Insights of Bangladeshi Doctors on Usage, Acceptance and Future Prospects of Vonomax (Vonoprazan)
Dr. Faruque Ahmed*,1, Mohammod Shafiqul Islam2, Md. Muksidul Haque2, Dr. Zayer Rashid3, Dr. Mohaiminur Rahman4, Md. Ahsanur Rahman5, Md. Mustafizur Rahman6
1Professor, Department of Gastroenterology, Sheikh Russel Gastro Liver Institute & Hospital, Dhaka, Bangladesh.
2Department of Pharmacy, University of Dhaka, Dhaka, Bangladesh.
3Department of Medicine, Rajshahi Medical College, Rajshahi, Bangladesh.
4Department of Medicine, Dhaka Medical College, Dhaka, Bangladesh.
5Department of Pharmacy, University of Development Alternative, Dhaka, Bangladesh.
6Department of Pharmacy, University of Asia Pacific, Dhaka, Bangladesh.
*Corresponding Author: Dr. Faruque Ahmed, Professor, Department of Gastroenterology, Sheikh Russel Gastro Liver Institute & Hospital, Dhaka, Bangladesh.
Received: 25 August 2025; Accepted: 31 August 2025; Published: 30 September 2025
Article Information
Citation: Dr. Faruque Ahmed, Mohammod Shafiqul Islam, Md. Muksidul Haque, Dr. Zayer Rashid, Dr. Mohaiminur Rahman, Md. Ahsanur Rahman, Md. Mustafizur Rahman. Insights of Bangladeshi Doctors on Usage, Acceptance and Future Prospects of Vonomax (Vonoprazan). Archives of Internal Medicine Research. 8 (2025): 312-316.
View / Download Pdf Share at FacebookAbstract
Introduction: Acid-related gastrointestinal disorders such as gastritis, peptic ulcer, and gastroesophageal reflux disease (GERD) are commonly encountered in clinical practice throughout Bangladesh. While proton pump inhibitors (PPIs) have long been the standard treatment, many physicians report limitations, including delayed symptom relief and variable efficacy. Vonoprazan, a potassium-competitive acid blocker, has emerged as a promising alternative due to its rapid onset, ensuring desired actions from the very first day, along with sustained acid suppression and a favorable safety profile.
Aim of the Study: This study aimed to explore the real-world usage, physician perceptions, and clinical experiences regarding Vonomax (vonoprazan) as a treatment for acid-related disorders among doctors in Bangladesh.
Methods: A cross-sectional survey was conducted among 500 practicing physicians from various specialties, including gastroenterology, internal medicine, and cardiology. The structured questionnaire collected information on prescribing habits, perceived effectiveness and safety, patient compliance, and dosage patterns. Data was compiled and analyzed to identify trends in clinical practice and physician preferences.
Results: Out of 500 surveyed physicians, 72% reported prescribing Vonomax as a first-line treatment for acid-related gastrointestinal complications, while 25% used it frequently in clinical practice. The vast majority (99%) considered it effective, with 87% rating it as highly effective. Only 1.4% of doctors reported mild side effects such as nausea, fast gastric emptying, or headache, while 98.6% observed no adverse reactions. Physicians commonly used Vonomax for GERD, gastric ulcers, and Helicobacter pylori eradication, adhering to standard dosing protocols. Compared to traditional PPIs, respondents favored Vonomax for its faster onset of action, improved patient compliance, consistent efficacy, and better safety profile across a wide range of patients, including those with renal or hepatic impairment.
Conclusion: The findings suggest a strong and growing preference for Vonomax among physicians in Bangladesh, supported by its rapid symptom relief, excellent tolerability, and practical dosing flexibility. These insights highlight the evolving landscape of acid-suppressive therapy and support broader integration of vonoprazan into clinical practice.
