Short-Term Outcome of Ischemic Stroke after Thrombolytic Therapy with Recombinant Tissue Type Plasminogen Activator in a Hospital in Sylhet, Bangladesh
Md. Nazrul Islam1, Md. Matiur Rahman2, Rahat Amin Chowdhury3, Shiuli Rani Deb4, Tania Islam5 , Md. Hafiz Ehsanul Hoque6, K M Hafizur Rahman7, Tamanna Tabassum Turna8 , Md Ariful Haque9, Tasnim Mahmud10
1Professor & Head, Department of Neurology, Parkview Medical College Hospital, Sylhet, Bangladesh.
2Professor (Ex.) & Chief Consultant, Department of Neurology, Mount Adora Hospital, Sylhet, Bangladesh.
3Associate Professor & Head, Department of Neurology, Sylhet Women’s Medical College Hospital, Sylhet, Bangladesh.
4Assistant Registrar, Department of Neurology, Mount Adora Hospital, Sylhet, Bangladesh.
5Medical Officer, Department of Neurology, Mount Adora Hospital, Sylhet, Bangladesh.
6Associate Professor, Department of Community Medicine, Sylhet MAG Osmani Medical College, Sylhet, Bangladesh.
7Senior Lecturer, Department of Pharmacology, Sylhet Women’s Medical College Hospital,
Sylhet, Bangladesh.
8Assistant Registrar, Parkview Medical College Hospital, Sylhet, Bangladesh.
9Department of Public Health, Atish Dipankar University of Science and Technology, Dhaka, Bangladesh, Department of Orthopaedic Surgery, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, Yunnan, China
*Corresponding author: Md. Nazrul Islam. Professor & Head, Department of Neurology, Parkview Medical College Hospital, Sylhet, Bangladesh.
Received: 12 November 2025; Accepted: 18 November 2025; Published: 21 November 2025
Article Information
Citation: Md. Nazrul Islam, Md. Matiur Rahman, Rahat Amin Chowdhury, Shiuli Rani Deb, Tania Islam, Md. Hafiz Ehsanul Hoque, K M Hafizur Rahman, Tamanna Tabassum Turna, Md Ariful Haque, Tasnim Mahmud. Short-Term Outcome of Ischemic Stroke after Thrombolytic Therapy with Recombinant Tissue Type Plasminogen Activator in a Hospital in Sylhet, Bangladesh. Archives of Internal Medicine Research. 8 (2025): 334-338.
View / Download Pdf Share at FacebookAbstract
Background:
The incidence of ischemic stroke is increasing worldwide, including Bangladesh. Evidence showed that early initiation of thrombolysis therapy correlated with better functional outcome of the patients. The drug recombinant tissue type plasminogen activator (rtPA) is using in thrombolysis therapy successfully for several years. The objective of this study was to compare short-term outcome of thrombolysis therapy by rtPA between the patients’ group of with and without thrombolysis therapy.
Methodology:
This open-labeled non-randomized negatively controlled clinical trial conducted at Neurology unit of Mount Adora Hospital,Sylhet. The investigating group was treated with intravenous rtPA at a dose of0.6mg/kgbody-weight and administered within 4.5 hours of onset of disease and after proper diagnosis. Age and sex matched negatively controlled group used to compare. The modified Rankin Scale (mRS) was the outcome variable.
Result:
Thirty-one patients of acute ischemic stroke included for rtPA therapy and they were compared with similar number of agesex matched ischemic stroke patients those who came after the window period of rtPA therapy or not agreed to take the drug therapy. Both the study groups found matched for their age (p = 0.92), sex (p = 1.00), hypertension (p = 1.00), diabetes mellitus (p = 0.47), ischemic heart disease (p = 1.00) and smoking habit (p = 0.31) in this study. Clinical evaluation before starting the therapy found similarity of clinical laterality (p = 0.45), cranial nerve involvement (p = 0.25), sensory affected (p = 0.47), motor deficit (p = 0.20), number of lesion (p = 0.42), TOAST subtype (p = 0.89) in this study. The Modified Ranking Scale (mRS) score before starting the therapy was 30.71 among rtPA group and 32.29 in non rtPA group, which was not significantly differ (p = 0.723). The mean rank found significant on discharge (36.68 in non rtPA groupand 26.32 in rtPA group, p = 0.021) and after 3 months of the therapy (37.76 in non rtPA groupand 25.24 in rtPA group, p = 0.006). There was no serious adverse effect of rtPA observed in this study.
