Study of Aetiology of Acute Respiratory Tract Infection in Adult Outpatients in Bangladesh Institute of Tropical and Infectious Diseases (BITID) in Chattogram, Bangladesh by Filmarray Respiratory Panel Assay in Nasopharyngeal Swab
Dr. Istiak Ahmad1*, Prof. Dr. Md Shakeel Ahmed2, Prof. Dr. Md Mamunur Rashid3, Dr. Md Zakir Hossain4, Dr. Ratan Kumar Nath5, Dr. Saifuddin Mahmud6, Dr. Tabassum Ferdousi 7, Dr. Farzin Akhter8, Dr. Mohammad Rifat Kamal9, Zahirul Islam10
1Jr. Consultant (Medicine), Department of Medicine, Chattogram Medical College Hospital, Chattogram, Bangladesh.
2Professor and Head, Department of Microbiology, Institute of Applied Health Sciences, Chattogram, Bangladesh.
3Professor and Head, Department of Clinical Tropical Medicine, Bangladesh Institute of Tropical and Infectious Diseases, Chattogram, Bangladesh.
4Assistant Professor, Department of Microbiology and Immunology, Bangladesh Institute of Tropical and Infectious Diseases, Chattogram.
5Associate Professor, Department of Clinical Tropical Medicine, Bangladesh Institute of Tropical and Infectious Diseases, Chattogram, Bangladesh.
6Jr. Consultant (Medicine), Department of Medicine, Chattogram Medical College Hospital, Chattogram, Bangladesh.
7Assistant Professor, Department of Tropical Hygiene, Bangladesh Institute of Tropical and Infectious Diseases, Chattogram, Bangladesh.
8Jr. Consultant (Medicine), Bangladesh Institute of Tropical and Infectious Diseases, Chattogram, Bangladesh.
9Registrar, Department of Neurology, Chattogram Medical College Hospital, Chattogram, Bangladesh.
10Senior Research Officer, Bangladesh Institute of Tropical and Infectious Diseases, Chattogram, Bangladesh.
*Corresponding author: Dr. Istiak Ahmad, Jr. Consultant (Medicine), Department of Medicine, Chattogram Medical College Hospital, Chattogram, Bangladesh.
Received: 23 June 2025; Accepted: 30 June 2025; Published: 07 July 2025
Article Information
Citation: Dr. Istiak Ahmad, Prof. Dr. Md Shakeel Ahmed, Prof. Dr. Md Mamunur Rashid, Dr. Md Zakir Hossain, Dr. Ratan Kumar Nath, Dr. Saifuddin Mahmud, Dr. Tabassum Ferdousi, Dr. Farzin Akhter, Dr. Mohammad Rifat Kamal, Zahirul Islam. Study of Aetiology of Acute Respiratory Tract Infection in Adult Outpatients in Bangladesh Institute of Tropical and Infectious Diseases (BITID) in Chattogram, Bangladesh by Filmarray Respiratory Panel Assay in Nasopharyngeal Swab. Archives of Internal Medicine Research. 8 (2025): 192-199.
View / Download Pdf Share at FacebookAbstract
Background:
According to World Health Organization (WHO) respiratory infection account for 6% of total global disease burden. In Bangladesh acute respiratory tract infection (ARTI) are one of the leading causes of heath care visit for both adult and children. ARTI can be caused by a heterogeneous group of viruses and bacteria that produce similar clinical presentations. Although viruses are the major pathogen that causes upper respiratory tract infections and acute bronchitis, the extensive use of antibiotics among patients with ARTI threatens to erode the efficacy of these medications.
Objectives:
This study aims to identify and describe the etiological agents associated with acute respiratory tract infections (ARTIs) by FilmArray respiratory panel assay in nasopharyngeal swab in adults attending in outpatients of Bangladesh Institute of Tropical and Infectious Diseases Hospial, Chattogram, Bangladesh and their associated risk factors such as clinical symptoms, signs, sociodemographic characteristics and reduce inappropriate antibiotic use in viral infections.
Methods:
This cross-sectional observational study was conducted in Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh from 1st July 2023 to 31st December 2023. A total of 100 patients with ARTI who attended in outpatient department of the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh were enrolled in this study. Nasopharyngeal swabs for FilmArray respiratory panel assay obtained from all patients. Data were collected over a period of 6 months and analyzed by appropriate computer based programmed software Statistical Package for the Social Sciences (SPSS), version 25.