Keywords
<p>Vonoprazan, Vonomax, Bangladesh</p>
Article Details
Introduction
Stomach-related problems like acid reflux, gastritis, peptic ulcers, and indigestion are common complaints in medical chambers across Bangladesh.(1) These conditions affect a wide range of people, often causing discomfort, disrupting daily life, and requiring long-term treatment. For years, doctors have relied on proton pump inhibitors (PPIs) as the standard solution to reduce stomach acid.(2) While PPIs have certainly helped millions of patients, many physicians now recognize their limitations—slow onset of action, inconsistent symptom relief, and sometimes reduced effectiveness in certain individuals.(3) As medical science continues to evolve, newer treatment options have emerged. One such advancement is the introduction of a new class of medicine known as potassium-competitive acid blocker.(4) This drug offers faster and more dependable acid control compared to traditional PPIs. Among them, vonoprazan has received significant attention due to its quick action, longer duration of acid suppression, and ability to work well even in patients who don’t respond consistently to PPIs.(5) In Bangladesh, vonoprazan has been introduced under the brand name Vonomax marketed by Popular Pharmaceuticals PLC. Since its launch, many doctors across the country have started using it in their regular practice. Vonomax’s unique mechanism of action allows it to start working within hours, unlike PPIs, which may take several days to become fully effective. It also works regardless of food intake and is less affected by genetic variations that influence how PPIs are metabolized in the body. Globally, clinical trials have shown that vonoprazan performs as well as—or in some cases better than—PPIs for treating conditions like GERD, ulcers, and H. pylori infections.(5) But while there’s strong international data supporting vonoprazan’s use, very little is known about how it’s being used in real-world practice here in Bangladesh. Are doctors confident in prescribing Vonomax? Do they see good results in their patients? What do they think about its safety and long-term value? This study, “Vonomax: Insights of Bangladeshi Doctors on Usage, Acceptance and Future Prospects,” set out to explore those very questions. By surveying 500 doctors from a variety of specialties and practice settings, we aimed to get a clearer picture of how Vonomax is being used across the country. Their responses provide valuable insight into current prescribing habits, perceived benefits and challenges, and expectations for the future role of Vonomax in managing acid-related disorders in Bangladesh.
Methods
This study employed a cross-sectional observational design to assess physician practices and perspectives regarding vonoprazan (Vonomax) utilization in clinical settings across Bangladesh. A structured survey was administered to 500 actively practicing physicians representing gastroenterology, internal medicine, and cardiology specialties. The questionnaire was designed to capture real-world clinical experiences, including prescribing patterns, therapeutic decision-making factors, and observations of treatment outcomes in routine practice settings. Survey items explored various aspects of acid suppression therapy management while specifically focusing on the integration of vonoprazan into treatment protocols. The study protocol emphasized chamber-based clinical experiences to ensure practical relevance to daily patient care scenarios. Data collection procedures were implemented to maintain respondent confidentiality while gathering comprehensive information about therapeutic approaches for acid-related disorders. All collected responses underwent systematic compilation and analysis to identify prevailing practice trends and clinical perspectives within the surveyed physician population.
Results
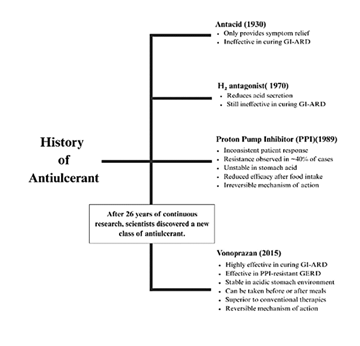
This historical overview illustrates the gradual evolution of acid suppression therapy, highlighting key developments from traditional antacids to H2 antagonists, PPIs, and the emergence of potassium-competitive acid blocker like vonoprazan. The timeline emphasizes the need for more potent, faster-acting, and safer alternatives, setting the stage for Vonomax’s clinical relevance.
Table 1: Demographic Profile of Survey Participants
|
Specialization |
Number of Doctors |
Percentage (%) |
|
Gastroenterology |
207 |
41.4% |
|
Medicine (General) |
143 |
28.6% |
|
Cardiology |
94 |
18.8% |
|
Others |
56 |
11.2% |
|
Total |
500 |
100% |
The majority of respondents were gastroenterologists (40%) and general medicine practitioners (30%), followed by cardiologists and other specialists. This distribution reflects a broad cross-section of clinical perspectives relevant to acid-related disorder management, ensuring that the survey captures diverse real-world practices across Bangladesh.
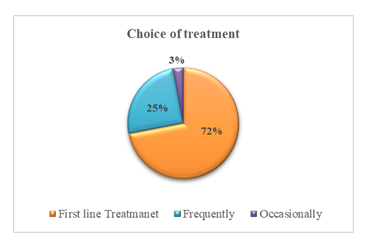
A significant portion of physicians indicated a strong preference for Vonomax, reinforcing its acceptance in routine clinical practice. This figure underscores the growing confidence among clinicians in using vonoprazan as a primary treatment option over conventional therapies.
