Tocilizumab Effect in COVID-19 Hospitalized Patients: A Systematic Review and Meta-Analysis of Randomized Control Trials
Basheer Abdulrahman1, Waleed Th Aletreby1*, Ahmed F Mady1, 2, Alfateh Mohammed Noor1, Mohammed Lhmdi1, Fahad Faqihi1, Abdulrahman M Alharthy1, Mohammed A Al-Odat1, Dimitrios Karakitsos1, 3, Ziad A Memish5, 6, 7
1Department of Critical Care, King Saud Medical City, Riyadh, Saudi Arabia
2Department of Anesthesia, Faculty of Medicine, Tanta University, Tanta, Egypt
3Department of Medicine, South Carolina University School of Medicine, SC, USA
4Department of Critical Care, Keck School of Medicine, University of Southern California, CA, USA
5Research and Innovation Centre, King Saud Medical City, Riyadh, Saudi Arabia
6Hubert Department of Global Health, Rollins School of Public Health, Emory University, GA, USA
7College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
*Corresponding author: Waleed Th Aletreby, Department of Critical Care, King Saud Medical City, Riyadh, Saudi Arabia
Received: 25 May 2022; Accepted: 07 June 2022; Published: 13 June 2022
Article Information
Citation: Basheer Abdulrahman, Waleed Th Aletreby, Ahmed F Mady, Alfateh Mohammed Noor, Mohammed Lhmdi, Fahad Faqihi, Abdulrahman M Alharthy, Mohammed A Al-Odat, Dimitrios Karakitsos, Ziad A Memish. Tocilizumab Effect in COVID-19 Hospitalized Patients: A Systematic Review and Meta-Analysis of Randomized Control Trials. Archives of Internal Medicine Research 5 (2022): 210-232.
View / Download Pdf Share at FacebookAbstract
Since the emergence of the first cases of COVID-19 viral pneumonia late 2019 several studies evaluated the benefits of different treatment modalities. Early in the pandemic, the interleukin 6 (IL-6) receptor antibody Tocilizumab was considered in view of the cytokine release syndrome associated with COVID-19 infection. Several early observational studies showed beneficial effect of treatment with Tocilizumab on mortality, however, results from well-designed randomized clinical trials (RCT) were contradicting.
Objectives: To perform a systematic literature review and meta-analysis of RCTs utilizing Tocilizumab in the treatment of COVID-19 pneumonia, with in-hospital mortality as a primary objective, while secondary objectives included composite outcome of mortality, intubation, or ICU admission, another secondary outcome was super added infection.
Method: This was a random effects model (DerSimonian and Laird) model of relative risk (RR), along with corresponding 95% confidence intervals, p values, and forest plots of both primary and secondary outcomes. A fixed effect sensitivity test was performed for the primary outcome, in addition to subgroup and meta-regression analyses with predefined criteria.
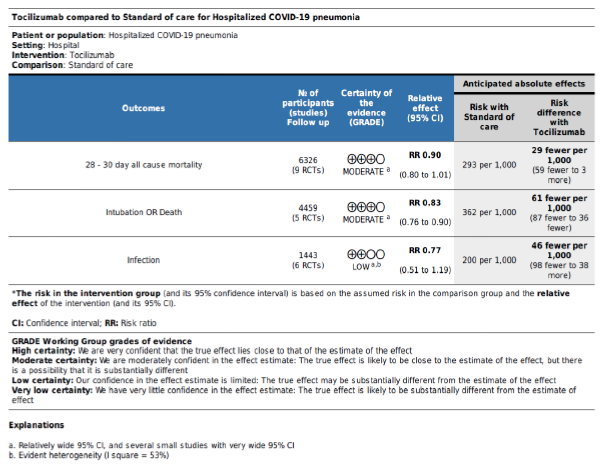
Results: The primary outcome of mortality showed statistically insignificant reduction of mortality with Tocilizumab (RR = 0.9, 95% CI: 0.8 – 1.01; p = 0.09) although with an unmistakable apparent clinical benefit. There was a significant reduction in the RR of the secondary composite outcome (RR = 0.83, 95% CI: 0.76 – 0.9; p < 0.001), and no difference between groups in super-added infection (RR = 0.77, 95% CI: 0.51 – 1.19; p = 0.24). Treatment protocol allowing a second dose was the only significant predictor of improved mortality in the meta-regression analysis. Certainty of evidence was reduced to moderate for the primary outcome and the secondary outcome of clin
Keywords
<p>COVID-19, Tocilizumab, Meta Analysis, Mortality</p>
Article Details
1. Introduction
Since the first case of severe acute respiratory syndrome coronavirus 2 (COVID-19) infection was identified at the end of 2019, COVID-19 has become a huge threat to global health [1, 2]. The full spectrum of clinical manifestations of COVID-19 ranges from asymptomatic carriage and mild acute respiratory disease, to severe pneumonia and even acute respiratory distress syndrome (ARDS)[3]. Although, different mortality reports were coming, most of the deaths were attributed to severe COVID-19 cases [4]. COVID-19 is a novel emerging infectious disease associated with a complicated pathogenesis; however, laboratory evidence of severe COVID-19 infections suggests that cytokine release syndrome (CRS) plays a crucial pathogenic role [5]. Although many proinflammatory cytokines are involved in CRS, interleukin-6 (IL-6) is the most important, although it was also found to be a poor prognostic factor [6].