Results:
In this study, most of the patients 50 (50.0%) lies between 18 years to 30 years. Mean ± SD of the patients was 21.2 ± 7.3 years. Most of the patients 67 (67.00%) were male and 33 (33.00%) were female. Common symptoms were cough 87 (87.0%), fever 81(81.0%), runny nose 66 (66.0%), muscle ache 43 (43.0%) and lethargy 45 (45.0%). Most of the patients 72 (72.0%) had a high temperature, respiratory rate was increased in 7 (7.0%), high blood pressure was recorded in 8 (8.0%) patients. Result of FilmArray was positive in 69 (69.0%) patients, among them 47 (68.1%) patients were male and 22 (31.9%) patients were female. Viruses are the major pathogen, among 69 positive cases 67 (97.1%) were viral infections and only 2 (2.9%) were bacterial infections and 17 (24.6%) were mixed infection. About 11 types of viruses were identified according to the result of FilmArray. Among them most of the viruses were Influenza B (26), Influenza A H3 (15), Human Rhinovirus/Enterovirus (11), Influenza A H1-2009 (6), SARS CoV2 (5), Adenovirus (5), RSV (3),Influenza A (2), Human Metapneumovirus (2),Parainfluenza virus 3 (1) and Coronavirus NL63 (1) . Only 2 types of bacteria were identified which were Chlamydia pneumoniae (1) and Bordetella pertussis (1). Among total 100 patients, 58 (58.0%) patients used antibiotic, among antibiotic receiver 34 (58.62%) patients were male and 24 (41.37%) patients were female and 42 (42.0%) patients did not use antibiotic. Among positive 69 patients, 43 (62.3%) patients used antibiotic, among antibiotic receiver 26 (60.46%) patients were male and 17 (39.54%) patients were female. Azithromycin was the commonly used antibiotic 26 (26%) and Ciprofloxacin was used by 9 (9%) patients among antibiotic user.
Conclusion:
By implementing FilmArray respiratory panel assay in acute respiratory tract infections microorganism can be precisely diagnosed and inappropriate use of antibiotics can be reduced.
Keywords
<p>Acute respiratory tract infection; FilmArray respiratory panel assay; Viral infection, Influenza</p>
Article Details
Introduction
Infections of the respiratory tract are the most common diseases in primary medical care. Acute respiratory tract infection (ARTI) is one of the major infectious diseases that may occur at any age and accounts for 3.5 million deaths worldwide [1]. ARTIs are classified as acute upper respiratory tract infection (URTI) (such as laryngitis, pharyngitis, nasopharyngitis, and rhinitis), acute bronchitis or pneumonia.
Diagnosis of ARTI except pneumonia is largely based on clinical signs and symptoms, because viruses, the most commonly causative pathogens of URTI and acute bronchitis, are difficult to detect. The clinical manifestations of respiratory infections caused by the common bacterial and viral agents are similar in most cases, including cough, fever ≥ 38°C, rhinitis, sneezing, nasal congestion, headache, weakness and/or difficult breathing. On the other hand, the clinical spectrum is variable, ranging from mild infections, which can be treated on an outpatient basis, to more serious forms that require hospitalization, particularly in patients with older age, immunosuppressive condition or metabolic diseases [2,3].
Among different types of ARTI, for bacterial pneumonia, prompt treatment with antibiotics can improve outcomes, and delay in effective antimicrobial therapy is associated with increased mortality and length of hospital stay [4]. Current guidelines recommend empirical use of antimicrobial therapy among patients with suspected pneumonia [5]. While empirical antibiotics are critical for patients with pneumonia, the extensive use of antibiotics among patients with respiratory conditions threatens to erode the efficacy of these medications. Recent estimates suggest that half of antibiotic prescriptions for acute respiratory conditions are unnecessary, primarily in the setting where antibiotics are given for viral infections [6]. Unnecessary antibiotic use has led to a rise in the number of antibiotic-resistant infections, which now affect over 2.8 million people each year and result in over 35,000 deaths annually [7]. Rapid diagnostic testing for pneumonia and other ARTIs has the potential to guide clinical decisions and reduce the use of broad-spectrum antibiotics; however, these benefits are dependent upon the accuracy of these test methods.