Conclusion:
This study revealed favorable functional outcome of rtPA therapy in acute ischemic stroke patients when given within the window period of the therapy.
Keywords
Acute ischemic stroke; rtPAt herapy; Outcome
Article Details
Introduction
Globally 15 million people are suffering from stroke each year. Among them 5 million die and another 5 million left permanently disabled [1]. Over the years, prevalence of the stroke patients increased due to changes in demographic pattern which showed increase number of aged populations not only in the developed countries but also in the developing countries. Ischemic stroke is the commonest type of stroke which accounts for 87% of all strokes worldwide [2]. Among the acute ischemic stroke patients, 13.5% die within a month [3] and 13.4% suffer from severe and total disability [4]. Thrombolysis with intravenous (IV) recombinant tissue type plasminogen activator (rtPA) is an FDA approved treatment option for acute ischemic stroke. This treatment method is endorsed by American Heart Association and American Stroke Association. Window period for starting hrombolytic therapy in acute ischemic stroke patients is 4.5 hours from the onset of symptoms [5,6]. Initiation of thrombolysis increase perfusion of the area vulnerable to necrosis after acute ischemic stroke. In a randomized controlled trial, administering IV rtPA within window period showed better functional outcome of the patients but after the window period the benefit is uncertain [7-10].
Though there is significant benefit of thrombolysis of acute ischemic patients, there is still risk of fatal intracranial hemorrhage [8]. Despite this initial elevated risk of hemorrhage due to thrombolysis, the mortality rate of the patient’s having thrombolysis is comparable with the patients did not have thrombolysis after 3 months’ period [8]. Recommended dose of thrombolysis by rtPA approved by FDA is 0.9 mg per body weight of the patient. In Japan, the approved dose of rtPA(alteplase) for acute ischemic stroke is 0.6mg/kg, based up on the results of a small open-label study suggesting that this dose was associated with a lower risk of devastating adverse effects and provide similar efficacy compared with the standard dose of 0.9 mg/kg [10].
Thrombolysis therapy in acute stroke patients required effective coordination between emergency, indoor, laboratory division to minimize time so that required thrombolytic therapy can be given within window period. This is very challenging in low resource setting. Though there is clear evidence of thrombolysis therapy in developed countries, little evidence is available from the patients in Bangladesh. As the aged population will be increased in future, vascular events among them will also be increased in Bangladesh. The stroke events, and stroke associated mortality and morbidity will also be increased. So, it is time demanding to know the effectiveness of thrombolysis therapy among acute ischemic stroke patients in our setting. This study aimed to compare short-term outcome of thrombolysis therapy in acute ischemic stroke patients and the patients without having thrombolysis therapy.
Methodology
This open-labeled negatively controlled clinical trial conducted at the Neurology unit of Mount Adora Hospital, Sylhet from 2022 to 2024. The treatment group comprised of the patients of acute ischemic stroke who were treated with rtPA after admission; and the control group was age and sex matched ischemic stroke patients who were admitted after the window period and not treated with thrombolysis therapy. Patient with focal neurological deficit, arriving within 4 hours from onset of symptoms at the hospital, was immediately called to the stroke team and undergone MRI of brain with DWI to exclude hemorrhagic stroke. Ischemic stroke patients eligible for rtPA offered thrombolysis. The contraindications for rtPA therapy were excluded on the basis of AHA guideline. Japanese recommended dose that is 0.6 milligram per kg body weight was applied due to Asian population. Initially 10%of total dose was given intravenously within 1 minute, and then 90% of dose was administered over 1 hour via syringe pump. The patients were clinically assessed for general health and neurologic health. The Glasgow Coma Scale(GCS) used to assess the consciousness level and National Institute of Health Stroke Scale (NIHSS)toassessthestrokeseverityandmodifiedRankinscale(mRS)tomeasurethefunctional ability of the patients. Patient’s BP, Pulse, NIHSS score, any bleeding manifestations were strictly monitored in every 15 minutes’ interval for 2 hours, thenhourlyfornext24hours. Systolic blood pressure was maintained <180 mmHg and diastolic blood pressure <105 mmHg. No antiplatelet was given within 24 hours of rtPA Therapy. Patients received rtPA had undergone another scan after 24 hours to assess spontaneous intracranial hemorrhage (sICH) due to rtPA. Patient's was discharged when Modified Rankin Score was satisfactory and there were also no other complications. Follow up of the patient was done after 3 months with Modified Rankin Score. Data was collected by trained physician through a questionnaire, which includes socio demographic information, clinical, radiological information and treatment related information.