Tocilizumab (TCZ) is a humanized monoclonal anti-
body that can target both membrane-bound and soluble forms of the IL-6 receptor, and several studies have evaluated its efficacy in the treatment of severe COVID-19,tocilizumab use showed a rapid and sustained response and was also associated with signi-ficant clinical improvement. By neutralizing a key inflammatory factor in the cytokine release syndrome (CRS), this molecule may block the cytokine storm during the systemic hyper-inflammation stage and reduce disease severity [7, 8]. In another study by Ramaswamy etal., although tocilizumab-treated patients displayed higher levels of biomarkers [C-reactive protein (CRP) and IL-6] indicative of cytokine storm at initial presentation, tocilizumab still provided a short-term survival benefit [9], such results of observational studies were also reflected in syste-matic reviews [10]. However, contradicting results are emerging from well-designed randomized clinical trials (RCT), indicating lack of such benefit [11], in opposition to other RCTs clearly reflecting clinical and mortality benefits [12]. In view of the conflicting evidence, and the increasing number of published RCTs we aimed to conduct the current systematic literature review to try and fill in the gap of evidence, and consider it as an update of previous reviews that included only observational studies, or those that included both observational and randomized trials.
1.1 Objectives
The primary objective of the review was to compare 28 – 30 day all-cause mortality among hospitalized patients with confirmed COVID-19 infection whom were given TCZ, to the mortality of similar control patients. Secondary objectives included the compar-ison of the same groups with regards to: Combined outcome of death, intubation, or intensive care unit (ICU) admission, and incidence of super-infection.
2. Method
We utilized the PRISMA checklist of preferred reporting items for systematic reviews and meta-analysis.
2.1 Study selection criteria
We included only RCTs that compared head to head at least two arms, one receiving TCZ (as intervention arm) and another not receiving TCZ (as the control), if TCZ was being compared to another medication, that medication’s group was considered the control, but if the study included more than two arms, the TCZ arm was compared to the control only, without conside-ration of the third arm, regardless of TCZ dosing regimen. The included studies must have recruited adult patients (at least 18 years old) with confirmed COVID-19 infection, regardless of other inclusion or exclusion criteria pertaining to the severity of the condition (such as oxygen requirement), or pre-specified laboratory tests’ values. Included studies must have also reported at least one of the objectives of this review.
2.2 Search strategy
We systematically searched for eligible studies in PUBMED, EMBASE, and medical archives (medR-xiv) using the keywords: “COVID-19”, “SARS-CoV-2”, and “Tocilizumab” (details of Pubmed search in supplementary file). Furthermore, we reviewed the list of references of each potentially eligible article for additional studies. The final search list was reviewed by three authors (AB, MA, AW) for final inclusion in the review, any disagreements were resolved by discussion.
2.3 Risk of bias (RoB) and quality of evidence assessment
Each included study was evaluated independently by 2 authors for RoB using the modified version of the Cochrane Collaboration Tool [13], the tool is built in within the Review Manager ® software. The Cochrane Collaboration tool assesses RoB in each included study with regards to 7 domains, namely: random sequence generation, allocation concealment, blinding of participants, blinding of assessors, attrition bias, selective reporting bias, in addition to other sources of bias. Each one of the 7 domains can be evaluated on a 3 level scale as low, unclear, or high risk of bias. The RoB evaluation of each study as well as a summary RoB of included studies were graphically presented. As for the quality of evidence, we evaluated each outcome according to the GRADE methodology [14], very briefly the methodology evaluates certainty of evidence for a particular outcome after the conside-ration of 5 criteria: Individual study risk of bias, direct-ness, consistency, precision, and publication bias. Accordingly, generated evidence of each outcome can be graded as: high, moderate, low, or very low.
2.4 Data abstracting
Each included study was abstracted twice by two independent authors for comparison and validation of consistency. Each author summarized an included study on a pre-prepared excel sheet that included the following information: Last name of first author, year of publication, country, number of patients in intervention and control groups, as well as total number of patients. Data abstracting also included details of inclusion and exclusion criteria, dosing regimen of TCZ, and a list of reported outcome.
2.5 Publication bias assessment
We assessed publication bias of the primary outcome
using Egger’s test (considered significant for publication bias if p value < 0.05), according to the test result we presented a trim-and-fill funnel plot.