Table 2: Treatment Preferences for Acid-Related Disorders
|
Preference Category |
Percentage (%) |
Key Findings |
|
First-line Treatment |
72% |
Vonomax preferred over PPIs/H2 blockers. |
|
Frequently Prescribed |
25% |
High adoption in clinical practice. |
|
Occasionally Prescribed |
3% |
Limited use in specific cases. |
Vonomax is used as the first-line treatment by 72% of surveyed doctors, with an additional 25% prescribing it frequently. This widespread adoption reflects high clinical trust in its efficacy and supports its emerging role as a frontline therapy in Bangladesh for acid-related gastrointestinal disorders.
Table 3: Efficacy and Safety Perceptions of Vonomax
|
Parameter |
Percentage (%) |
Details |
|
Highly Effective |
87% |
Management of GI symptoms. |
|
Effective |
12% |
Moderate symptom relief. |
|
Minimal Side Effects |
1.4% |
Nausea, fast gastric emptying, headache (Page 8). |
|
No Side Effects |
98.6% |
Excellent tolerability. |
Physicians overwhelmingly rated Vonomax as either highly effective (87%) or effective (12%) in managing GI symptoms. Additionally, 98.6% reported no side effects, indicating excellent tolerability and reinforcing its suitability for long-term use in diverse patient populations.
Table 4: Dosage Recommendations for Vonomax
|
Indication |
Dose |
Duration |
|
GERD (Erosive Esophagitis) |
20 mg OD |
4–8 weeks |
|
Gastric Ulcer |
20 mg OD |
8 weeks |
|
Helicobacter pylori Eradication (Triple Therapy) |
20 mg BD |
14 days |
|
Severe Renal Impairment (eGFR <30 mL/min) |
10 mg OD |
As needed |
The dosage patterns align with global clinical guidelines, indicating appropriate usage for conditions like GERD, ulcers, and H. pylori infection. The inclusion of renal dosing also highlights physician awareness of safety in patients with impaired kidney function, suggesting cautious yet confident application in high-risk populations.
Table 5: Molecular Mechanism Comparison (Vonoprazan vs. Conventional PPIs)
|
Characteristics |
Conventional PPIs |
Vonoprazan |
Interpretation |
|
Type of Drug |
Pro-drug |
Active-drug |
Vonoprazan works faster, higher bioavailability. |
|
Binding Nature |
Irreversible |
Reversible |
Vonoprazan has higher efficacy and safety. |
|
H+/K+ ATPase Inhibition |
Active phase only |
Active + resting phases |
Vonoprazan provides maximum acid control. |
|
Half-life |
0.5–2.1 hours |
9.0 hours |
Vonoprazan ensures longer duration of action. |
|
pKa Value |
3.8–5.0 |
9.06 |
Vonoprazan shows higher concentration at canaliculi. |
|
Full Effect Achievement |
Requires repeat doses |
Single dose |
Vonoprazan provides optimum efficacy from Day 1. |
|
CYP2C19 Polymorphism |
Affected (risk of failure) |
Unaffected |
No genetic variability in response. |
The comparison clearly illustrates Vonomax’s (vonoprazan) pharmacological superiority over conventional PPIs across multiple parameters—including onset of action, bioavailability, and resistance profile. These advantages support Vonomax’s positioning as a next-generation therapy for acid suppression with better long-term patient outcomes.
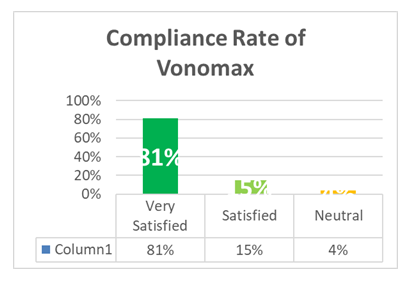
The figure shows that 81% of patients demonstrated high compliance with Vonomax therapy, with minimal adverse effects. These findings reinforce the drug’s practical benefits in everyday use, contributing to its strong adoption in clinical practice.