The early detection of the potential causative agents of ARTI is essential for appropriate treatment, helping to reduce the overuse of antibiotics therapy, and prevent outbreaks or reinfection [8]. In this context, polymerase chain reaction (PCR) assays have shown to be a sensitive and specific tool for detection of these agents. However, the limitations of traditional PCR are that it can only target a single pathogen and long turn around time [9]. In recent years, these problems have been overcome due to several multiplex platforms using PCR, and assays for nucleic acid amplification for the simultaneous identification of two or more viruses have been established. PCR methods, especially multiplex real-time PCR (RT-PCR) techniques, have been applied in the laboratory to provide the high-speed detection of multiple respiratory organisms from patient specimens in an easy workflow [10]. In addition, an expanded range of viral and bacterial targets can be differentiated and identified by PCR assay. There are also several multiplex PCR platforms available with the potential to identify multiple pathogens in a single reaction [11].
The FilmArray Biofire respiratory 2.1 panel (FARP) is a multiplexed, fully automated nested PCR assay, which can detect eighteen common respiratory virus and four bacterial pathogens with a turn around time of approximately 1 hour [12]. Previous studies have shown that FARP assay reveals excellent clinical utility over the more traditional laboratory methods of virus culture and direct antigen tests [13-14]. Owing to the sensitive detection of respiratory viruses, more and more clinical laboratories have introduced this technique to solve intractable cases for clinicians.
Data about FARP application in the detection of ARTI pathogens in adult outpatients in Bangladesh is still unclear. In this regard, the present study is designed to identify and describe the etiological agents associated with ARTIs by FilmArray RP in adults in outpatients of Bangladesh Institute of Tropical and Infectious Diseases Hospital in Chattogram and their associated risk factors, such as clinical symptoms or sociodemographic characteristics and reduce inappropriate antibiotic use in viral infections.
Methodology:
Study design
This cross-sectional Observational study was conducted in outpatient departments of Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh from 1st July 2023 to 31st December 2023.
Study population and settings
A total of 100 patients with ARTI who attended in outpatient department of the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh were enrolled in the study. Patients were enrolled based on clinical suspicion of acute respiratory tract infection in adult outpatients.
Inclusion criteria encompassed patients presenting with the following symptoms of respiratory tract infection such as cough, fever, rhinitis, sore throat, nasal congestion, sneezing, headache, wheeze, throat discomfort, muscle ache, lethargy, chest tightness, shortness of breath as an acute onset of symptoms within 7 days suspected as respiratory tract infection and age more than 18 yrs. Failure to obtain consent from the patients, age < 18 years old and patient presenting with severe infection like severe pneumonia were excluded in this study.
Data Collection and Laboratory Analysis
Nasopharyngeal swabs for FilmArray RP obtained from all patients. According to the instruction, nasopharyngeal secretion (NPS) samples were collected on the basis of standard technique from these enrolled patients by clinicians and immediately placed in viral transport media (VTM). Specimens in VTM were processed and tested as soon as possible. If storage is required, specimens in VTM can be held at refrigerator temperature (2–8 °C) for up to 3 days. The FARP assay was performed by multiplex PCR according to the manufacturer’s instructions (BioMérieux, France). In short, after hydrating the pouch with 1 mL of hydration solution, 300 ml of respiratory sample (VTM) was diluted in 0.5 ml of sample buffer, of which 300 ml was injected into the sample pouch, which was then loaded on the instrument. After entering the sample identification, the instrument was started and the result was available in approximately 1 hour. The testing pouch contains all the reagents for nucleic acid extraction, reverse transcription, first step multiplex PCR amplification and second step real time PCR amplification with single specific primer pair. For each target agent, the second step PCR was performed in triplicate. The software automatically analyzes the melting curve of the second step PCR to report the result as positive or negative. The following organism types and subtypes are identified: adenovirus, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43,SARS CoV 2, human metapneumovirus, human rhinovirus/enterovirus, influenza A virus, influenza A virus A/H1, influenza A virus A/ H1-2009, influenza A virus A/H3, influenza B, parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3, parainfluenza Virus 4, respiratory syncytial virus, Bordetella pertussis, Bordetella parapertussis, Chlamydia pneumoniae and Mycoplasma pneumoniae. However, human rhinovirus and human enterovirus was reported as indistinguishable since these are closely related viruses and cross positivity between those viruses is possible with the FARP assay.