2.6 Statistical method
Both the primary and secondary objectives of this review were dichotomous outcomes, accordingly, were presented as risk ratio (RR), with corresponding 95% confidence interval (CI). In view of our antici-pation of existing between studies differences, at least in terms of studied populations and TCZ dosing, we used DerSimonian and Laird method in a random effects model to pool the effect size of each outcome, and presented corresponding forest plots, along with corresponding 95% CI and p values. For each reported outcome we evaluated heterogeneity using I2 test, and considered heterogeneity among included studies to be high if I2 was higher than 50%. Regardless of the value of I2 test, we a priori planned to perform sub-group analysis of the primary outcome according to severity of enrolled patients, accordingly, included studies were divided into two subgroups, based on whether endotracheal intubation and ICU admission was an exclusion criteria or it was allowed during enrollment, another subgroup analysis was planned based on TCZ dose (single or multiple). Furthermore, we planned a meta-regression analysis for the primary outcome based on the following criteria: patients’ severity (dichotomous), TCZ dose (dichotomous single or multiple), and number of recruited patients (contin-uous). We presented log odds ratio (LogOR), 95% CI, and p values of the meta-regression. As a sensitivity test for the primary outcome we also presented RR of the less conservative (narrower CI) fixed effect model [15]. All statistical tests and graphs were generated using STATA 14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) and Review Manager (RevMan) [Computer program]. Version 5.3. Copen-hagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014. The protocol of this study was reviewed and approved by the local institutional review board at King Saud Medical City, Riyadh, Saudi Arabia, under the registration number: H1R1 – 22 – Feb 21-01.
3. Results
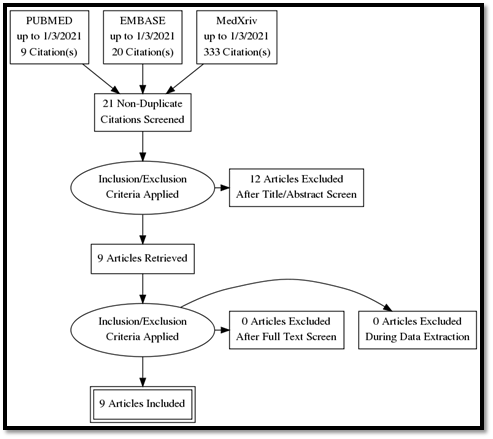
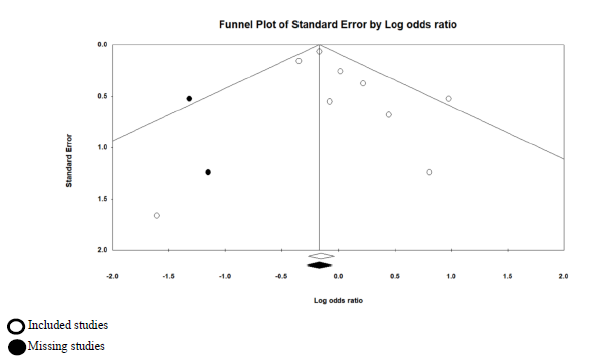
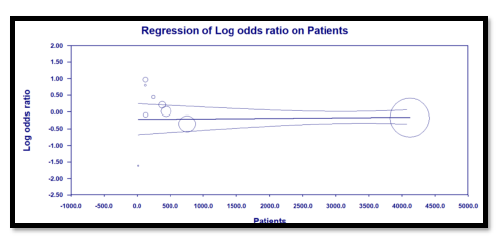
Our systematic search in PUBMED, EMBASE, and medXriv databases resulted in the inclusion of 9 articles [11, 12, 16-22]. Eight articles were duplicates between PUBMED and EMBASE, while one article [12] was unique to MedXriv, figure 1 shows the flow diagram of studies’ inclusion (details of excluded studies provided in table S1, supplementary file). All studies were randomized clinical trials according to our inclusion criteria with only three double blind studies [11, 17, 21]. Three studies [17, 21, 22] were multinational studies, the remaining studies although performed in a single country were all multicenter. The included studies recruited a total of 6326 patients, of whom 3272 patients were randomized to the intervention, and 3054 patients were randomized to control group. Included studies had multiple discre-pancies among them, the most striking was the mechanical ventilation status of recruited patients, as four studies excluded patients if they were mech-anically ventilated [11, 16-18], whereas in the other five studies patients could be enrolled if they were mechanically ventilated [12, 19-22]. Only two studies provided TCZ as a single dose [11, 19], in the rest of the studies a second dose could be given if the patients were not improving clinically. It’s worth noting that the primary outcome of our review (28 – 30 day all-cause mortality) was the primary outcome for only one study [12] (table 1: Details of included studies). Publication bias assessment was done for the nine included studies which all contributed to the primary outcome, and despite fairly visually apparent lack of studies on the left (TCZ) side and apparent asymmetry, the p value of Egger’s test was insignificant (p = 0.201), indicating that there is no effect of small studies, however, trim and fill test indicated that 2 studies were missing on the left side (Figure S1, supplementary file).