Data were analyzed by SPSS (version 25.0). Comparison of thrombolysis group and control group was assessed by chi square and t test as applicable. Mann-Whitney U test (Non parametric test) was used to measure the differences in mRs score between the groups. A p- value of <0.05 at 95% confidence interval was considered as statistically significant.
Written consent was taken from the respondents or from the relative of the patient during follow up after explaining the purpose of the study. As the study was open-labeled, patient’s relative was allowed to choose to take the therapy options. All of them provided sufficient information regarding the investigating drug therapy. Ethical approval was obtained from institutional ethical review board.
Results
Thirty-one acute ischemic stroke patients treated with rtPA included in Group A and age sex matched31ischemicstrokepatientsnottreatedwithrtPAincludedinGroupB.Table1showed the demographic variables were similar in the groups. Comorbidity (Hypertension, Diabetes mellitus and Ischemic heart disease) among the patients were not significantly differed between the groups. Smoking behavior also not differed between rtPA and non rtPA groups.
Table1: Demographic and other characteristics of rtPA and non rtPA group.
|
Variable |
GroupA (rtPA) |
Group B (NonrtPA) |
pvalue |
|
Ageinyears(Mean±SD) |
61.3±13.1 |
61.7±13.4 |
0.92 |
|
Male |
10,32.3% |
10,32.3% |
1 |
|
Hypertension |
28,90.3% |
28,90.3% |
1 |
|
DM |
18,58.1% |
14,45.2% |
0.47 |
|
IHD |
6,19.4% |
6,19.4% |
1 |
|
Smokingever |
13,41.9% |
18,58.1% |
0.31 |
Clinical characteristics of the patients of stroke were depicted in the table 2. It showed clinical laterality (Right side 41.9%vs51.6%),cranial nerve involvement (32.3%vs19.4%), sensory (9.7% vs 19.4%) and motor (87.1% vs 74.2%) deficit, location of stroke (Right carotid 58.1% vs 51.6%), number of lesion (Single 61.3% vs 71.0%) and TOAST subtypes of stroke (Large artery 35.5% vs 32.3%) were not differed between the groups. The duration of hospital stay was differed as it showed mean stay was more in rtPA group (5.48 vs 3.35 days) and the difference was significant.
Table2: Clinical and other characteristics of rtPA and non rtPA group.
|
Variable |
rtPAgroup |
Non rtPA group |
pvalue |
|
Clinical laterality, Right |
13,41.9 |
16,51.6 |
0.45 |
|
Cranial nerve involvement |
10,32.3 |
6,19.4 |
0.25 |
|
Sensory affected |
3,9.7 |
6,19.4 |
0.47 |
|
Motor deficit |
27,87.1 |
23,74.2 |
0.2 |
|
Location |
|||
|
RtCarotid |
18,58.1 |
16,51.6 |
|
|
LtCarotid |
10,32.3 |
15,48.4 |
|
|
Vertebrobasilar |
2,6.5 |
||
|
Undetermined |
1,3.2 |
||
|
Number of lesions |
0.42 |
||
|
Single |
19,61.3 |
22,71.0 |
|
|
Multiple |
12,38.7 |
9,29.0 |
|
|
Mechanism |
|||
|
Thrombotic |
28,90.3 |
27,87.1 |
1 |
|
Embolic |
3,9.7 |
4,12.9 |
|
|
TOAST |
|||
|
subtype |
|||
|
Largeartery |
11,35.5 |
10,32.3 |
0.89 |
|
Cardioembolic |
2,6.5 |
3,9.7 |
|
|
Smallartery |
18,58.1 |
18,58.1 |
|
|
Duration of hospital stay (Mean±SD)* |
5.48±4.2 |
3.35±2.26 |
0.015 |
*independent‘t’test
Modified Rankin Scale was used to measure disability among the patients during admission, during discharge from hospital and follow up after 3 months. mRS scores were converted to ranked data and Independent Samples Mann Whitney U test was used to compare the scores.