Statistical Analysis
Purposive sampling was done according to the availability of the patients who fulfilled the selection criteria. Face to face interview was done to collect data with a semi-structured questionnaire. After collection, the data were checked and cleaned, followed by editing, compiling, coding, and categorizing according to the objectives and variable to detect errors and to maintain consistency, relevancy and quality control. Statistical evaluation of the results used to be obtained via the use of a window-based computer software program devised with Statistical Packages for Social Sciences (SPSS-25).
Ethical Considerations
This study received approval from the institutional ethical committee. Informed consent was obtained from all participants before data collection. Patient confidentiality was strictly maintained and all data used solely for research purposes.
Result:
The study was successfully conducted among 100 adult outpatients presenting with acute respiratory tract infections.
Table I: Distribution of the patients according to age (n = 100)
|
Age (years) |
Frequency |
% |
|
18 - 30 |
50 |
50.0 |
|
31 - 40 |
25 |
25.0 |
|
41 - 50 |
7 |
7.0 |
|
> 50 |
18 |
18.0 |
|
Total |
100 |
100.0 |
|
Mean ± SD: 21.2 ± 7.3 years |
||
Table I shows that, most of the patients 50 (50.0%) lies between 18 years to 30 years. Mean ± SD of the patients was 21.2 ± 7.3 years.
Figure I show that, most of the patients 67 (67.00%) were male and 33 (33.00%) were female.
Table II: Distribution of the patients according to symptoms (n = 100).
|
Symptoms |
Frequency |
% |
|
Cough |
87 |
87.0 |
|
Fever |
81 |
81.0 |
|
Runny nose |
66 |
66.0 |
|
Lethargy |
45 |
45.0 |
|
Sneezing |
43 |
43.0 |
|
Muscle ache |
43 |
43.0 |
|
Nasal congestion |
32 |
32.0 |
|
Sore throat |
22 |
22.0 |
|
Sputum |
21 |
21.0 |
|
Headache |
19 |
19.0 |
|
Shortness of breath |
7 |
7.0 |
|
Chest tightness |
6 |
6.0 |
|
Wheeze |
6 |
6.0 |
|
Conjunctivitis |
3 |
3.0 |
|
Haemoptysis |
2 |
2.0 |
Table II shows that, Common symptoms were cough 87 (87.0%), fever 81(81.0%) runny nose 66 (66.0%) and lethargy 45 (45.0%).
Table III: Distribution of the patients according to sign (n=100)
|
Variables |
Frequency |
% |
|
Raised temperature |
72 |
72.0 |
|
RR increased |
7 |
7.0 |
|
BP elevated |
8 |
8.0 |
Table III shows that, most of the patients 72 (72.0%) had a high temperature, respiratory rate was increased in 7 (7.0%), high blood pressure was recorded in 8 (8.0%) patients.
Table IV: Distribution of the patients according to result of FilmArray (n = 100).
|
Result of FilmArray |
Frequency |
% |
|
Positive |
69 |
69.0 |
|
Positive in male |
47 |
68.1 |
|
Positive in female |
22 |
31.9 |
|
Negative |
31 |
31.0 |
Table IV shows that, Result of FilmArray was positive in 69 (69.0%) patients, among them 47 (68.1%) patients were male and 22 (31.9%) patients were female.
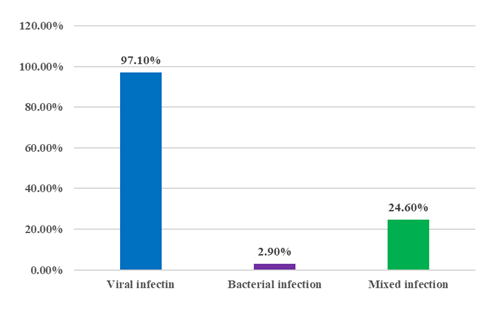
Figure II shows that, viruses are the major pathogen, among 69 positive cases 67 (97.1%) were the viral infection and only 2 (2.9%) were bacterial infection and 17 (24.6%) were mixed infection ( 2 or more viral infection or viral plus bacterial infection).