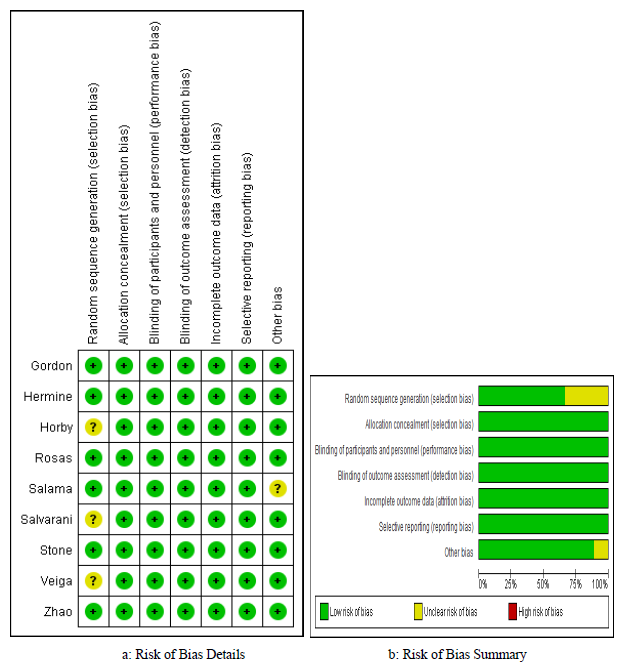
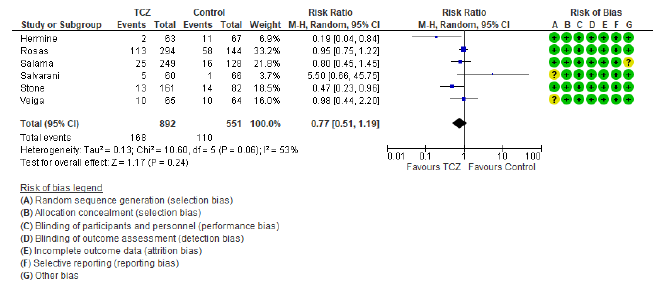
Our assessment of RoB of the included studies was low in five domains for all studies, whereas the domain of random sequence generation (selection bias) was assessed as unclear in three studies [12, 18, 19] since these studies didn’t report the number of screened patients for eligibility, RoB was also deemed unclear in the “Other” domain for one study, in view of the significant involvement of the sponsor in the study’s design, conduct, data collection, and analysis [17]. This means that the overall RoB was 100% low in 5 domains, 67% low in the selection bias, and 89% low in “Other” domain (figure 2 a and b).
3.1 Primary outcome: 28-30 day all-cause mortality
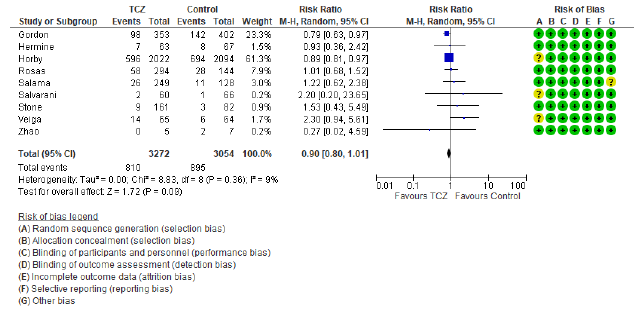
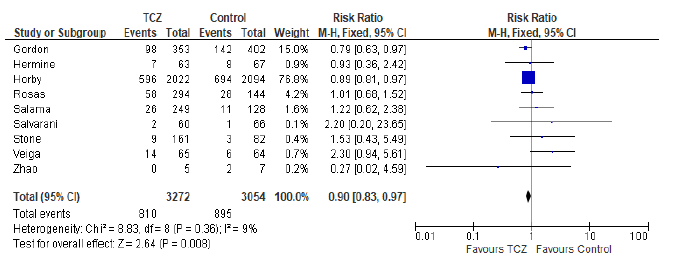
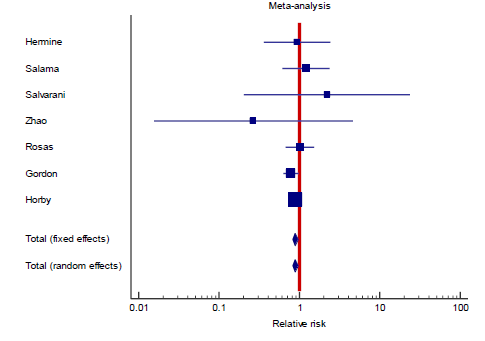
In the intervention (TCZ) pooled arm 810 incidences of death occurred within 28-30 days follow up period out of a total of 3272 patients, whereas in the pooled control arm 895 incidences of death occurred within the same follow up period out of 3054 patients. Intuitively, this result indicates lower mortality in the TCZ arm, however, the result just missed statistical significance in the random effects model with RR = 0.9 (95% CI: 0.8 – 1.01; p = 0.09) (Figure 3). Hetero-geneity among the studies included in the primary outcome was very low at I2 value of only 9%, this low heterogeneity is also reflected in an insignificant p value (0.36) of chi square test of heterogeneity. However, this statistically insignificant impact of TCZ on 28-30 day mortality was not robust in our predefined sensitivity test of fixed effect. In the fixed effect model RR = 0.9 (95% CI: 0.83 – 0.97; p = 0.008).
