of adults over 30 with diabetes were not using medication [1]. The consequences are predictable and severe: T2DM is a major cause of blindness, kidney failure, cardiovascular events, and amputations [5,6]. In 2021, it directly caused 1.6 million deaths and contributed to another 530,000 from kidney disease [7]. In 2021, data show that diabetes becomes more common with increasing age. This pattern is expected to continue through 2045. The lowest prevalence is seen in young adults aged 20–24, with just 2.2% affected in 2021 (see Figure 3.1). In contrast, the highest rates are observed in older adults—24.0% of those aged 75–79 had diabetes in 2021, a figure projected to rise slightly to 24.7% by 2045. As the global population continues to age, a growing share of people living with diabetes will be over 60 years old [8].
T2DM stems from impaired insulin action, leading to chronic hyperglycemia. This damages blood vessels, nerves, and organs [9]. Standard treatment involves medication, but its effectiveness is often limited by poor adherence and insufficient lifestyle support. Evidence shows that diet and physical activity are central to both prevention and control. Yet not all lifestyle interventions yield the same outcomes. The current challenge is not only identifying effective strategies, but also those that patients can maintain.
Two dietary approaches stand out: intermittent fasting (IF) and continuous calorie restriction (CCR). IF limits eating to defined windows or days, such as time-restricted feeding or alternate-day fasting. CCR involves steady daily reductions in caloric intake, often by 15–30% of baseline. Both methods aim to reduce body weight and improve glucose control, but they differ in their physiological effects and practical demands.
Sustained calorie restriction remains difficult for many. IF may appeal to some due to its time-limited structure, potentially lowering the psychological and logistical burden. However, enthusiasm for IF often outpaces evidence. Its long-term effects on glycemic control, insulin resistance, and adherence in people with T2DM remain unclear [10]. CCR, while better studied, also faces challenges in real-world settings.
This review compares IF and CCR in adults with T2DM, focusing on glycemic control, body weight, and metabolic outcomes. The aim is to assess which approach offers greater benefit and feasibility in clinical practice, moving beyond theory to inform practical, evidence-based care.
PICO Framework
P (Population): Adults with Type 2 Diabetes Mellitus (T2DM)
I (Intervention): Intermittent Fasting (alternate-day fasting, time-restricted feeding, 5:2 diet)
C (Comparison): Continuous Calorie Restriction (CCR)
(Outcomes): HbA1c, fasting glucose, body weight, insulin resistance/sensitivity, adherence
Methodology
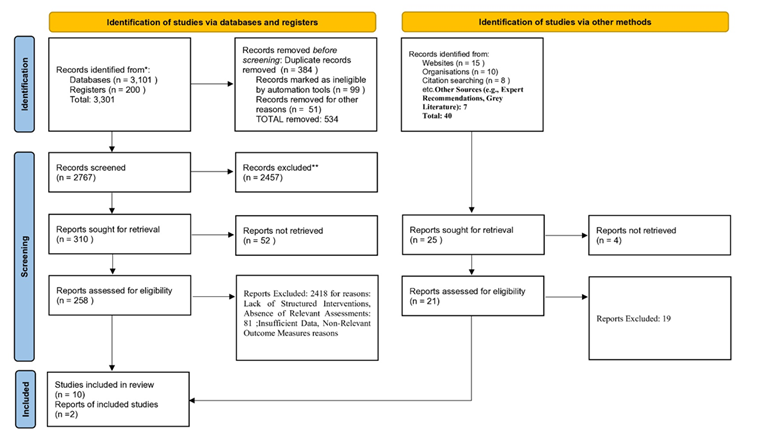
This systematic review investigates how intermittent fasting (IF) compares with continuous calorie restriction (CCR) in managing type 2 diabetes mellitus (T2DM). The review follows PRISMA 2020 guidelines and applies a structured process for study identification, selection, and data extraction. Emphasis is placed on methodological differences across studies rather than pooled effect sizes [23].