Table V: Distribution of the patients according to result of FilmArray (n = 100)
|
Name of organism |
Male |
Female |
Total |
|
Influenza B |
17 |
9 |
26 |
|
Influenza A virus A/H3 |
14 |
1 |
15 |
|
Human rhinovirus/enterovirus |
7 |
4 |
11 |
|
Influenza A virus A/ H1-2009 |
3 |
3 |
6 |
|
SARS CoV 2 |
3 |
2 |
5 |
|
Adenovirus |
2 |
3 |
5 |
|
RSV |
3 |
0 |
3 |
|
Influenza A virus |
2 |
0 |
2 |
|
Human metapneumovirus |
2 |
0 |
2 |
|
Parainfluenza virus 3 |
1 |
0 |
1 |
|
Coronavirus NL63 |
1 |
0 |
1 |
|
Chlamydia pneumoniae |
0 |
1 |
1 |
|
Bordetella pertussis |
1 |
0 |
1 |
Table V shows that, 11 types of virus and 2 types of bacteria were identified according to result the of FilmArray. Among them most of the viruses were Influenza B (26), Influenza A virus A/H3 (15), Influenza A virus A/ H1-2009 (6), SARS CoV 2 (5), Adenovirus (5) and RSV (3). Only 2 types of bacteria were identified which were Chlamydia pneumoniae (1) and Bordetella pertussis (1).
Table VI: Distribution of the patients according to use of antibiotic (n = 100).
|
Use of antibiotic |
Frequency |
% |
|
|
Among total 100 case |
Yes |
58 |
58.0 |
|
Male |
34 |
58.62 |
|
|
Female |
24 |
41.37 |
|
|
No |
42 |
42.0 |
|
|
Among positive 69 case |
Yes |
43 |
62.3 |
|
Male |
26 |
60.46 |
|
|
Female |
17 |
39.54 |
|
|
No |
26 |
37.7 |
Table VI shows that, among total 100 patients, 58 (58.0) patients used antibiotic, among 58 antibiotic receiver 34 (34.0%) patients were male and 24 (24.0%) patients were female and 42 (42.0) patients did not use antibiotic. Among positive 69 patients, 43 (63.3) patients used antibiotic, among them 26 (37.7%) patients were male and 17 (24.6%) patients were female and 26 (37.7) patients did not use antibiotic.
Table VII: Distribution of the patients according to type of antibiotic (n = 100)
|
Type of antibiotic |
Frequency |
% |
|
Azithromycin |
26 |
26.0 |
|
Ciprofloxacin |
9 |
9.0 |
|
Cefixime |
7 |
7.0 |
|
Cefuroxime + clavulanic acid |
6 |
6.0 |
|
Amoxicillin + clavulanic acid |
5 |
5.0 |
|
Amoxicillin |
2 |
2.0 |
|
Levofloxacin |
2 |
2.0 |
|
Moxifloxacin |
1 |
1.0 |
Table VII shows that, Azithromycin was the commonly used antibiotic 26 (26.0), Ciprofloxacin was used by 9 (9.0) patients
Discussion:
Both bacteria and viruses have been identified as the agents of ARTI; however, it is known that most of these infections have a viral origin. But antibiotics have been prescribed in many patients with ARTI. Inappropriate prescription of antibiotics promotes antibiotic resistance. Therefore, these viruses should be detected. The early detection of the potential causative agents of ARTI is essential for appropriate treatment, helping to reduce the overuse of antibiotics therapy and prevent outbreaks or reinfection. Conventional methods used to diagnose respiratory tract infection include viral or bacterial culture, rapid antigen test, direct fluroscent antibody assay and molecular assays based on PCR. Each of these methods has advantages and disadvantages with sensitivities and specificities ranging from 44 to 99% and 74 to 100%, respectively requiring additional testing of negative specimens. In addition, these assays can be subjective, require expertise for interpretation of cytopathic effect, have a limited range of detection and turnaround time as long as 14 days. The FilmArray Respiratory panel assay is the first and only FDA cleared assay for the qualitative detection of nucleic acid targets from both viruses and bacteria in nasopharyngeal swab specimen.