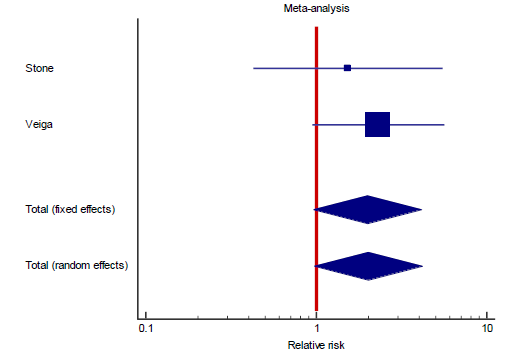
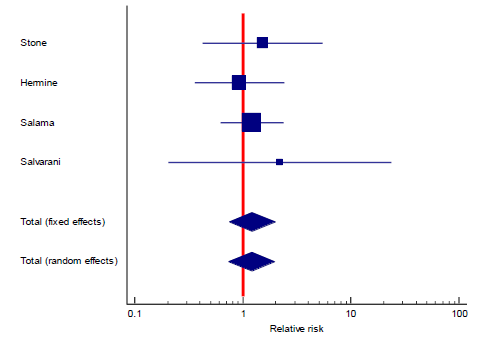
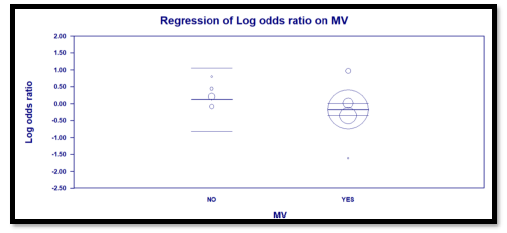
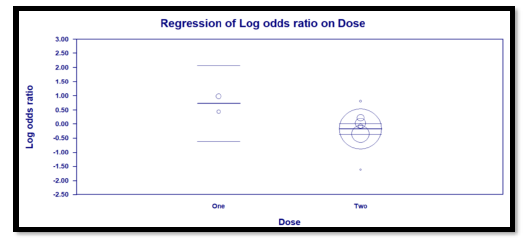
Interestingly, the primary outcome had very low heterogeneity despite the fact that different popul-ations were included, different doses of TCZ given, and substantially variable sample size for each study. Accordingly, we decided to proceed as planned with our sub-group and meta-regression analyses. Sub-group analyses based on the number of TCZ doses indicates that in the two studies allowing only one dose RR = 2.011 (95% CI: 0.97 – 4.2; p = 0.06) that is to say there was no statistical difference although clini-cally the effect was in favor of the control group. On the contrary, in the subgroup of studies that allowed more than one dose of TCZ, there was a statistically significant reduction of mortality in the TCZ group (RR = 0.9, 95% CI: 0.81 – 0.96; p = 0.003). The second predefined subgroup analysis was based on criteria of inclusion, in the subgroup of studies not recruiting mechanically ventilated patients there was no differ-ence between both groups with regards to mortality (RR= 1.2, 95% CI: 0.7 – 2; p = 0.4). Similarly, in the subgroup allowing recruitment of mechanically ventil-ated patients there was no difference in mortality as well (RR = 0.9, 95% CI: 0.8 – 1.1, p = 0.24). The supp-lementary file has more details in figures S3 – S6).
3.2 Meta regression
Three predefined variables were used to perform the meta-regression, number of TCZ doses (as a binary variable), whether recruitment of mechanically ventil-ated patients was allowed (as a binary variable), and the total number of recruited patients in each study (as a continuous variable). The only variable with statistical significance was the use of more than one dose of TCZ, as it showed a significant reduction of mortality. For this variable, the coefficient was -0.91 (95% CI: -0.04 to -1.77; p = 0.04). The other two variables were not statistically significant (Details in table S2 and figures S7 – S9 in supplementary file).
3.3 Secondary outcomes
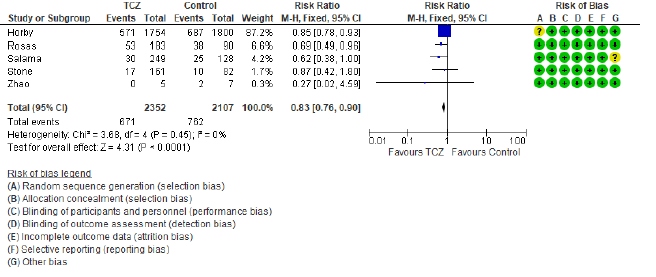
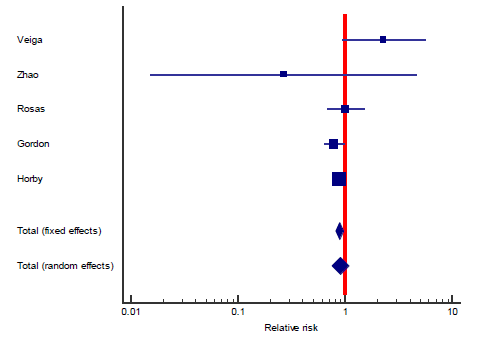
The first secondary outcome was the combined out-come of either death, intubation, or admission to ICU (we collectively call this outcome: Clinical worse-ning). Only five out of the included nine studies contributed toward this outcome (figure 4) including 2352 patients in the TCZ arm and 2107 patients in the control arm. There was a statistically significant reduction of this composite outcome with the admini-stration of TCZ, as RR = 0.83 (95% CI: 0.76 – 90; p < 0.001), there was no heterogeneity detected between studies contributing to this outcome (I2 = 0%, and chi square test of heterogeneity had an insignificant p value of 0.45). The second secondary outcome was the safety outcome of super-added infection, six studies contributed to this outcome, that have reported the incidence of infection out of a total of 892 patients who received TCZ, and 551 patients in the control arm. This outcome showed statistically insignificant RR between both arms (RR = 0.77, 95% CI: 0.51 – 1.19; p = 0.24). Figure 5 shows the forest plot of the superadded infection outcome.