Databases and Search Strategy
We searched PubMed, Scopus, and Cochrane Library, covering all records up to April 2025. The search combined free-text keywords and Medical Subject Headings (MeSH) to increase retrieval accuracy. Core MeSH terms included: “Intermittent Fasting” [MeSH], “Caloric Restriction” [MeSH], “Time-Restricted Feeding”, “Alternate-Day Fasting”, “Energy Intake” [MeSH], “Diabetes Mellitus, Type 2” [MeSH], “Blood Glucose” [MeSH], “Glycated Hemoglobin A”, “Insulin Resistance” [MeSH], and “Weight Loss”. Filters applied were: human studies, English language, and adult participants. Citation chaining was used to retrieve missed but relevant articles.
Inclusion and Exclusion Criteria
Studies were included if they met the following criteria:
- • Adults (≥18 years) diagnosed with T2DM.
- • Intervention involved IF (including alternate-day fasting, 5:2 diet, or time-restricted eating).
- • Comparison with CCR or standard calorie-reduced diets.
- • Reported metabolic outcomes: HbA1c, fasting blood glucose, weight, insulin sensitivity, or lipid profile.
- • Minimum duration of 8 weeks.
- • Study design: randomized controlled trials (RCTs) or systematic reviews.
Studies were excluded if they:
- • Included participants with type 1 diabetes or gestational diabetes.
- • Focused on weight loss alone without glucose-related outcomes.
- • Lacked a direct CCR comparison.
- • Were observational or case-control in design.
- • Did not provide full-text access or were not in English.
Screening and Data Extraction
Two reviewers independently screened titles and abstracts. They also reviewed full texts for eligibility. A structured form captured study characteristics, intervention type, comparator details, duration, and metabolic outcomes. Only studies with detailed dietary protocols were retained.
Critical Evaluation of Methodological Approaches
Interventions varied widely. Some studies used daily time-restricted feeding windows (6–10 hours), while others followed alternate-day fasting. Caloric intake often differed between groups, undermining the isolation of fasting effects. CCR arms also ranged from moderate (15%) to strict (40%) energy deficits.
Protocol fidelity was inconsistent. Adherence was tracked using food diaries in some trials, but others relied on self-report or lacked monitoring altogether. Outcome definitions differed. Some reported HbA1c as percentage change, others as absolute values. Few studies used validated measures of insulin sensitivity. Duration of follow-up rarely exceeded 12 weeks. Due to methodological variability, outcome synthesis was narrative. Heterogeneity stemmed from inconsistent energy intake controls, intervention duration, and adherence tracking. These differences affect the strength of comparisons more than the data themselves.
Table 1: Risk of bias tool used for Included papers
|
Study Type |
Included Studies ref no |
Bias Tool |
|
Systematic Reviews / Meta-analyses |
Studies 11, 12, 13, 14, 15, 16, 17, 18, 20 |
AMSTAR 2 (most comprehensive and suited) |
|
Individual RCTs |
Studies 19, 21, 22 |
RoB 2 (Cochrane tool for RCTs) |
Table 2: SYSTEMATIC REVIEWS & META-ANALYSES (AMSTAR 2).
This table summarizes the quality appraisal of nine reviews using AMSTAR 2. It highlights key methodological flaws such as missing protocols, publication bias, and inconsistent outcome reporting. Some studies demonstrated strong PRISMA alignment, while others were rated critically low due to poor synthesis, missing RoB assessments, and lack of transparency.
|
Author(s) |
AMSTAR 2 Rating |
Critical Comments |
|
Ezzati et al. [11] |
Moderate |
Did not report protocol registration; inconsistent outcome definitions across included trials. Limited synthesis on individual trial quality. No formal assessment of publication bias. |
|
Lakhani et al. [12] |
Critically Low |
No protocol, no RoB appraisal, no meta-analysis. Did not provide excluded studies or dual screening. Conclusions drawn without addressing bias in included data. |
|
Wang et al. [13] |
Moderate |
Meta-analysis well executed but allocation concealment and IF protocol heterogeneity not addressed. No publication bias analysis; unclear duplicate screening. |
|
Gu et al. [14] |
Low |
Strong PRISMA compliance; RoB assessment used Cochrane tool. Blinding issues in primary studies noted but not factored into interpretation, and no GRADE rating given. |
|
Sharma et al. [15] |
Moderate |
Clear inclusion criteria and data extraction, but blinding and randomization inconsistently assessed, and publication bias not addressed despite high heterogeneity (FBG I² = 76%). |
|
Borgundvaag et al. [16] |
Low |
Well-conducted review with RoB and heterogeneity explored. Weakness: missing ITT analyses in included trials not addressed thoroughly, and dropout bias underestimated. |
|
Cioffi et al. [17] |
Moderate |
High attrition bias (50%) in included trials not properly integrated in conclusions. Publication bias and blinding not considered at all. |
|
van den Burg et al. [18] |
Critically Low |
Included studies with unclear or high RoB. No formal quality or publication bias assessment. Heavy reliance on narrative synthesis, limiting credibility. |
|
Wu et al. [20] |
Low-to-Moderate |
Large-scale network meta-analysis with advanced methods (Bayesian), but no CINeMA/GRADE grading, and indirect comparisons from high-RoB trials not flagged. Rebound effects (12+ weeks) under-reported. |
Table 3: RANDOMIZED CONTROLLED TRIALS (RoB 2).