This cross-sectional Observational study was conducted in Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh from 1st July, 2023 to 31st December, 2023. A total of 100 adult patients with ARTI who attended in outpatient department of the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh were enrolled in the study. Nasopharyngeal swabs for FilmArray RP obtained from all patients.
In this study, most of the patients 50 (50.0%) lies between 18 years to 30 years. Mean ± SD of the patients was 21.2 ± 7.3 years. Most of the patients 67 (67.00%) were male and 33 (33.00%) were female. Common symptoms were fever 81(81.0%), cough 87 (87.0%), runny nose 66 (66.0%), muscle ache 43 (43.0%) and lethargy 45 (45.0%). Most of the patients 72 (72.0%) had a high temperature, respiratory rate was increased in 7 (7.0%), high blood pressure was recorded in 8 (8.0%) patients. Result of FilmArray was positive in case of 69 (69.0%) patients, among them 47 (68.1%) patients were male and 22 (31.9%) patients were female. Viruses are the major pathogen, among 69 positive cases 67 (97.1%) were viral infections and only 2 (2.9%) were bacterial infection and 17 (24.6%) were mixed infection. About 11 types of viruses were identified according to result the of FilmArray. Among them most of the viruses were Influenza B (26), Influenza A virus A/H3 (15), Influenza A virus A/ H1-2009 (6), SARS CoV 2 (5), Adenovirus (5) and RSV (3). Only 2 types of bacteria were identified which were Chlamydia pneumoniae (1) and Bordetella pertussis (1). Among total 100 patients, 58 (58.0) patients used antibiotic, among 58 antibiotic receiver 34 (34.0%) patients were male and 24 (24.0%) patients were female and 42 (42.0) patients did not use antibiotic. Among positive 69 patients, 43 (63.3) patients used antibiotic, among them 26 (37.7%) patients were male and 17 (24.6%) patients were female and 26 (37.7) patients did not use antibiotic. Azithromycin was the commonly used antibiotic 26 (26.0), Ciprofloxacin was used by 9 (9.0) patients.
In another study, FilmArray RP identified the infections in 56% of adult outpatient cases in the research. When restricted to URTI patients, the detection rate rise to 85% [14].The consensus positive rate of infections in URTI patients was 80.5% in the earlier study on FilmArray RP, Genmark eSensor RVP, Luminex TAG RVP v1, and Luminex Fast Multiplex Assays [15]. However, when this trial was restricted to patients with pneumonia, the detection rate dropped to 27%. As a result, FilmArray RP was advised for URTI patients but not for pneumonia patients. However, lower respiratory materials, like bronchoalveolar lavage fluids, could be used to improve the performance of FilmArray RP in patients with pneumonia [16-18].
The most frequent pathogen identified in the FilmArray RP is influenza virus. Six (42.9%) of the 14 patients with influenza virus in the FilmArray RP tested negative for the influenza antigen. The reported sensitivity of influenza antigen was 62.3% [19]. Furthermore, in a study comparing FilmArray RP and conventional culture, the former detected influenza viruses in all 24 influenza culture-positive cases, with a 100% predictive value [20].
Result of influenza antigen test results in the six patients may be false-negative. To make up for the limited sensitivity of the influenza antigen test, loop-mediated isothermal amplification or multiplex PCR was used. However, because to its tedious and manual procedures, traditional NAAT has not been widely employed as point-of-care testing (POCT).
For NAAT, FilmArray RP is a fully automated platform that produces results in less than an hour. In-house real-time PCR takes 200 minutes, whereas FilmArray RP just takes 2 minutes to operate [21].
When compared to the in-house real-time PCR, which had a mean turnaround time of 26.5 hours (P < 0.001), the mean turnaround time of FilmArray was 2.1 hrs [22]. After conducting the FilmArray, the mean time to test results was much shorter and the percentage of patients having results in the emergency department was higher than those before implementation, according to the earlier comparison study on FilmArray RP and in-house real-time PCR [23]. Therefore, FilmArray RP can be utilized as a POCT in patients with URTI due to its great sensitivity, simplicity, and speed.