3.4 Certainty of evidence
We utilized the GRADE approach to evaluate the certainty of evidence for our review’s three outcomes. The primary outcome of 28-30 day all-cause mortality and the secondary outcome of composite death/ intubation/ICU admission were both downgraded once to moderate in view of their relatively wide 95% CI and the inclusion of several small studies. The secondary outcome of super-added infection was downgraded twice due to the same reason as the previous two in addition to high heterogeneity with I2 = 53%. Table S3 in supplementary file provides further details.
Table 1: Characteristics of included studies.
4. Discussion
This systematic literature review and meta-analysis may not be unique in investigating the effects of TCZ on the outcomes of COVID-19 hospitalized patients, it is –however- the most updated in terms of inclusion of RCTs on the topic, other reviews [23] had fewer published RCTs available to them at the time of their publication, hence, this review could be considered as an update of previously published information. In this review we included a total of 6326 patients from 9 RCTs that compared receiving TCZ plus standard of care (3272 patients) to standard of care alone (3054 patients) in the management of hospitalized COVID-19 pneumonia. The intuition of a presumed benefit of Il-6 receptor antagonists arisen early in the COVID-19 era in view of similarities of the pathophysiology of COVID-19 pneumonia to other conditions associated with cytokine release syndrome, such as haemo-phagocytic lymphohistiocytosis and macrophage activation syndrome [24] and that this cytokine release syndrome is responsible for the multi-organ failure commonly associated with COVID-19 infection, particularly its severe forms [24], accordingly, the use of humanized monoclonal antibodies against IL-6 receptors such as TCZ may mitigate the dysregulated host immune response in COVID-19 infection, and subsequently avoid associated lung tissue damage [5].
These benefits of TCZ particularly on mortality were demonstrated by several observational studies as well as reviews including observational studies [7, 10], however, such results are questionable in view of the inherent limitations of observational studies in terms of design, in addition to patients’ severity and clinical condition variations [23], furthermore, other reviews failed to demonstrate such benefits, the review by Lan SH et al [25] concluded no additional benefits of TCZ on mortality, mechanical ventilation, and ICU admis-sion, although these results should also be looked at cautiously, in view of the substantial heterogeneity in all three outcomes, and of course the observational nature of the included studies. What was agreed upon by almost all the early studies and reviews was the need for well-designed randomized clinical trials.
In our review the outcome of short term (28 – 30 day) mortality showed no statistical significance of TCZ, however, this result should be meticulously examined, as it only reports statistical significance, while over-looking potential clinical benefit, the RR was found to be 0.9, however, the 95% CI was 0.8 – 1.01, with an overall effect p value of 0.09. While statistically insignificant, we should understand that the signifi-cance was only missed by 0.01 in the 95% CI, more importantly, the result should not be interpreted as lack of effect, but rather as not enough evidence to reject the null hypothesis, and consequently, a clinically meaningful effect can’t just be ruled out based solely on statistical results [26]. Furthermore, the statistically insignificant result of our primary outcome didn’t withstand the sensitivity test of fixed effect model (in fixed effect model RR = 0.9, and 95% CI ranged between 0.83 and 0.97; p = 0.008), although the random effects model is the most valid model of the two in view of differences between studies at least in terms of patients’ conditions and doses of TCZ [27]. What further strengthens the impression that there could be a meaningful clinical effect of TCZ on mortality despite a statistically insignificant overall effect is the fact that the trim and fill funnel plot (figure S1, supplementary file) indicates two missing studies in favor of TCZ (the left side), that is to say our result may be underpowered. This is supported by the findings of a similar review [23] in which only 5 RCTs were included, and the overall effect on mortality was highly insignificant (RR = 1.09, 95% CI: 0.8 – 1.49; p = 0.57), in the study by RECOVERY Collaborative group [12] a meta-analysis section of previously published RCTs was included, in that section the addition of three more RCTs yielded a statistically significant result in favor of TCZ (RR = 0.87, 95% CI: 0.79 – 0.96; p = 0.005). While the addition of a ninth small RCT in our review [20] that showed no difference in mortality resulted in widening of the 95% CI to miss statistical significance.