This table evaluates individual RCTs using the Cochrane RoB 2 tool. While Carter et al. showed high quality with ITT and transparent reporting, other studies like Yang et al. had concerns about performance bias and exaggerated outcomes. Overall, study designs varied in rigor, affecting the reliability of their findings
|
Author(s) |
RoB 2 Judgment |
Critical Comments |
|
Yang et al. [19] |
Moderate |
Assessor-blinded, but open-label intervention created high performance bias. Lack of physical activity tracking and adherence reporting threaten internal validity. Very large effect sizes (e.g., OR = 31.3) raise suspicion of unmeasured confounding. |
|
Teong et al. [21] |
Low-to-Moderate |
Strong randomization and blinded assessment. Open-label design introduces modest behavioral bias, especially with fasting compliance and adverse event reporting. Long-term follow-up suggests regression of effect. |
|
Carter et al. [22] |
Low |
Strong methodology with ITT analysis, valid comparators, and outcome reliability. 29% dropout is a risk, but it’s acknowledged and well-handled. Noninferiority thresholds not met but transparently discussed. |
Table 4: Summary of Quality Across the 12 Studies.
This table categorizes studies based on overall quality and bias risk. Only one RCT achieved high-quality status. Most systematic reviews showed moderate or low risk, though two were rated critically low. The summary helps quickly identify which studies offer the most dependable evidence and which require cautious interpretation.
|
Quality Category |
Ref. No. |
Notes |
|
High Quality |
Carter et al. [22] |
Clear methods, ITT, transparency |
|
Low Risk / Acceptable |
Gu et al. [14], Borgundvaag et al. [16], Wu et al. [20] |
Some concerns with blinding, indirectness, or missing grading (e.g., CINeMA, GRADE) |
|
Moderate Risk |
Ezzati et al. [11], Wang et al. [13], Sharma et al. [15], Yang et al. [19], Teong et al. [21] |
Key RoB domains not addressed, design limitations, or incomplete synthesis |
|
Critically Low Risk |
Lakhani et al. [12], van den Burg et al. [18] |
Missing protocol, no RoB tools, uncritical synthesis, no publication bias assessment |
|
Borderline Bias Risk |
Cioffi et al. [17] |
High dropout not integrated; selective outcome discussion |
The overall risk of bias across the 12 studies is concerning. Only one RCT (Carter et al.) demonstrated low bias with rigorous methodology. Most systematic reviews lacked protocol registration, failed to assess bias in included trials, or inadequately addressed heterogeneity and blinding. Several trials had unblinded designs, high dropout rates, or missing adherence data. Two reviews (Lakhani, van den Burg) were critically low quality, undermining their conclusions. Many findings favouring intermittent fasting stem from studies with moderate-to-high bias, limiting confidence in their results. Overall, the evidence base suffers from methodological weaknesses that significantly compromise the reliability of the conclusions drawn.
