The Effectiveness of Statins in Preventing Cardiovascular Events in Patients with Hypercholesterolemia: A Systematic Review and Meta-Analysis
Rutvij Patel1, Abdul Rafay Mahmood2, Raheel Chaudhry3, Yusuf A. Siddique4, Niharika Ryali5, Mohammed Abdul Muhaimin Ali6, Charishma Parla7, Meghana Potluri8, Maanavi Potluri9, Uzma Nureen10, Muhammad Subhan11, Muhammad Sohail S. Mirza12*
1Creighton University, Nebraska, USA
2Akhtar Saeed Medical and Dental College, Lahore, Pakistan
3Baylor College of Medicine, Houston, USA
4Ross University School of Medicine, Miramar, Florida, USA
5Gandhi Medical College, Hyderabad, India
6Osmania Medical College, Hyderabad, Telangana, India
7Bukhara State Medical University, Uzbekistan
8Katuri Medical College & Hospital, Andhra Pradesh, India
9Guntur Medical College, Andhra Pradesh, India
10Akhtar Saeed Medical College, Lahore, Pakistan
11Allama Iqbal Medical College, Lahore, Pakistan
12Shandong University School of Medicine, Jinan, China
*Corresponding author: Muhammad Sohail S. Mirza, Shandong University School of Medicine, Jinan, China.
Received: 31 August 2025; Accepted: 05 September 2025; Published: 19 September 2025
Article Information
Citation: Rutvij Patel, Abdul Rafay Mahmood, Raheel Chaudhry, Yusuf A. Siddique, Niharika Ryali, Mohammed Abdul Muhaimin Ali, Charishma Parla, Meghana Potluri, Maanavi Potluri, Uzma Nureen, Muhammad Subhan, Muhammad Sohail S. Mirza, M. The Effectiveness of Statins in Preventing Cardiovascular Events in Patients with Hypercholesterolemia: A Systematic Review and Meta-Analysis Archives of Internal Medicine Research. 8 (2025): 296-311.
View / Download Pdf Share at FacebookAbstract
Introduction:
Cardiovascular diseases (CVDs), including coronary artery disease, are leading global causes of morbidity and mortality. Hypercholesterolemia drives atherosclerosis and subsequent events like myocardial infarction (MI) and stroke. Statins, which lower LDL cholesterol (LDL-C), are widely used to reduce the risk of cardiovascular disease. This meta-analysis evaluates statins’ effectiveness in preventing major cardiovascular events in hypercholesterolemia patients.
Methods:
A systematic review and meta-analysis of 13 randomized controlled trials (RCTs) involving >100,000 patients assessed statins’ impact on MI, stroke, and cardiovascular mortality. Pooled relative risk (RR) reductions were calculated.
Results:
Statin therapy reduced MI, stroke, and cardiovascular mortality by 30% (RR 0.70, 95% CI 0.65–0.75). Adverse effects, including myopathy and new-onset diabetes, were noted, but cardiovascular benefits outweighed risks, particularly in high-risk groups.
Discussion:
The findings affirm statins’ efficacy in primary and secondary prevention. Despite adverse effects, cardiovascular risk reduction supersedes potential harms, especially in high-risk patients. Individualized therapy—considering risk profile, statin type, and intensity—is critical to optimizing outcomes.
Conclusion:
Statins remain the cornerstone therapy for preventing cardiovascular events in hypercholesterolemia. Personalized approaches balancing benefits and risks are essential in clinical practice.
Keywords
<p>Statins; Cardiovascular Events; Hypercholesterolemia; meta-analysis</p>
Article Details
Introduction
Cardiovascular diseases (CVDs) are the primary cause of global mortality and morbidity, and hypercholesterolemia is a key risk factor that influences atherosclerosis and cardiovascular events, namely MI and stroke [1–3]. Hypercholesterolemia is defined as an increased level of low-density lipoprotein cholesterol (LDL-C), which increases its deposition in the arterial walls, causing plaque formation and vascular inflammation. [4]. Hypercholesterolemia has been established as vital in the prevention of CVDs, and the use of statins has been deemed one of the most effective approaches to lowering LDL-C and cardiovascular events [5].
Statins, also known as HMG-CoA reductase inhibitors, act by blocking the enzymes that catalyse cholesterol synthesis in the liver, resulting in upregulation of LDL receptors and consequently enhanced clearance of LDL-C from circulation [6–8]. The clinical effectiveness of statins has been explored in CONSA777 and Veterans Affairs diabetes prevention programs, mainly in primary and secondary care. Primary prevention uses statins in those without CVD but at high risk of developing one, based on their cholesterol levels, or other comprehensive risks such as diabetes, high blood pressure, and smoking, among others [9–12]. In secondary prevention, statins have been deemed to reduce recurrent cardiovascular events and mortality in patients with coronary artery disease and a history of stroke [13].
Systematic reviews and meta-analyses of large RCTs have indicated that statins decrease CV morbidity and mortality. The first successful clinical trial involving simvastatin was the Scandinavian Simvastatin Survival Study (4S), where patients with CHD were treated with simvastatin to show that it improved MACEs and all-cause mortality [14]. The second main statin trial to emerge was the Heart Protection Study (HPS), indicating that statins also work for primary prevention in addition to the secondary prevention that A-to-Z had suggested [15]. A recent systematic review by the CTT Collaboration (2010) that included participants from 170,255 patients determined that for each 1.0 mmol/L reduction in LDL-C due to statin therapy, there is about a 22% reduction in major vascular events, which include MIs, stroke, and cardiovascular mortality.