Discussion
This study offers important real-world insights into how Bangladeshi doctors are using vonoprazan (Vonomax) in their everyday practice to treat acid-related gastrointestinal conditions like GERD, gastritis, and peptic ulcers. With 500 physicians participating from a wide range of specialties, the findings help us understand not just the clinical utility of Vonomax, but also the confidence and experience doctors have when using it. One of the key takeaways from this survey is that nearly three-quarters (72%) of doctors consider Vonomax their first-line treatment, while another 25% report frequently prescribing it. This signals a shift in prescribing habits, moving away from the long-standing use of proton pump inhibitors (PPIs) and toward newer potassium-competitive acid blocker like vonoprazan. This trend isn’t unique to Bangladesh. Similar findings have been reported internationally—for example, a study by Laine et al. showed better symptom control and mucosal healing in GERD patients treated with vonoprazan compared to PPIs like lansoprazole.(6) Data on effectiveness and safety also back the strong clinical confidence seen in this study. An overwhelming 99% of physicians found Vonomax to be effective, with 87% rating it as highly effective. Even more reassuring is the safety profile: only 1.4% of doctors reported minor side effects such as nausea or headache. This mirrors results from earlier clinical trials, such as those conducted by Tian et al., where vonoprazan not only matched but often outperformed traditional PPIs in healing ulcers while maintaining excellent tolerability.(7) What sets vonoprazan apart is its unique mechanism of action. While PPIs need to be activated in an acidic environment and often take days to show full effect, vonoprazan works faster and more reliably. It binds reversibly to the acid-producing proton pumps and suppresses acid secretion in both active and resting H+/K+ ATPase phases. Studies like the one by Sakurai et al. have shown that vonoprazan can bring symptom relief much earlier than esomeprazole, often within the first few days of treatment.(8) This rapid relief is especially valuable in chamber-based settings, where patients expect fast results and doctors need to manage symptoms efficiently. Another practical advantage is that vonoprazan’s effectiveness is not influenced by genetic variations such as CYP2C19 polymorphisms, which can affect how patients metabolize PPIs. In countries like Bangladesh, where genetic testing isn't commonly done before starting treatment, this consistency is a major plus. A study by Graham et al. reinforced that vonoprazan maintains strong and consistent acid suppression regardless of genetic background.(9) In terms of long-term use, physicians in this survey expressed confidence even in patients with renal or liver impairment. This aligns with pharmacokinetic studies showing that vonoprazan can be safely used in these populations without requiring significant dose adjustments. Sugano et al. have also reported a favorable safety profile in patients on long-term maintenance therapy.(10) However, despite these clinical advantages, some challenges remain. A small number of physicians (3%) reported using Vonomax only occasionally. This limited use could stem from several factors, including availability in rural or less-resourced areas, or institutional restrictions in government facilities. In a healthcare system where affordability often drives prescribing behavior, particularly in underprivileged or remote regions, access could influence how widely Vonomax is adopted in the future. Addressing these barriers through policy interventions, pricing strategies, and awareness could help ensure more equitable access to this newer generation of acid-suppressive therapy.
Limitations of the study
This study relied on doctors’ self-reported experiences, so it didn’t directly track patient outcomes or long-term follow-up.
Conclusion
The findings of this nationwide survey indicate that Vonomax (vonoprazan) is widely accepted by Bangladeshi physicians as a first-line treatment for acid-related gastrointestinal disorders due to its rapid onset, high efficacy, excellent safety profile, and ease of use. Its growing adoption reflects a shift in clinical practice toward more effective and patient-friendly acid suppression therapy.
Recommendation
To build on these encouraging results, future research should look at how patients themselves are responding to Vonomax, especially in terms of symptom relief, quality of life, and long-term safety. It would also help to raise awareness among more general physicians, particularly in remote areas, so that this promising treatment can benefit a wider group of patients across the country.
Reference
- Hossain T. Prescription Pattern of Acid Suppressive Medications in Bangladesh. East West University (2012).
- Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 11 (2017): 27–37.
- Lacy BE. Proton Pump Inhibitor Nonresponders. Gastroenterol Hepatol 11 (2015): 483–5.
- Wong N, Reddy A, Patel A. Potassium-Competitive Acid Blockers: Present and Potential Utility in the Armamentarium for Acid Peptic Disorders. Gastroenterol Hepatol 18 (2022): 693–700.
- Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil 24 (2018): 334–44.
- Laine L, DeVault K, Katz P, et al. Vonoprazan versus lansoprazole for healing and maintenance of healing of erosive esophagitis: a randomized trial. Gastroenterology 164 (2023): 61–71.
- Tian L, Xiang D, Yue F, et al. Efficacy and safety of vonoprazan versus proton pump inhibitors in the treatment of peptic ulcer disease: a systematic review and network meta-analysis for randomized controlled trails. Front Nutr 11 (2024): 1436993.
- Sakurai K, Suda H, Fujie S, et al. Short-Term Symptomatic Relief in Gastroesophageal Reflux Disease: A Comparative Study of Esomeprazole and Vonoprazan. Dig Dis Sci 64 (2019): 815–22.
- Graham DY, Dore MP. Update on the use of vonoprazan: a competitive acid blocker. Gastroenterology 154 (2018): 462–6.
- Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Ther Adv Gastroenterol 11 (2018): 1756283X17745776.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 