In addition to the FilmArray, Verigene and GeneXpert were additional fully automated technologies for respiratory pathogen detection; however, they were only able to identify a smaller number of pathogens. The sensitivity and specificity of FilmArray RP were shown to be equivalent to those of Verigene RV+ in a comparison research [24], however another investigation found that the sensitivity of FilmArray RP in identifying influenza virus was better than that of Verigene [25]. The positive predictive value for FilmArray RP was nearly the same as that of Xpert Flu (FilmArray RP, 98.3% and XpertFlu, 100%, respectively), according to a comparative research on FilmArray RP and GeneXpert (Xpert Flu) for the detection of influenza virus [26]. Moreover, the sensitivity and specificity of FilmArray RP were comparable to those of GeneXpert (Xpert Flu/RSV XC) in a prior investigation on the detection of influenza virus and respiratory syncytial virus [27]. So this study showed that numerous viruses produce ARTI even during influenza epidemics, FilmArray may be a more effective method of pathogen detection than Verigene and GeneXpert.
In another study only three individuals is tested positive during sputum culture, but nine patients who tested positive for the film array RP received antibiotic treatment. Prescription antibiotics for six patients may not be acceptable based on these findings. Since improper antibiotic use has resulted in the growth of multidrug-resistant bacteria, it has become a global concern. Antibiotics have been often recommended in many patients with URTI or acute bronchitis, despite viruses being the most frequent causative microorganisms for ARTI [28-30]. According to a study, the mean duration of antibiotic use was noticeably shorter following the installation of FilmArray than it was prior to its introduction [23]. Additionally, the combination of serum procalcitonin with FilmArray RP demonstrated the potential to enhance antibiotic prescribing [22].
The results of this study showed that by using FilmArray RP many viruses were identified causing acute respiratory tract infections, where antibiotics was used inappropriately and injudicious use of antibiotics is a global thread for antibiotic resistance.
Conclusion:
The importance of FilmArray RP in patients with ARTI was demonstrated in this study. By using FilmArray RP acute respiratory tract infections can be accurately detected and improper antibiotic use can be decreased.
Conflict of interest: There is no conflict of interest.
Funding: We would like to express our profound gratitude to Rodolphe Merieux Laboratory, France, for their contributions to the completion of my project titled “Study of Aetiology of Acute Respiratory Tract Infection in Adult Outpatients in Bangladesh Institute of Tropical and Infectious Diseases (BITID) in Chattogram, Bangladesh, by FilmArray Respiratory Panel Assay in Nasopharyngeal Swab.”
References
- Jin X, Ren J, Li R, et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. E Clinical Medicine 37 (2021): 100986.
- Wen X, Huang Q, Tao H, et al. Clinical characteristics and viral etiologies of outpatients with acute respiratory infections in Huzhou of China: a retrospective study. BMC infectious diseases 19 (2019): 1-8.
- Correia W, Dorta-Guerra R, Sanches M, et al. Study of the Etiology of Acute Respiratory Infections in Children Under 5 Years at the Dr. Agostinho Neto Hospital, Praia, Santiago Island, Cabo Verde. Frontiers in Pediatrics 9 (2021): 716351.
- Houck PM, Bratzler DW, Nsa W, et al. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Archives of internal medicine 164 (2004): 637-644.
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. American journal of respiratory and critical care medicine 200 (2019): e45-67.
- Neill JI. Tackling drug-resistant infections globally: final report and recommendations-The review on antimicrobial resistance. Date of access 16 (2016): 1-9.
- CDC A. Antibiotic resistance threats in the United States. US Department of Health and Human Services: Washington, DC, USA. 2019 Feb 21.
- Harris AM, Hicks LA, Qaseem A, High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Annals of internal medicine 164 (2016): 425-434.
- Fraga D, Meulia T, Fenster S. Real-time PCR. Current protocols essential laboratory techniques 8 (2014): 10-30.
- Hanson KE, Couturier MR. Multiplexed molecular diagnostics for respiratory, gastrointestinal, and central nervous system infections. Clinical Infectious Diseases. 2016;63(10): 1361-1367.