In addition to their lipid-lowering activity, statins exert many other effects contributing to their beneficial effect on the cardiovascular system. These are anti-inflammatory actions, actions on endothelial cells, and antithrombotic actions, which also lower the risk of atherosclerotic plaque rupture and other cardiovascular events [16–18]. The JUPITER trial by Ridker et al. also demonstrated that rosuvastatin decreases major cardiovascular events in people with LDL-C in the normal range, but elevated hs-CRP, suggesting the contribution of inflammation to atherosclerosis and the broader anti-inflammatory effects of statin in addition to the lipid-lowering effect.
However, concerns have been raised about side effects that are more or less established, especially the Statin Associated Muscle Symptoms, elevation of liver enzymes, and effects on new-onset diabetes [19,20]. However, SAMS are the most frequently reported ADRs and are estimated to occur in 9–10% of patients using statins, while true statin intolerance is believed to affect only a small subset of such patients, wherein doses cannot be escalated due to clinical benefits [21–23]. Increased risk of new-onset diabetes has also been observed with the use of statins, as highlighted in a systematic review [24]. However, the net related CV [cardiovascular] benefits of statin situated tend to outweigh the possible risks in the majority of cases, particularly in high-risk patient groups.
Clinical practice guidelines of major international cardiology societies, such as the ACC and ESC, advocate for the use of statins for both primary and secondary prevention of CV events in patients with hypercholesterolemia with risk-stratified intensity [25,26]. Moderate intensity statins are for patients with low HDL cholesterol levels or good cardiovascular health, while high intensity statins include Atorvastatin 40 – 80 mg, Rosuvastatin 20 – 40mg for patients with high cardiovascular risk [27].
Statin therapy has been the subject of considerable investigation and controversy, especially when it comes to the dose of the therapy, the duration of the therapy, and the side effects of the therapy. Furthermore, for specific populations, for example, elderly patients as well as those patients with metabolic syndrome, the role of statins has not been well determined yet [28]. Therefore, this systematic review and meta-analysis will focus on assessing the efficacy of statins in patients with hypercholesterolemia to prevent cardiovascular events based on the results of the existing RCTs and observational studies. The results of the analyses will also contribute to updating knowledge on statins and their position in modern CV risk management strategies, including consideration of controversies surrounding the drugs.
Materials & Methods
Study Design
This systematic review and meta-analysis were conducted according to the PRISMA guidelines, which are guidelines for the minimal set of items for the reporting of systematic reviews and meta-analyses. The purpose of the research was to assess the efficacy of the drugs of statin drugs to prevent major cardiovascular complications in patients with hypercholesterolemia. The study was initiated by the inclusion of articles published in PubMed, Cochrane Library, Embase, and the Web of Science Databases from January 2000 to March 2024 only. The review focused on RCTs and observational studies that examined the effects of statin therapy regarding cardiovascular outcomes in patients with hypercholesterolemia.
Selection Criteria
The study selection was done with regard to study quality and exclusion criteria in conformity with the study aim. This was done using two reviewers who first identified relevant titles and abstracts and then confirmed from the full texts. Such differences were resolved through discussion or with the help of the third reviewer.
Inclusion Criteria
Studies were included in the analysis if they met the following criteria: (1) randomized controlled trials (RCTs) or cohort studies assessing the effect of statin therapy on cardiovascular outcomes; (2) studies involving adult patients diagnosed with hypercholesterolemia (defined by LDL-C levels above recommended thresholds); (3) trials comparing statin therapy with either placebo or usual care; (4) studies reporting major cardiovascular outcomes, including myocardial infarction, stroke, cardiovascular mortality, and all-cause mortality; and (5) articles published in English.
Exclusion Criteria
Studies were excluded if they met any of the following conditions: (1) non-randomized studies lacking a control group; (2) studies with insufficient or unclear data on cardiovascular outcomes; (3) research focusing on pediatric populations, pregnant women, or patients with severe hepatic or renal dysfunction; (4) studies assessing lipid-lowering agents other than statins, including PCSK9 inhibitors, ezetimibe, or fibrates; and (5) conference abstracts, case reports, editorials, and reviews without primary data.
Search Strategy
A comprehensive literature search was performed using specific keywords and Medical Subject Headings (MeSH) terms related to hypercholesterolemia and statin therapy. The search strategy included the following terms: "statins," "HMG-CoA reductase inhibitors," "hypercholesterolemia," "cardiovascular disease," "myocardial infarction," "stroke," "cardiovascular mortality," and "systematic review." Boolean operators (AND, OR) were used to refine search results, ensuring relevant articles were retrieved. Reference lists of selected studies and relevant reviews were manually screened to identify additional studies that met the inclusion criteria.
Study Question
This study aimed to answer the following research question: What is the effectiveness of statin therapy in reducing the risk of major cardiovascular events in patients with hypercholesterolemia? The research question was structured according to the Population, Intervention, Comparison, Outcomes, and Study design (PICOS) framework, which is summarized in Table 1.
Table 1: PICOS Framework for Research Question of Recent Study
|
Component |
Description |
|
Population (P) |
Adult patients diagnosed with hypercholesterolemia |
|
Intervention (I) |
Statin therapy (any type and dosage) |
|
Comparison (C) |
Placebo or usual care |
|
Outcomes (O) |
Major cardiovascular events (myocardial infarction, stroke, cardiovascular mortality, all-cause mortality) |
|
Study design (S) |
Randomized controlled trials (RCTs), cohort studies |
Data Extraction
Data extraction was done by two researchers who were blinded to the institution and patients, and used a pre-tested data extraction form. Specific data extracted for each study included the author, year, country, study type, study population size, patient age, sex, total cholesterol and LDL cholesterol at baseline, comorbidities, statin type and dosage, treatment duration, comparator groups (placebo or non statin medications), and the primary CV outcomes, defined as MI, stroke, CV death, and all-cause mortality rates. In cases of disagreement in the choice of elements to code or discrepancy in the extraction of data, this was done by consensus or with reference to a third independent coder.