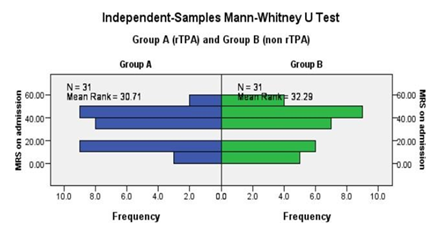
Figure 1 revealed the mean rank among rtPA group was30.71 and 32.29 in nonrtPA group and difference was not significantly differed (Table 3).
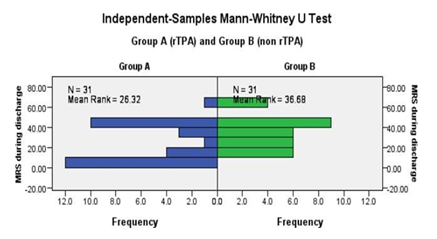
Modified Rankin Scale (mRS) score during discharge demonstrated in figure 2. Mean rank of nonrtPA group was 36.68 whereas 26.32 in rtPA group and the difference was statistically (p value 0.021) significant. (Figure 2 and Table 3).
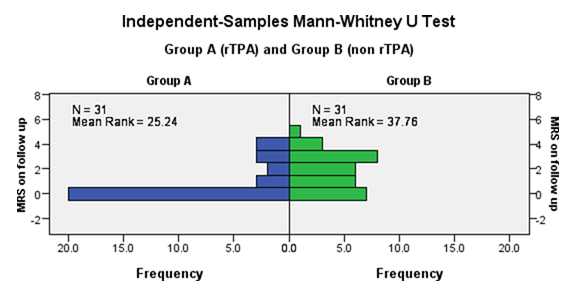
The difference between mean rank score in rtPA and nonrtPA group after 3 months was different statistically and difference was highly significant statistically (p value 0.006, Figure 3 and Table 3).
Table3: Differences in mean rankinrtPA and nonrtPA group by MannWhitneyUtest.
|
mRS |
GroupA(rtPA) |
GroupB(NonrtPA) |
Pvalue |
|
On admission |
30.71 |
32.29 |
0.723 |
|
On discharge |
26.32 |
36.68 |
0.021 |
|
3months follow up |
25.24 |
37.76 |
0.006 |
Discussion
This study was conducted at a private hospital in Sylhet, North-East part of Bangladesh. A total of 31 patients received rtPA for the management of acute ischemic stroke was included in the study and age sex matched comparison group also included to compare the functional outcome of the patients. The mean age was 61.3 years which is similar to studies conducted in Egypt by Elsayed et al. (Mean age 60.2 years) [11]. A study conducted in Singapore by Yeo et al. [12] found median age of 65 which was also near to this study finding (Median 62 years). In this study there were one third of the patients who were male (32.3%). However, more male patients observed in Egypt study (60%) [11] and a study conducted in Tamilnadu, India (95.6%) by Sivanandy et al. [13]. A study in Singapore revealed 44% male [12], which showed similarity to this study. The deviation might be due to small sample size and the patients who consent to receive rtPA therapy. This study revealed 90.3% had hypertension and 58.1% had DM. The findings were much higher than the study of Egypt (65.3% HTN and 36% DM) [11] and India (43.4% HTN and 21.7% DM) [13] but near to the findings of Singapore study (75.4% HTN and 29.1% DM) [12].