- Dabisch-Ruthe M, Vollmer T, Adams O, et al. Comparison of three multiplex PCR assays for the detection of respiratory viral infections: evaluation of xTAG respiratory virus panel fast assay, RespiFinder 19 assay and RespiFinder SMART 22 assay. BMC infectious diseases. 2012; 12 (2012): 1-10.
- Tomoaki N, Ryosuke I, Atsushi K, et al. Investigating viral involvement in immunocompromised patients using comprehensive infectious disease testing including FilmArray Respiratory Panel 2.1 on Bronchoscopy: A retrospective study 15 (2023): e38820.
- Azadeh N, Sakata KK, Brighton AM, et al. FilmArray respiratory panel assay: comparison of nasopharyngeal swabs and Bronchoalveolar lavage samples. J Clin Micro biol 53 (2015): 3784-3787.
- Kaku N, Hashiguchi K, Iwanaga Y, et al. Evaluation of FilmArray respiratory panel multiplex polymerase chain reaction assay for detection of pathogens in adult outpatients with acute respiratory tract infection. J Infect Chemother 24 (2018): 734-738.
- Popowitch EB, O'Neill SS, Miller MB. Comparison of the Biofire FilmArray RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP fast multiplex assays for detection of respiratory viruses. J Clin Microbiol 51 (2013): 1528e33.
- Ruggiero P, McMillen T, Tang Y-W, et al. Evaluation of the BioFire Fil- mArray respiratory panel and the GenMark eSensor respiratory viral panel on lower respiratory tract specimens. J Clin Microbiol 52 (2014): 288e90.
- Hammond SP, Gagne LS, Stock SR, et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J ClinMicro biol 50 (2012): 3216e21.
- Azadeh N, Sakata KK, Brighton AM, et al. FilmArray respiratory panel assay: comparison of nasopharyngeal swabs and bronchoalveolar lavage samples. J Clin Micro biol 53 (2015): 3784e7.
- Chartrand C, Leeflang MM, Minion J, et al. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Annals of internal medicine 156 (2012): 500-511.
- Chiu S-C, Lin Y-C, Wang H-C, et al. Surveillance of upper respiratory infections using a new multiplex PCR assay compared to conventional methods during the influenza season in Taiwan. Int J Infect Dis 61 (2017): 97e102.
- Renaud C, Crowley J, Jerome KR, et al. Comparison of FilmArray Respiratory Panel and laboratory-developed real-time reverse transcription- polymerase chain reaction assays for respiratory virus detection. Diagn Microbiol Infect Dis 74 (2012): 379e83.
- Gilbert D, Gelfer G, Wang L, et al. The potential of molecular diagnostics and serum procalcitonin levels to change the antibiotic management of community-acquired pneumonia. Diagn Microbiol Infect Dis 86 (2016): 102e7.
- Rogers BB, Shankar P, Jerris RC, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med 139 (2015): 636e41.
- Butt SA, Maceira VP, McCallen ME, et al. Comparison of three com- mercial RT-PCR systems for the detection of respiratory viruses. J Clin Virol 61 (2014): 406e10.
- Van Wesenbeeck L, Meeuws H, Van Immerseel A, et al. Comparison of the FilmArray RP, Verigene RV+, and prodesse ProFLU+/FAST+ multiplex platforms for detection of influenza viruses in clinical samples from the 2011-2012 influenza season in Belgium. J Clin Microbiol 51 (2013): 2977e85.
- Stellrecht KA, Nattanmai SM, Butt J, Maceira VP, Espino AA, Castro AJ, et al. Effect of genomic drift of influenza PCR tests. J Clin Virol 93 (2017): 25e9.
- Wahrenbrock MG, Matushek S, Boonlayangoor S, et al. Comparison of cepheid Xpert Flu/RSV XC and BioFire FilmArray for detection of influenza A, influenza B, and respiratory syncytial virus. J Clin Microbiol 54 (2016): 1902e3.
- Schroeck JL, Ruh CA, Sellick Jr JA, et al. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrobial agents and chemotherapy 59 (2015): 3848-3852.
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Annals of internal medicine 163 (2015): 73-80.
- Silverman M, Povitz M, Sontrop JM, et al. Antibiotic prescribing for nonbacterial acute upper respiratory infections in elderly persons. Annals of internal medicine 166 (2017): 765-774.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 