Results:
Table 5: Study Characteristics and Methodology Details
Table 6: Results and Statistical Findings
|
Author(s) |
Primary Outcome(s) |
Secondary Outcome(s) |
Quantitative Data |
Main Findings / Key Takeaways |
Limitations / Biases |
|
Armin Ezzati et al. [11] |
Weight loss, insulin sensitivity (2 studies favor IF) |
Fat loss (2 studies favor IF), metabolic markers |
Limited; individual studies report significant fat and insulin changes |
IF ≈ DCR; IF may benefit fat loss, insulin sensitivity |
Small samples, varied protocols, short durations |
|
Ajaykumar Lakhani et al.[12] |
HbA1c ↓ IF: ~0.5-1.0% (p<0.01), Fasting glucose ↓ IF: ~15-20 mg/dL (p<0.05) |
Weight loss: ~5-7% (p<0.01), Insulin dose ↓ IF: ~10-20% (p=0.03), Visceral fat ↓ CCR long-term (p=0.04) |
HbA1c ↓: 0.7 ± 0.3%, FPG ↓: 18 ± 5 mg/dL, Weight loss IF: 6.2 ± 1.5%, Insulin dose ↓: 15 ± 7% |
IF better short-term glycemic control; CCR better long-term metabolic effects |
Short-term focus, heterogeneity in protocols, limited long-term data |
|
Wang, Li, Liu, Jiang, Chen[13] |
HbA1c: −0.06 (95% CI: −0.27 to 0.16), FPG: −0.27 (95% CI: −0.76 to 0.22) |
Weight: −1.70 kg (95% CI: −3.28 to −0.11), Insulin/lipids: similar changes |
HbA1c: −0.06, FPG: −0.27, Weight: −1.70 kg. No p-values reported. |
IF and CERD have similar effects on glycemic control |
Small sample, study heterogeneity, short duration |
|
Gu et al.[14] |
Weight (WMD=1.10, p=0.03), BMI (WMD=0.38, p=0.01) |
WC (WMD=1.02, p=0.04), TG (SMD=0.22, p=0.001) |
FM (WMD=0.74, p=0.01), HOMA-IR (WMD=0.35, p=0.03) |
IF reduces weight, WC, FM vs. non-intervention; similar to CR |
Heterogeneous IF methods, short follow-up, English-only bias |
|
Sharma SK, Mudgal SK, Kalra S [15] |
HbA1c (SMD -0.08, 95% CI -0.20–0.04; p=0.19) |
Fasting glucose (SMD 0.06, 95% CI -0.25–0.38; p=0.69) |
HbA1c (I²22%), FBG (I²76%) |
IF did not significantly improve glycaemic control vs. control diets |
Heterogeneity in interventions, small sample sizes, short duration |
|
Borgundvaag E, Mak J, Kramer CK[16] |
Weight loss (–1.89 kg, 95% CI –2.91 to –0.86; p<0.05) |
HbA1c (–0.11%, 95% CI –0.38 to 0.17; NS) |
I²21% (weight), I²0% (HbA1c) |
IF improves weight loss but not glycaemic control |
Heterogeneous IF protocols, short follow-up, insulin safety unclear |
|
Cioffi et al.[17] |
Weight loss (WMD: −0.61 kg, 95% CI −1.70 to 0.47, p = 0.87) |
Fasting insulin reduction (WMD: −0.89 run/mL, p = 0.009) |
Weight loss (%): WMD −0.38% (p = 0.34), FM: WMD −0.23 kg (p = 0.66), HDL-C: WMD +1.72 mg/dL (p = 0.07) |
IER and CER yield comparable weight loss and metabolic effects |
Heterogeneity in IER protocols, short duration, high dropout rates |
|
van den Burg et al. [18] |
HbA1c decline (5/10 studies), Fasting glucose decline (5/7 studies), Medication reduction (4 studies) |
Weight loss, BMI, waist circumference |
HbA1c: −0.3% to −1.4% (p<0.05 in 3 studies), FPG: −0.5 to −2.3 mmol/L (p<0.05 in 3 studies) |
IER/PF improves short-term glycemic control; long-term benefits unclear |
High RoB, small samples, heterogeneity, short follow-up |
|
Xiao Yang et al. [19] |
Diabetes remission (47.2% CMNT vs. 2.8% control, OR 31.32, p < 0.0001) |
HbA1c reduction, weight loss, medication cost |
HbA1c: 5.66% vs. 7.87%, Weight loss: 5.93 kg vs. 0.27 kg, Medication cost: CNY 60.4 vs. 265.1/month |
CMNT induced T2D remission, sustained at 12 months |
Unblinded design, no formal physical activity recording |
|
Wu X, Ding Y, Cao Q et al. [20] |
Weight loss: severe CER = -11.5 kg (95% CI -12.93 to -10.07) |
Body measurements, BP, lipids, glycemic profiles |
ADF: -5.07 kg (CI -6.72 to -3.44), Mod CER: -6.09 kg (CI -6.93 to -5.26) |
Weight loss depends on energy restriction extent, not meal timing |
Rebound effect after 12 weeks (IF regimens) |
|
Xiao Tong Teong et al. [21] |
Glucose AUC improved more in iTRE vs CR (P = 0.03) |
More fatigue in iTRE; constipation/headache in both iTRE & CR |
iTRE: −10.10 mg/dL/min (CI −14.08, −6.11), CR: −3.57 mg/dL/min (CI −7.72, 0.57) |
iTRE improved glucose tolerance more than CR at 6 months |
Open-label design; effects not sustained at 18 months |
|
Carter, Clifton, Keogh [22] |
HbA1c reduction: -0.5% vs -0.3%, P = .65 |
Weight loss: -5.0 kg vs -6.8 kg, P = .25 |