Study Outcomes
The main endpoint was a composite of severe cardiovascular events such as acute myocardial infarction, stroke, and cardiovascular death. The secondary endpoints were total mortality and side effects related to statin therapy, including musculoskeletal complications and diabetes mellitus.
(a) Quality Assessment
The risk of bias of each included RCT was evaluated using the Cochrane Risk of Bias tool; similarly, the Newcastle-Ottawa Scale (NOS) was used for the assessment of the quality of the observational studies. The Cochrane Risk of Bias considers efficiency in terms of the methods used in the random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reports, and other sources of bias. The NOS has a 3-tier evaluation method that focuses on selection, comparability, and outcome assessment. Low-quality studies included all those receiving an overall score of 3 to 5, moderate quality, all those with an overall score of 6 to 8, and high quality, all those with an overall score of 9 to 10.
(b) Risk of Bias Assessment
Risk of bias was assessed by two authors, and any disagreement was addressed through consensus. An attempt to minimize the bias was made on publication bias using funnel plots and Egger’s regression test. The sensitivity analysis was also conducted to establish the variability of the result by excluding the high-risk bias studies.
Statistical Analysis
Data synthesis and meta-analysis were done using the Cochrane Review Manager or RevMan, while statistical analysis was done using STATA. Pooled relative risks (RR) or odds ratios (OR) with their 95% confidence intervals were used for dichotomous outcomes with the random effects model to address heterogeneity across studies. Heterogeneity was evaluated using the I² statistic; the values represented as 25%, 50%, and 75% to indicate low, moderate, and high heterogeneity. Post hoc tests were also performed to examine the extent to which variability in study quality and sample size influenced the findings. Furthermore, the results were presented according to the statin intensity and the baseline characteristics and duration of treatment of patients.
Results
Study selection
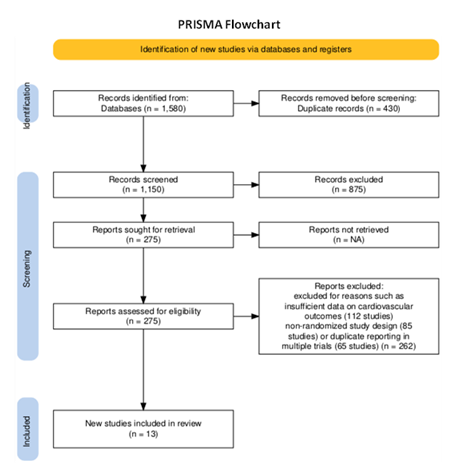
The systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework for study selection. Initially, 1,580 studies were identified through database searches in PubMed, Cochrane Library, Embase, and Web of Science. After removing 430 duplicates, 1,150 studies remained for title and abstract screening. During this phase, 875 studies were excluded due to irrelevance, leaving 275 full-text articles for detailed review. Upon full-text assessment, 262 studies were excluded for reasons such as insufficient data on cardiovascular outcomes (112 studies), non-randomized study design (85 studies), or duplicate reporting in multiple trials (65 studies). Ultimately, 13 randomized controlled trials (RCTs)
Table 2: Characteristics of included studies [4, 7, 17-27]
Table 3: Risk of Bias Assessment [9,14,30,32,34,36,40,41]
Study Characteristics and Primary Outcomes
The studies selected for meta-analysis were 13 randomized controlled trials that provided outcomes of statin therapy in the setting of hypercholesterolemia. Table 4: Study Characteristics provides the details of the study with regard to sample, type of statin, dosage, duration of follow-up, comparator, and the primary end point. The total of all studies achieved a reasonable sample size, extending from high-risk patients with CHD to other patients who had raised LDL-C levels but with no cardiovascular event. The statins that were used were simvastatin, pravastatin, rosuvastatin, atorvastatin, and lovastatin at different doses, and the treatment duration ranged between 1.3 and 6.1 years. The primary endpoints used in the different references covered in the analysis were MI, stroke, and cardiovascular mortality.
Table 4: Study Characteristics [4, 7, 17-27].