Despite the fact that heterogeneity was substantially low (9%), subgroup analysis a priori determined indicated both a statistically significant and a clinically meaningful reduction of mortality among patients treated with more than one dose of TCZ, regardless of whether critically ill (mechanically ventilated) patients were recruited or not (figure S4, supplementary file). This finding was confirmed in our meta regression analysis, where doses of TCZ (strictly one dose versus possible second dose) was the only significant predi-ctor of lower mortality, in contrast to the severity of recruited patients which proved insignificant in both subgroup and meta regression analyses. Both of our secondary objectives showed results in favor of treatment with TCZ both statistically and clinically, RR of composite outcome of death, mechanical ventil-ation, or ICU admission was lower in the group treated with TCZ, while there was no difference in the incidence of super-added infection as a safety mea-sure. Notably, those two secondary outcomes included only five and six studies respectively, indicating under-power. It is worth mentioning that the certainty of evidence of the primary outcome, and the secondary composite outcome of death, intubation, or ICU admission were both downgraded to “Moderate” in view of imprecision due to the inclusion of small sized studies with few events, and wide confidence inter-vals. The secondary outcome of super-added infection was downgraded twice to “Low” because of imprec-ision (previously described) and inconsistency with an I2 test of heterogeneity of 53%.
5. Conclusion
We conclude that moderate certainty of evidence suggest no statistically significant improvement of 28-30 day all-cause mortality of hospitalized COVID-19 patients treated with TCZ, although there may be clinically important value. Moderate certainty of evidence suggest lowered relative risk of a composite outcome of death or clinical deterioration, while, low grade evidence indicate no increase in the risk of super-added infection associated with TCZ treatment. A protocol allowing two doses of TCZ shows evidence of improved mortality as compared to a strictly single dose protocol.
Strengths and Limitations
Our review included only RCTs and all available RCTs on the topic to our best knowledge, they were all well-designed with very low levels of RoB. We utilized rigorous statistical methods of meta-analysis, subgroup analysis, and meta-regression, despite low between studies statistical heterogeneity, since clinical heterogeneity was clearly evident at least with regards to study design, blinding, and inclusion criteria. However, our review is subject to several limitations as well, we included data from one preliminary report of a study available at medRxiv but is still not officially published and accordingly not peer reviewed, although with a high level of validity since it was a very well designed study by a highly trusted group. We also included a small study from China that recruited a limited number of patients (12 patients), and this particular study showed no difference in mortality, but had a very wide 95% CI, that had an obvious impact on the overall effect in the primary outcome. Our review examined three outcomes only, the outcomes that appeared to be most patient centered, however, many more outcomes were studied by the included articles, yet we didn’t include them as they were not consistent in the included articles, and investigating them would have resulted in a small number of studies in each outcome. Finally, the primary outcome of our review was in fact the primary outcome of only one included study, in other words, eight out of nine studies were not sufficiently powered to detect the impact of TCZ on short term mortality.
Financial and Conflicts of Interest Declaration
All authors declare no conflicts of interest during writing of this manuscript. No personal or institutional financial support was received during the writing of this manuscript.
Acknowledgment
The authors would like to extend their gratitude to:
Mrs. Huda Mhawish, nursing supervisor of ICU at King Saud Medical City.
Ms. Katrina Baguisa, study coordinator.
Ms. Alva M. Alcazar. Study coordinator.
References
- Aletreby WT, Alharthy AM, Faqihi F, et al. Dynamics of SARS-CoV-2 outbreak in the Kingdom of Saudi Arabia: A predictive model. Saudi Crit Care J 4 (2020): 79-83.
- Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents (2020).
- Alharthy A, Aletreby W, Faqihi F, et al. Clinical Characteristics and Predictors of 28-Day Mortality in 352 Critically Ill Patients with COVID-19: A Retrospective Study. J Epidemiol Glob Health (2020).
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382 (2020): 1708-1720.
- Mady A, Aletreby W, Abdulrahman B, et al. Tocilizumab in the treatment of rapidly evolving COVID-19 pneumonia and multif-aceted critical illness: A retrospective case series. Ann Med Surg (Lond) 60 (2020): 417-424.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (2020): 1054-1062.
- Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med 76 (2020): 31-35.
- Klopfenstein T, Zayet S, Lohse A, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect (2020).
- Ramaswamy M, Mannam P, Comer R, et al. Off-label real world experience using tocili-zumab for patients hospitalized with COVID-19 disease in a regional community health system: a case–control study. MedRxiv (2020).
- Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID?19: A systematic review and meta?analysis. J Med Virol 93 (2021): 1620?1630.
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 383 (2020): 2333-2344.
- RECOVERY Collaborative Group, Peter W Horby, Guilherme Pessoa-Amorim, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv (2021).
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. In: The Cochrane Collaboration (2011).
- Schünemann H, Bro?ek J, Guyatt G, et al. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group (2013).
- Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol 150 (1999): 469-475.