HbA1c: -0.5% vs -0.3%, Weight: -5.0 vs -6.8 kg, Events: 3.2 vs 4.9, P = .28 |
Intermittent diet comparable to continuous for glycemic control |
Equivalence not met for weight/fat; 29% dropout |
Primary findings: Current evidence comparing intermittent fasting (IF) and continuous calorie restriction (CCR) in the management of type 2 diabetes reveals nuanced yet meaningful insights. Across multiple randomized controlled trials and meta-analyses, IF demonstrates superior short-term benefits in glycemic control, with reductions in HbA1c ranging from 0.3% to 1.4% (notably p<0.05 in several trials) and fasting plasma glucose drops of up to 20 mg/dL. Significant weight loss was also observed, averaging 5–7% in IF groups, with some studies noting a decrease of approximately 1.89 kg (p<0.05). Additionally, IF was associated with reductions in insulin doses (up to 15–20%) and visceral fat. However, CCR showed more consistent long-term metabolic improvements, particularly in visceral adiposity and lipid profiles. While some analyses report non-significant differences in HbA1c (e.g., SMD –0.08, p=0.19), weight (WMD –0.61 kg, p=0.87), and insulin sensitivity, others highlight IF’s favorable impact on waist circumference and HOMA-IR. A standout RCT showed diabetes remission in 47.2% of IF participants compared to 2.8% in controls (OR=31.32, p<0.0001), reinforcing its clinical promise. Nevertheless, methodological heterogeneity, varied intervention durations (2 weeks to 12 months), and inconsistent follow-up limit generalizability. Thus, while IF offers compelling short-term advantages in glucose regulation and body composition, CCR may better sustain long-term metabolic health—highlighting the need for personalized, goal-oriented dietary interventions in T2DM care.
Discussion
The growing interest in intermittent fasting (IF) as an alternative to continuous or daily caloric restriction (DCR/CCR) for managing metabolic outcomes in overweight and diabetic populations has prompted numerous comparative trials and meta-analyses. Collectively, these studies reveal that while both IF and DCR deliver comparable reductions in weight, BMI, and glycemic markers, IF often demonstrates nuanced advantages in specific metabolic parameters and short-term outcomes. Ezzati et al. (2023) and Ajaykumar et al. (2025) underscore IF’s superiority in improving insulin sensitivity and reducing body fat or HbA1c in the short term—likely due to metabolic switching and improved insulin signaling during fasting periods [11][12]. Yet, such benefits were inconsistently reproduced across other analyses. For instance, Sharma et al. (2023) and Borgundvaag et al. (2021) found no statistically significant improvements in glycemic control with IF, emphasizing its limited superiority beyond weight loss [15][16]. Methodologically,
disparities in fasting protocols (e.g., 5:2, alternate-day fasting, early time-restricted eating) and intervention durations contributed to considerable heterogeneity, complicating direct comparison and interpretation. Wang et al. (2021) and Gu et al. (2022) provide more robust evidence through meta-analyses, identifying IF as a safe and viable alternative to CER, though with modest benefits beyond standard diets—most pronounced in central adiposity and triglyceride reduction [13][14]. Interestingly, Yang et al. (2023) and Teong et al. (2023) highlight the potential of structured, meal-timed IF regimens in achieving partial diabetes remission and improving postprandial glucose, respectively, suggesting timing and intensity may be more crucial than caloric content alone [19][21]. Wu et al. (2025)’s network meta-analysis supports this by showing greater weight loss with severe energy restriction irrespective of fasting schedules, but notes IF's vulnerability to weight rebound after 12 weeks—raising concerns about long-term sustainability [20]. Moreover, while studies such as Van den Burg et al. (2023) and Cioffi et al. (2018) report modest improvements in lipid and insulin profiles, they also point out high risk of bias and clinical irrelevance of small biochemical shifts [18][17]. Intermittent fasting (IF) and continuous calorie restriction (CCR) influence