Mean Modified Rankin Score (mRS) during admission was not differed between the groups but the score differed significantly during discharge (Mean rank of non rtPA group was 36.68 whereas 26.32 in rtPA group, p = 0.021). The difference found highly significant after 3 month follow up as rtPA group showed marked functional ability with mRS (25.19vs37.00, p=0.006). The result consistent with study conducted by Geisennegger et al. in Austria [14] which demonstrates functional improvement of the ischemic patients who received rtPA increases over time and about half of the patients become functionally independent (mRS score 0-2) within 3 months’ post-stroke. Egypt study found functional improvement in mild stroke group only, they did not find significant improvement in moderate and severe stroke patients compare to non rtPA group [11]. A multicenter RCT study in Europe (ECASS III) also showed significant functional outcome of ischemic patients treated with rtPA (52.4%vs45.2%, p=0.04)
[15]. Similar finding also demonstrated in a study in Spain (53.8%vs24.1%, p=0.001) [16].
This study analyzed 31 cases who received rtPA after ischemic stroke and none of them developed subsequent hemorrhage. Subsequent intracranial hemorrhage is an adverse effect ofrtPA therapy as revealed in several researches [11,16]. Though the studies revealed hemorrhage as an adverse event following rtPA therapy, studies revealed the mortality outcome is similar with the non rtPA group. The reason of not developing any hemorrhage in this study might be due to lower dose of rtPA (0.6 mg per kg body weight) used for thrombolysis.
Conclusion
This study revealed good functional outcome of the ischemic stroke patients receiving rtPA like other studies. The rtPA therapy should be used to treat patients having acute ischemic stroke that will decrease the disability of the patients. Further studies recommended with larger sample size to supplement the finding of this study.
Conflict of interest:
The authors declare there is no conflict of interest.
References
- World Health Organization. WHO EMRO. Stroke, Cerebrovascular accident. Health topics [Internet]. World Health Organization - Regional Office for the Eastern Mediterranean. 2022. Available from: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html
- Benjamin EJ, Virani SS, Lee CW, et al. Heart disease and stroke statistics – 2018 update: A report from the American heart association. Circulation 137 (2018): E67-E492
- Zhang, R., Wang, Y., Fang, J., et al. Worldwide 1 -month case fatality of ischaemic stroke and the temporal trend. Stroke and vascular neurology, 5 (2020): 353-360.
- Lv Y, Sun Q, Li J, et al. Disability Status and Its Influencing Factors Among Stroke Patients in Northeast China: A3-YearFollow-UpStudy. NeuropsychiatricDiseaseand Treatment 17 (2021): 2567–2573.
- Jauch EC, Saver JL, Adams HP, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 44 (2013): 870–947.
- Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association/ American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke 46 (2015): 3020-3035
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue Plasminogen activator for acute ischemic stroke. N Engl J Med 333 (1995): 1581-1587
- WardlawJM, MurrayV, BergeE, et al. Thrombolysis for acute ischemic stroke. Cochrane Database SystRev 7 (2014): CD000213.
- Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischemic stroke: a meta-analysis of individual patient fata from randomized trials. Lancet 384 (2014): 1929-1935.
- Alper BS, Malone-Moses M, McLellan JS, et al. Thrombolysis in acute ischemic stroke: time for rethink? BMJ 350 (2015): 1-7.
- Elsayed MA, Salah H, Sabbah A, et al. Early functional outcome after IV rTPA administration in Egyptian acute ischemic stroke patients. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 55 (2019): 1-7.
- Yeo LLL, Paliwal P, Teoh HL, et al. Timing of Recanalization After Intravenous Thrombolysis and Functional Outcomes After Acute Ischemic Stroke. JAMA Neurology 70 (2013): 353–358.
- Sivanandy P, Thomas B, Krishnan V, Arunachalam S. Safety and Efficacy of Thrombolytic Therapy Using rt-PA (Alteplase) in Acute Ischemic Stroke. ISRN Neurology (2011): 1–6.
- Marko M, Dominika Miksova, Ebner J, et al. Temporal Trends of Functional Outcome in Patients with Acute Ischemic Stroke Treated with Intravenous Thrombolysis. Stroke 53 (2022): 3329-3337.
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. New England Journal of Medicine 359 (2008): 1317-1329.
- Iglesias-ReyR, Rodríguez-YáñezM, Rodríguez-CastroE, et al. Worse Outcome in Stroke Patients Treated with rt-PA Without Early Reperfusion: Associated Factors. Translational Stroke Research 9 (2017): 347–55.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 