- Hermine O, Mariette X, Tharaux PL, et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Rand-omized Clinical Trial. JAMA Intern Med 181 (2021): 32-40.
- Salama C, Han J, Yau L, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 384 (2021): 20-30.
- Salvarani C, Dolci G, Massari M, et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med 181 (2021): 24-31.
- Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 372 (2021): n84.
- Zhao H, Zhu Q, Zhang C, et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed Pharmacother 133 (2021): 110825.
- Rosas IO, Bräu N, Waters M, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med (2021).
- REMAP-CAP Investigators, Gordon AC, Mouncey PR, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med (2021).
- Tleyjeh IM, Kashour Z, Damlaj M, et al . Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect 27 (2021): 215-227.
- Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 217 (2020): e20200678.
- Lan SH, Lai CC, Huang HT, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents 56 (2020): 106103.
- Schober P, Bossers SM, Schwarte LA. Statistical Significance Versus Clinical Importance of Observed Effect Sizes: What Do P Values and Confidence Intervals Really Represent?. Anesth Analg 126 (2018): 1068-1072.
- Jakobsen JC, Wetterslev J, Winkel P, et al. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 120 (2014).
Supplementary
|
Study Reference |
Reason of exclusion |
|
AndrewTsai, et al. Impact of tocilizumab administration on mortality in severe COVID-19. medRxiv2020.07.30.20114959;doi:https://doi.org/10.1101/2020.07.30.20114959 |
COHORT |
|
Garth W.Strohbehn, et al. COVIDOSE: Low-dose tocilizumab in the treatment of Covid-19. medRxiv2020.07.20.20157503;doi:https://doi.org/10.1101/2020.07.20.20157503 |
Historical Controls |
|
Emily C Somers, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medRxiv2020.05.29.20117358;doi:https://doi.org/10.1101/2020.05.29.20117358 |
COHORT |
|
VictorCarvalho, et al. Effects of Tocilizumab in Critically Ill Patients With COVID-19: A Quasi-Experimental Study. medRxiv2020.07.13.20149328;doi:https://doi.org/10.1101/2020.07.13.20149328 |
Historical controls |
|
Francesco Perrone, et al. Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 prospective phase 2 trial. medRxiv2020.06.01.20119149;doi:https://doi.org/10.1101/2020.06.01.20119149 |
Single arm, not compared to standard. |
|
Malgorzata Mikulska, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. medRxiv2020.06.22.20133413;doi:https://doi.org/10.1101/2020.06.22.20133413 |
Observational |
|
ManuelRubio-Rivas, et al. Beneficial Effect of Corticosteroids in Preventing Mortality in Patients Receiving Tocilizumab to Treat Severe COVID-19 Illness medRxiv2020.08.31.20182428;doi:https://doi.org/10.1101/2020.08.31.20182428 |
Observational |
|
NafisaWadud. Et al. Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. medRxiv2020.05.13.20100081;doi:https://doi.org/10.1101/2020.05.13.20100081 |
Retrospective |
|
Martínez-Sanz J, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. Clin Microbiol Infect. 2021;27(2):238-243. doi:10.1016/j.cmi.2020.09.021 |
COHORT |
|
Tian J, et al. Repurposed Tocilizumab in Patients with Severe COVID-19. J Immunol. 2021;206(3):599-606. doi:10.4049/jimmunol.2000981 |
Retrospective observational. |
|
Pomponio G, et al. Tocilizumab in COVID-19 interstitial pneumonia. J Intern Med. 2021 Jan 29. doi: 10.1111/joim.13231. |
Single arm, not compared to standard. |
|
Jordan SC, et al. Compassionate Use of Tocilizumab for Treatment of SARS-CoV-2 Pneumonia. Clin Infect Dis. 2020 Dec 15;71(12):3168-3173. doi: 10.1093/cid/ciaa812. |
Single arm, not compared to standard. |
Table S1: Excluded studies after review of Abstract/Full test.
|
Covariate |
Coefficient |
Standard Error |
95% Lower |
95% Upper |
Z value |
P value (2 sided) |
|
Intercept |
0.9609 |
0.4585 |
0.0623 |
1.8594 |
2.1 |
0.04 |
|
Total number of patients |
0.00 |
0.00 |
-0.0001 |
0.0001 |
0.39 |
0.7 |
|
Mechanical ventilation (Allowed) |
-0.2959 |
0.3131 |
-0.9096 |
0.3177 |
-0.95 |
0.3 |
|
TCZ Doses (two) |
-0.9069 |
0.4409 |
-1.7711 |
-0.0427 |
-2.06 |
0.04 |
Table S2: Results of Meta regression.
Random Effects: RR = 2.011 (95% CI: 0.97 – 4.2; p 0.06)
Random effects: RR = 0.9 (95% CI: 0.81 – 0.96; p = 0.003)
Random effects: RR = 1.2 (95% CI: 0.7 – 2, p =0.4)
Random Effects: RR = 0.9 (95% CI: 0.8 – 1.1, p = 0.24)


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 78.21%
Acceptance Rate: 78.21%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 