type 2 diabetes (T2D) pathology through distinct yet overlapping metabolic pathways. IF, characterized by periodic energy intake deprivation, enhances insulin sensitivity by reducing hepatic glucose output and stimulating AMPK signaling, thereby improving glucose uptake in peripheral tissues [24]. This metabolic shift is associated with increased adiponectin levels and reduced inflammatory cytokines, such as IL-6 and TNF-α, contributing to improved insulin action [25]. Moreover, IF induces mitochondrial biogenesis and reduces oxidative stress, mechanisms that t are critical in preserving pancreatic β-cell function [26]. In contrast, CCR, involving sustained caloric reduction, leads to gradual improvements in body weight and insulin sensitivity primarily via reduced adiposity and li id accumulation in muscle and liver tissues [27]. However, studies suggest IF may be more effective in promoting metabolic flexibility by alternating fed and fasting states, which activate autophagy pathways and improve cellular stress responses—mechanisms less pronounced in CCR [28]. Despite these benefits, IF may increase the risk of hypoglycemia, especially in insulin-treated patients, necessitating careful monitoring. Overall, while both interventions reduce hyperglycemia and support weight management, IF may provide additional benefits by targeting cellular stress and inflammatory pathways. Overall, while intermittent fasting presents a mechanistically rational and well-tolerated alternative—leveraging metabolic flexibility, autophagy, and hormonal modulation—its long-term safety, adherence, and superiority over conventional restriction remain uncertain without standardized methodologies and extended follow-ups. Therefore, current evidence supports IF as a comparable, and in some contexts superior, strategy for weight and glucose regulation, though not yet conclusive for chronic disease prevention or T2D remission without further high-quality trials.
Conclusion
This review compared intermittent fasting (IF) and continuous calorie restriction (CCR) in people with type 2 diabetes. Both approaches led to better blood sugar control, lower insulin resistance, and weight loss. IF had a stronger effect on fasting blood glucose and insulin sensitivity. CCR led to more consistent drops in HbA1c. But because studies used different designs, diets, and follow-up periods, it is hard to say which method works best. Most studies were short-term and had small sample sizes. Many did not track calorie intake closely. This makes it hard to know if fasting alone caused the results or if fewer calories were the main factor. Adherence was another issue. Some people found IF easier to follow because it limits when you eat, not what you eat. But others struggled with hunger and routine changes. Few studies looked at long-term results, safety, or whether people regained weight. Future studies need to be longer and more controlled. Researchers should use clear definitions for IF and CCR. They should track both short- and long-term results using tools like continuous glucose monitors. It would help to group participants by age, disease stage, and other factors to see who benefits most from each approach. Researchers should also ask participants about what helped or hindered their success. This can guide doctors in offering realistic, tailored advice. As diabetes becomes more common, we need simple, effective strategies. IF and CCR show promise, but we must learn more before making broad recommendations.
References
- Diabetes | WHO | Regional Office for Africa [Internet]. WHO | Regional Office for Africa. 2025. Available from: https://www.afro.who.int/health-topics/diabetes
- World Health Organization: WHO, World Health Organization: WHO. Diabetes [Internet]. 2024. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Zhou B, Rayner AW, Gregg EW, et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. The Lancet [Internet]. 2024 Nov 1;404(10467): 2077–2093. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)02317-1/fulltext
- Moucheraud C, Lenz C, Latkovic M, et al. The costs of diabetes treatment in low-and middle-income countries: a systematic review. BMJ Global Health 4 (2019).