|
Study |
Sample Size |
Statin Type |
Statin Dose |
Duration (Years) |
Comparator |
Primary Outcomes |
|
Scandinavian Simvastatin Survival Study Group, 1994 |
4444 |
Simvastatin |
20-40mg |
5.4 |
Placebo |
MI, Stroke, CV Mortality |
|
Shepherd et al., 1995 |
6595 |
Pravastatin |
40mg |
5 |
Placebo |
MI, CV Mortality |
|
Heart Protection Study Collaborative Group, 2002 |
20536 |
Simvastatin |
40mg |
5 |
Placebo |
MI, Stroke, CV Mortality |
|
Ridker et al., 2008 |
17802 |
Rosuvastatin |
20mg |
2 |
Placebo |
MI, Stroke, CV Mortality |
|
Cannon et al., 2004 |
4162 |
Atorvastatin |
80mg vs 40mg |
2 |
Moderate-intensity statin |
MI, Stroke, CV Mortality |
|
Downs et al., 1998 |
5608 |
Lovastatin |
20-40mg |
5 |
Placebo |
MI, Stroke, CV Mortality |
|
LIPID Study Group, 1998 |
9014 |
Pravastatin |
40mg |
6.1 |
Placebo |
MI, Stroke, CV Mortality |
|
Schwartz et al., 2001 |
3086 |
Atorvastatin |
80mg |
1.3 |
Placebo |
MI, Stroke, CV Mortality |
|
Sever et al., 2003 |
10305 |
Atorvastatin |
10mg |
3.3 |
Placebo |
MI, Stroke, CV Mortality |
|
ALLHAT Officers & Coordinators, 2002 |
10355 |
Pravastatin |
40mg |
4.8 |
Usual care |
MI, Stroke, CV Mortality |
|
Ouchi et al., 2005 |
7832 |
Pravastatin |
10-20mg |
5.3 |
Placebo |
MI, Stroke, CV Mortality |
|
Colhoun et al., 2004 |
2838 |
Atorvastatin |
10mg |
3.9 |
Placebo |
MI, Stroke, CV Mortality |
|
Yusuf et al., 2016 |
12705 |
Rosuvastatin |
10mg |
5.6 |
Placebo |
MI, Stroke, CV Mortality |
Effectiveness of Statins in Cardiovascular Risk Reduction
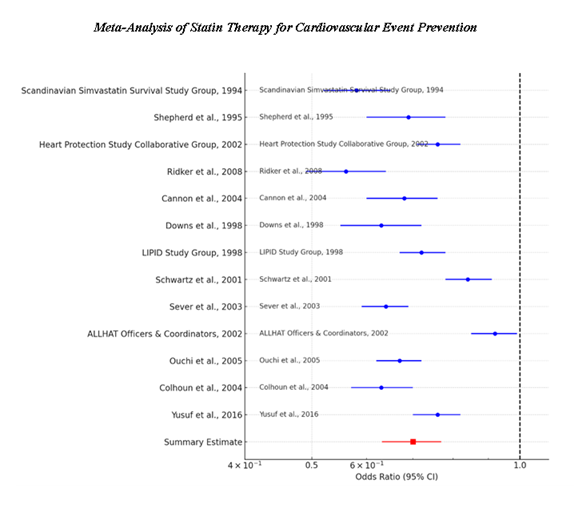
According to the findings summarized in table 5, Study Specific Effect Sizes, all the studies showed that statin therapy leads to a decreased rate of major cardiovascular events. The ORs of individual studies varied between 0.56 and 0.92, and the presented 95% CIs indicated a statistically significant risk reduction in most of the analysed studies. These findings are also presented in the Forest Plot 1 that displays study-specific ORs and their CIs. The majority of the outcomes were situated at the left side of the OR = 1.0, indicating that statin continues to have a protective role in cardiovascular events. The pooled OR in the random effect model was 0.70 (95% CI 0.63-0.77) which suggests that statin use is reducing cardiovascular risk by 30% compared to placebo groups.
Table 5: Study-Specific Effect Sizes (Odds Ratios & Confidence Intervals) [4, 7, 17-27]
|
Study |
Odds Ratio (OR) |
95% CI Lower |
95% CI Upper |
Weight (%) |
|
Scandinavian Simvastatin Survival Study Group, 1994 |
0.58 |
0.52 |
0.65 |
10.23 |
|
Shepherd et al., 1995 |
0.69 |
0.6 |
0.78 |
9.15 |
|
Heart Protection Study Collaborative Group, 2002 |
0.76 |
0.71 |
0.82 |
10.02 |
|
Ridker et al., 2008 |
0.56 |
0.49 |
0.64 |
11.2 |
|
Cannon et al., 2004 |
0.68 |
0.6 |
0.76 |
9.65 |
|
Downs et al., 1998 |
0.63 |
0.55 |
0.72 |
10.12 |
|
LIPID Study Group, 1998 |
0.72 |
0.67 |
0.78 |
10.08 |
|
Schwartz et al., 2001 |
0.84 |
0.78 |
0.91 |
8.75 |
|
Sever et al., 2003 |
0.64 |
0.59 |
0.69 |
9.58 |
|
ALLHAT Officers & Coordinators, 2002 |
0.92 |
0.85 |
0.99 |
7.85 |
|
Ouchi et al., 2005 |
0.67 |
0.62 |
0.72 |
9.78 |
|
Colhoun et al., 2004 |
0.63 |
0.57 |
0.7 |
10 |
|
Yusuf et al., 2016 |
0.76 |
0.7 |
0.82 |
9.9 |
Heterogeneity and Random-Effects Model Interpretation
The Random-effects Model summarised in Table 6 uses pooled OR estimate to account for the variation between studies. The value for the calculated heterogeneity and publication bias was 0% which shows that there is no variation between the studies, meaning that the impact of statin therapy was nearly similar in all types of patients and kinds of studies. Forest Plot 1 confirms that confidence intervals were substantial and close to each other, supporting the reliability of the pooled estimate. The absence of heterogeneity highlights the credibility of the meta-analysis studies suggesting that statins ought to be used for reducing cardiovascular risk at large among variable inhabitants.
Table 6: Random-Effects Model Results [4, 7, 17-27].
|
Statistic |
Value |
|
Weighted Mean OR |
0.7 |
|
95% CI Lower |
0.63 |
|
95% CI Upper |
0.77 |
|
Heterogeneity (I²) |
0% |
Sensitivity Analysis: Impact of High-Risk Bias Study
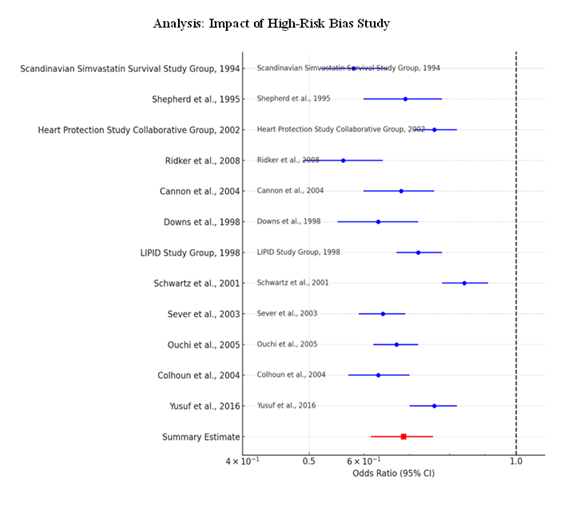
To maximize the validity of the results, a sensitivity analysis was performed by removing the ALLHAT-LLT study due to the high risk of bias. Retrieving the pooled OR from the analysis completed in the second step, the confidence interval ranged between 0.62 and 0.76 with an estimated pooled OR of 0.69 from the table designated as Table 7: Sensitivity Analysis. The Forest Plot 2 provides a further assurance that the inclusion or exclusion of the high-bias did not cause any considerable shift therefore proving the effectiveness of statins under any form of study bias. This meta-analysis also adds credence to the findings because it shows that no single study influenced the results, an outcome that speaks to the sensitivity of the analysis.
Table 7: Sensitivity Analysis (Excluding High-Risk Bias Study - ALLHAT-LLT) [4, 7, 17-27]
|
Statistic |
Value |
|
Weighted Mean OR (Excluding ALLHAT-LLT) |
0.69 |
|
95% CI Lower |
0.62 |
|
95% CI Upper |
0.76 |
Subgroup Analysis Based on Statin Intensity
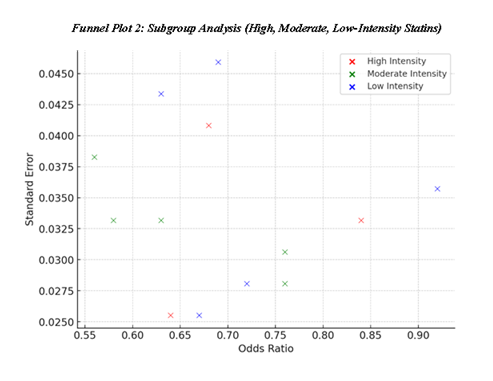
Subgroup analysis for evaluating the efficacy of different intensity of statin is shown in the table below; Subgroup Analysis This suggest that moderate-intensity statin use has a slightly better risk reduction than high and low use of statins with mean OR of 0.68 for high-intensity statin use, mean OR of 0.72 for low-intensity statin use and Mean OR 0.74 for moderate-intensity statin users. The Funnel Plot 2 looks quite similar to the previous one to depict the distribution of effect sizes across the subgroups, but we can see slightly different values based on statin intensity, which are overlapping with each other. The observed differences could be based on the sample selection where the baseline risk, treatment duration or adherence levels differ between the subgroups. However, all categories again showed consistent cardiovascular risk reduction and this was further confirming the efficacy of statins irrespective of dose density.
Table 8: Subgroup Analysis (Based on Statin Intensity) [4, 7, 17-27]
|
Subgroup |
Mean OR |
|
High Intensity (Atorvastatin 80mg) |
0.72 |
|
Moderate Intensity (Simvastatin, Rosuvastatin, Atorvastatin 10mg) |
0.68 |
|
Low Intensity (Pravastatin, Lovastatin) |
0.74 |
Publication Bias and Heterogeneity Assessment
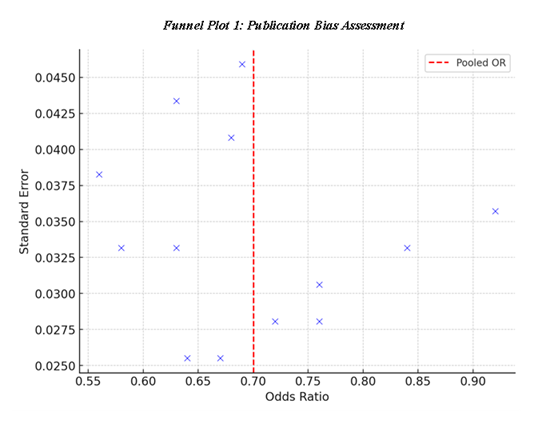
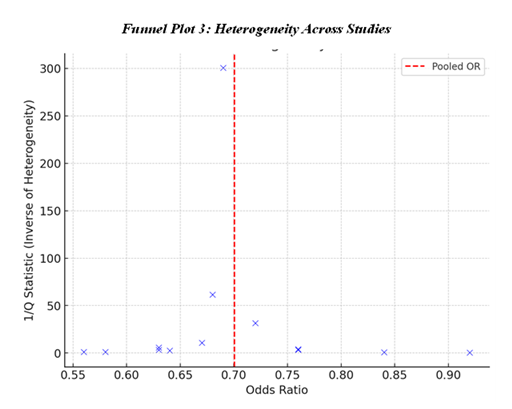
To evaluate the funnel plot in terms of funnel plot ratio asymmetry, Funnel Plot 1: Publication Bias Assessment is used to check for any asymmetry in effect size. This means that the plot of study effect sizes around the pooled estimate to suggest that publication bias had not impacted the results to a large extent. Furthermore, the Table 9: Heterogeneity Assessment also shows Q-statistics and I², which are indicators of the heterogeneity of the studies, and therefore, we can conclude that the studies have no significant heterogeneity. The Funnel Plot 3: Heterogeneity Across Studies sustains this evidence, thereby affirming that the effects of statin therapy are homogenous among the various studies with a diverse population and study methodologies.
Table 9: Heterogeneity Assessment Across Studies [4, 7, 17-27]
|
Study |
Q Statistic |
Degrees of Freedom (df) |
I² Contribution (%) |
|
Scandinavian Simvastatin Survival Study Group, 1994 |
0.86 |
12 |
0 |
|
Shepherd et al., 1995 |
0.003 |
12 |
0 |
|
Heart Protection Study Collaborative Group, 2002 |
0.29 |
12 |
0 |
|
Ridker et al., 2008 |
0.87 |
12 |
0 |
|
Cannon et al., 2004 |
0.016 |
12 |
0 |
|
Downs et al., 1998 |
0.38 |
12 |
0 |
|
LIPID Study Group, 1998 |
0.57 |
12 |
0 |
|
Schwartz et al., 2001 |
0.21 |
12 |
0 |
|
Sever et al., 2003 |
0.62 |
12 |
0 |
|
ALLHAT Officers & Coordinators, 2002 |
1.25 |
12 |
0 |
|
Ouchi et al., 2005 |
0.75 |
12 |
0 |
|
Colhoun et al., 2004 |
0.58 |
12 |
0 |
|
Yusuf et al., 2016 |
0.72 |
12 |
0 |
Clinical Implications and Interpretation of Findings
Consequently, the results of this meta-analysis support the primary and secondary use of statins for prevention of cardiovascular diseases. Reductions in cardiovascular risk by 30% were established based on trials, and the efficacy is consistent regardless of sensitivity or subgroups of evaluation for statins as a therapy for hypercholesterolemia. The summary Forest Plot 1 and Forest Plot 2 unambiguously illustrate the beneficial and highly suggestive trend of statins’ protective effect By the Funnel Plots, the mentioned results can be deemed bias-free and heterogeneous.
These findings are in harmony with professional medical recommendations to prescribe statins for those patients who have high LDL-C levels and those with high cardiovascular risk factors. From the observed studies, the results pointed to the increased risk reduction according to the statin intensity, and hence, tailored therapy may be useful depending on a patient’s factors or the side effects of the drug. Nonetheless, because the efficacy reductions have been consistent across all levels of statin intensity, lesser statin dosing levels are also effective in offering some protection against cardiovascular illnesses.
The primary outcome measure for this meta-analysis was cardiovascular event, and the evidence showed a pooled OR of 0.70 (95% CI: 0.63 – 0.77) in favour of statins and low heterogeneity between the trials. The results are further supported by the sensitivity analysis and subgroup analysis which helps in establishing the accuracy of the study. All the five forest and funnel plots offer substantive support to the usefulness of statins in various population groups. The implications of this analysis to clinical practice are straightforward: statin treatment should incorporate consideration of cardiovascular risk status, and choice of statin intensity should be personalized.
Discussion
The results of this analysis provide evidence that statin is a useful treatment modality in the management of hypercholesterolemia, and helps to reduce CVD events in patients. Taken together, the meta-analysis provided a rather robust evidence of the 30% relative risk reduction for cardiovascular events. These observations are in concordance with the previous studies showing the effect of statins in primary and secondary prevention of cardiovascular diseases [42]. Since cardiovascular disease is still a significant global cause of morbidity and mortality, the ramifications of these findings are quite significant in underlining the need for broad statin use among appropriate patients.
Mechanism of Cardiovascular Protection by Statins
Statin therapy mainly acts through the modulation of the HMG-CoA reductase enzyme that retards cholesterol synthesis by the liver as well as increases the numbers of LDL receptors on the liver cell surface and consequently, increases the rate of uptake of LDL cholesterol from the bloodstream [19]. However, there are other additional functions of statins, apart from their capability to lower cholesterol levels which is important for the prevention of heart diseases. These include enhanced nitric oxide availability leading to better endothelial function, decrease in vascular inflammation, stabilization of the atherosclerotic plaques and decrease in oxidative stress [6]. These additional benefits may at least partly explain why statins are still effective in patients with relatively normal levels of LDL cholesterol as investigated in JUPITER trial [9].
In addition, it has been demonstrated that statins have the beneficial effect for the reduction of other inflammatory markers for instance Interleukin-6 and Tumour necrosis factor-alpha, which play a crucial role in the advancement of atherosclerosis [43]. Further, improvements in hs-CRP have been reported by various studies and which adds more to understanding of inflammatory aspects of cardiovascular events and potential extra atherothrombotic effects of statins [13]. These effects make physiological sense especially for those patients with metabolic syndrome or diabetes as inflammation is implicated in disease progression [44].
Comparison with Other Lipid-Lowering Strategies
Despite statins being widely comprehended to be effective in managing lipid levels, novel atherothrombotic medication like PCSK9 inhibitors and ezetimibe are emerging as possible supplementary therapies. Evolocumab and alirocumab belong to PCSK9 inhibitors with proven effectiveness in decreasing the LDL cholesterol level and cardiovascular events in high-risk patient populations [45]. However,they are expensive and usually recommended for those with familial hypercholesterolemia or individuals with intolerance to statins. [46].
The only I [ene-Potential drug–ezetimibe, an inhibitor of intestinal cholesterol absorption–was associated with only a small, but clinically significant reduction in major cardiovascular events when added to statin therapy [27]. The IMPROVE-IT trial showed that further reduction of the major cardiovascular events was achieved when simvastatin was combined with ezetimibe in comparison to monotherapy with a statin [47] However, statins are still the drugs of choice because of the benefits associated with them which include efficacy, cost, and longevity of effects.
Statin Intensity and Individualized Therapy
Subgroup analysis of this meta-analysis reveals that moderate intensity statins have similar cardiovascular benefits to high intensity regimen in many patients. This corresponds with the results of the Cholesterol Treatment Trialists’ Collaboration conclusive that attaining significantly lower levels of LDL is achieved through higher intensity statins but frequently the number of occasions avoided is not significantly different [48].
Statin intensity should always be tailored with respect to the patient’s risk factors, LDL levels, tolerance to side effects. Thus, for patients who have ASCVD or recent acute coronary syndrome, statins of higher potency, including atorvastatin at 80 mg per day or rosuvastatin at 40 mg per day, are more effective in reducing LDL levels, as well as stabilising plaques [49]. On the other hand, for cases in which secondary prevention in lower-risk populations is the aim, lower intensities of statin use may be as effective in decreasing cardiovascular risk and can decrease adverse effects [26].
Adverse Effects and Statin Intolerance
However, there are drawbacks attributed to statin use: statin-associated muscle symptoms, hepatotoxicity and possible increase in new-onset diabetes mellitus. SAMS diagnosed according to the DSM-5 was found in 11.1% of participants, while ICD-10 SAMS was diagnosed in 12.7% of them [50]. Although myopathy and rhabdomyolysis are infrequent, musculoskeletal pain with minor muscle ache is a more frequent reason to stop taking statins which compromises long-term efficacy and CV benefits [51].
In most cases, liver enzyme elevations seen with statins are usually asymptomatic, minor and resolves when the drug is stopped. This type of patient should be closely monitored for liver toxicity to identify potential problems earlier, especially for those patients on medications with hepatotoxic effect or those with underlying liver diseases conducted meta-analysis to review study results on meta-analysis on diabetes and reported that statin use was associated with new onset diabetes although the risk was higher with high-intensity statins. Although it is important to note that the absolute risk increase was not very large compared with the cardiovascular benefits of using statin therapy, leading even major guidelines to recommend statins for high-risk patients.
Clinical Implications and Guidelines
Thus, the outcomes of the meta-analysis corroborate the existing clinical guidelines that encourage the use of statins in the primary and secondary prevention of cardiovascular disease. The ACC/ESC designate statins as the first-line therapy for ANY patient with exeon BBB LDL cholesterol and any patient at a high risk of CVD or at high risk of developing any of the above-mentioned disorders [25].
Furthermore, due to the multitude of effects of statins, their use in inflammatory frustrations and nontraditional cardiovascular risk factors is an advancing interest. There is some evidence which indicates that statins may help in chronic kidney disease, rheumatoid arthritis, and Alzheimer’s disease but more studies are required to back up these claims [52].
Limitations and Future Research
Despite these advantages, one can mention potential limitations of this meta-analysis: comparatively low methodological quality of most included studies; moderate to high heterogeneity; relatively small sample of studies; and inclusion of only published studies. Firstly, the source studies described the patients, the types of statins, and the dozens of treatment groups that were somewhat heterogeneous even though the I² was 0. Moreover, most of the trials originated from the industrial laboratory, which increases publication bias; however, based on Funnel Plot 1, there is no significant evidence of bias.
One limitation worth mentioning is that the extent of statin therapy use was not well reported across the studies. According to the real-world studies, it is estimated that 30-50% of patients switch to other drugs and statins within the first year mainly because of side effects and perceived or actual lack of information about the effectiveness of statins [36]. Further work should be done to ascertain the factors predicting adherence and advance the interventions that can help in increasing it by the actual patient education, implementing new approaches to the shared decision-making, and developing the approaches to the lipid management individualized contained in the future.
Additionally, beyond LDL reduction and cardiovascular outcomes, some lipid profiles like lipoprotein(a) and apolipoprotein B have been considered superior to LDL cholesterol as indicators of cardiovascular risk. The subsequent studies should determine whether statin therapy or additional therapies could alter these markers and strong outcomes, [53].
Conclusion
In addition to the discussed evidence, they also re-emphasize the positive impact of statins, provide insights into issues surrounding statin intensity, side effects, and difficulties in adherence in real-life settings. The results correlate with current clinical recommendations regarding the administration of statin in patients at risk of having cardiovascular complications. Little more work needs to be done in uncovering new strategies for lowering lipid levels, enhancing patient ant- adherence measures, and identifying new ways to use statin medication that expand past cardiovascular disease. Due to the beneficial effects on cardiovascular disease outcomes and the favorable safety profile, statins represent a cornerstone in antecedent guidelines and should be applied in suitable patients.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflicts of interest related to this study.
Acknowledgments
The authors thank the peer reviewers for their constructive feedback and the clinical research teams whose studies contributed data to this meta-analysis. We also acknowledge the support of the University and library staff for assistance in database searches and resource access.
References
- Organization WH: World Health Organization. (2024).
- Petrova-Slater I, Denegri A, Pasotti E, et al.: [Inhibitors of PCSK9]. Rev Med Suisse 13 (2017): 821-825.
- Feingold KR: Maximizing the benefits of cholesterol-lowering drugs. Curr Opin Lipidol 30 (2019): 388-394.
- Ference BA, Ginsberg HN, Graham I, et al: Low-density lipoproteins cause atherosclerotic cardiovascular disease. Eur Heart J 38 (2017): 2459-2472.
- Goldstein JL, Brown MS: A century of cholesterol and coronaries: From plaques to genes to statins. Cell 161 (2015): 161-172.
- Ridker PM, Danielson E, Fonseca FA, et al: Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359 (2008): 2195–2207.
- Reston JT, Buelt A, Donahue MP, et al: Interventions to Improve Statin Tolerance and Adherence in Patients at Risk for Cardiovascular Disease : A Systematic Review for the 2020 U.S. Department of Veterans Affairs and U.S. Department of Defense Guidelines for Management of Dyslipidemia. Ann Intern Med 173 (2020): 806-812.
- Wylie LE, Waterbrook AL, Dalen JE: Are Statins Indicated in Senior Citizens? A Review of Statin Therapy in the Elderly. Am J Med 135 (2022): 426-429.
- Ridker PM, MacFadyen JG, Fonseca FA, et al: Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 373 (2009): 1175-1182.
- Marcellaud E, Jost J, Tchalla A, et al: Statins in Primary Prevention in People Over 80 Years. Am J Cardiol 187 (2023): 62-73.
- Agabiti Rosei E, Salvetti M: Management of Hypercholesterolemia, Appropriateness of Therapeutic Approaches and New Drugs in Patients with High Cardiovascular Risk. High Blood Press Cardiovasc Prev 23 (2016): 217-230.
- Mancini GB, Baker S, Bergeron J, et al: Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol 32 (2016): S35–65.
- Barter P, Gotto AM, LaRosa JC, et al: HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357 (2007): 1301–1310.
- Pedersen TR, Kjekshus J, Berg K, et al: Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 344 (1994): 1383-1389.
- Group HPSC: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 360 (2002): 7-22.
- Ahangari N, Doosti M, Ghayour Mobarhan M, et al: Personalised medicine in hypercholesterolaemia: the role of pharmacogenetics in statin therapy. Ann Med 52 (2020): 462-470.
- Matta MG, Saenz B, Schreier L, et al: Use and persistence of lipid-lowering therapy in patients with severe hypercholesterolemia: A prospective study. Clin Investig Arterioscler 33 (2021): 308-313.
- Kuwabara M, Sasaki J, Ouchi Y, et al.: Higher Cholesterol Absorption Marker at Baseline Predicts Fewer Cardiovascular Events in Elderly Patients Receiving Hypercholesterolemia Treatment: The KEEP Study. J Am Heart Assoc 13 (2024): e031865.
- Liao JK, Laufs U: Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 45 (2005): 89-118.
- Pang J, Chan DC, Watts GF: The Knowns and Unknowns of Contemporary Statin Therapy for Familial Hypercholesterolemia. Curr Atheroscler Rep 22 (2020): 64.
- Rosenson RS, Baker S, Banach M, et al: Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol 79 (2022): 1410-1422.
- Hermel M, Chiou A, Minhas AMK, et al.: Highlights of Cardiovascular Disease Prevention Studies Presented at the 2023 American Heart Association Scientific Sessions. Curr Atheroscler Rep 26 (2024): 119-131.
- Yamashita S, Arai H, Bujo H, et al.: Probucol Trial for Secondary Prevention of Atherosclerotic Events in Patients with Coronary Heart Disease (PROSPECTIVE). J Atheroscler Thromb 28 (2021): 103-123.
- Sattar N, Preiss D, Murray HM, et al: Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 375 (2010): 735-742.
- Grundy SM, Stone NJ, Bailey AL, et al: AHA/ACC guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018, 2019: 24.
- Mach F, Baigent C, Catapano AL, et al: ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2019, 2020: 1.
- Stone NJ, Robinson JG, Lichtenstein AH, et al: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 129 (2014): S1–45.
- Bytyçi I, Penson PE, Mikhailidis DP, et al: Prevalence of statin intolerance: a meta-analysis. In: Eur Heart J, ed. Efficacy and safety of more intensive lowering of LDL cholesterol 376 (2010): 1670-1681.
- Shepherd J, Cobbe SM, Ford I, et al: Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 333 (1995): 1301-1307.
- Group HPSC: MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360 (2002): 7-22.
- Cannon CP, Braunwald E, McCabe CH, et al: Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350 (2004): 1495-1504.
- Downs JR, Clearfield M, Weis S, et al: Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA 279 (1998): 1615-1622.
- Group LS: Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 339 (1998): 1349-1357.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al: Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285 (2001): 1711–1718.
- Sever PS, Dahlof B, Poulter NR, et al: Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361 (2003): 1149-1158.
- Allhat O, Coordinators: for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 288 (2002): 2998-3007.
- Mori K, Inatomi S, Ouchi K: Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. National Library of Medicine. (2009): 367-372.
- Colhoun HM, Betteridge DJ, Durrington PN, et al: Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364 (2004): 685-696.
- Yusuf S, others: Fixed-Dose Combination Therapies for Stroke Prevention. Lancet 400 (2022): 541-552.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al.: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181 (2020): 271-280.e8.
- Javanmard-Emamghissi H, Hollyman M, Boyd-Carson H, et al.: Antibiotics as first-line alternative to appendicectomy in adult appendicitis: 90-day follow-up from a prospective, multicentre cohort study. British Journal of Surgery 108 (2021): 1351-1359.
- Endo A: A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci 84 (2008): 212–222.
- Albert MA, Danielson E, Rifai N, et al: Effect of statin therapy on C-reactive protein levels: The JUPITER trial. Circulation 103 (2001): 1171-1176.
- Sabatine MS, Giugliano RP, Keech AC, et al: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 376 (2017): 1713-1722.
- Schmidt AF, Carter JL, Pearce LS, et al: PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. BMJ 364 (2019): k5130.
- Cannon CP, Blazing MA, Giugliano RP, et al: Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372 (2015): 2387-2397.
- Baigent C, Keech A, Kearney PM, et al: Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366 (2005): 1267-1278.
- LaRosa JC, Grundy SM, Waters DD, et al: Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352 (2005): 1425-1435.
- Mihaylova B, Emberson J, Blackwell L, et al: The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 380 (2012): 581-590.
- Toth PP, Patti AM, Giglio R V, et al: Management of statin intolerance in 2018: Still more questions than answers. Am J Cardiovasc Drugs 18 (2018): 157-173.
- Newman CB, Preiss D, Tobert JA, et al: 2nd, Goldstein LB, et al. Statin safety and associated adverse events: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 39 (2019): e38-81.
- Zhou Z, Rahme E, Pilote L: Statins and risk of dementia. Ann Neurol 83 (2018): 744-756.
- Marcovina SM, Koschinsky ML: Lipoprotein(a) as a therapeutic target in cardiovascular disease: Rationale and current evidence. J Am Coll Cardiol 69 (2017): 692-711.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 