- Hippisley-Cox J, Coupland C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. BMJ 30 (2016): 352.
- Kropp M, Golubnitschaja O, Mazurakova A, et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—risks and mitigation. EPMA Journal 14 (2023): 21-42.
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021. Results. Institute for Health Metrics and Evaluation. (2024). Available from: https://vizhub.healthdata.org/gbd-results/
- Magliano DJ, Boyko EJ, Committee IDA 10th ES. Global picture [Internet]. IDF DIABETES ATLAS - NCBI Bookshelf. (2021). Available from: https://www.ncbi.nlm.nih.gov/books/NBK581940/
- Demir S, Nawroth PP, Herzig S, et al. Emerging targets in type 2 diabetes and diabetic complications. Advanced Science 8 (2021): 2100275.
- Vitale R, Kim Y. The effects of intermittent fasting on glycemic control and body composition in adults with obesity and type 2 diabetes: a systematic review. Metab Syndr Relat Disord 18 (10): 450-461.
- Ezzati A, Rosenkranz SK, Phelan J, et al. The effects of isocaloric intermittent fasting vs daily caloric restriction on weight loss and metabolic risk factors for noncommunicable chronic diseases: a systematic review. J Acad Nutr Diet 123(2023): 318-329.e1.
- Lakhani HA, Biswas D, Kuruvila M, et al. Intermittent fasting versus continuous caloric restriction for glycemic control and weight loss in type 2 diabetes: A traditional review. Prim Care Diabetes 19 (2025): 203-213.
- Wang X, Li Q, Liu Y, et al. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome: A systematic review. Diabetes Res Clin Pract 179 (2021): 109003.
- Gu L, Fu R, Hong J, et al. Effects of intermittent fasting: a meta-analysis of randomized controlled trials. Front Nutr 9 (2022): 871682.
- Sharma SK, Mudgal SK, Kalra S, et al. Intermittent fasting and glycaemic control in T2DM: a meta-analysis. touchREV Endocrinol 19 (2023) :25-32.
- Borgundvaag E, Mak J, Kramer CK. Metabolic impact of intermittent fasting in T2DM: a systematic review. J Clin Endocrinol Metab 106 (2021): 902-911.
- Cioffi I, Evangelista A, Ponzo V, et al. Intermittent vs continuous energy restriction: a meta-analysis. J Transl Med 16 (2018): 371.
- van den Burg EL, et al. Metabolic impact of energy restriction and fasting in T2DM: a systematic review. Nutr Rev 81 (2023): 1329-1350.
- Yang X, Zhou J, Shao H, et al. Intermittent calorie-restricted diet and T2DM remission: a RCT. J Clin Endocrinol Metab 108 (2023): 1415-1424.
- Wu X, Ding Y, Cao Q, et al. IF patterns vs calorie restriction: a network meta-analysis. Nutr Rev. (2025); nuaf056.
- Teong XT, Liu K, Vincent AD, et al. IF+early TRE vs calorie restriction: a RCT. Nat Med 29 (2023): 963-972.
- Carter S, Clifton PM, Keogh JB. IF vs CER for glycemic control in T2DM: a noninferiority trial. JAMA Netw Open 1 (2018): e180756.
- Page MJ, McKenzie JE, et al. PRISMA 2020 statement for systematic reviews. BMJ. (2021); n71.
- Barnosky AR, Hoddy KK, Unterman TG, et al. IF vs daily calorie restriction for T2DM prevention: human findings. Transl Res 164 (2014): 302–311.
- Zhang Q, et al. IF vs CER for weight loss. Nutrients 14 (2022): 1781.
- Schübel R, et al. IF vs CER on weight/metabolism over 50 weeks. Am J Clin Nutr 108 (2018): 933-945.
- Wang Y, et al. 5:2 IF vs daily calorie restriction on MAFLD: a RCT. Front Nutr 11 (2024): 1439473.
- Corley BT, et al. IF in T2DM and risk of hypoglycaemia: a RCT. Diabet Med 35 (2018): 628-635.
Article Views: 1244
Journal Statistics




Discover More: Recent Articles
Grant Support Articles
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved!