Vitamin D and respiratory infection in a rare genetic disorder. A systematic review and meta-analysis
Ghazala S. Virk, MD1, Yusuf A. Siddique2, Sarah Hack, MD3, Achbari Samia, MD4, Hemamalini Anand Murugesan, MBBS5, Huseyin Erdem Ak, MD6, Dinesh Aravind Rongali, MBBS/MDRD7, Yousif Jihad, MD8, Manaswi Modali, MBBS9, Sana Afzal, MBBS10
1Avalon University School of Medicine, Willemstad, Curaçao.
2Ross University School of Medicine, Florida, USA.
3St. George’s University, Grenada, West Indies.
4Mohammed V University, Rabat, Morocco.
5Government Vellore Medical College, Vellore, Tamilnadu, India.
6Bilecik Seyh Edebali University, Bilecik, Turkey.
7Rush University Medical Centre, Illinois, USA.
8United Lincolnshire Hospitals NHS Trust, Lincolnshire, UK.
9ACSR Government Medical College, Andhra Pradesh, India
10Lahore Medical and Dental College, Lahore, Pakistan.
*Corresponding author: Ghazala S. Virk, Avalon University School of Medicine, curacao.
Received: 15 May 2025; Accepted: 22 May 2025; Published: 23 June 2025
Article Information
Citation: Ghazala S. Virk, Yusuf A. Siddique, Sarah Hack, Achbari Samia, Hemamalini Anand Murugesan, Huseyin Erdem Ak, Dinesh Aravind Rongali, Yousif Jihad, Manaswi Modali, Sana Afzal. Vitamin D and respiratory infection in a rare genetic disorder. A systematic review and meta-analysis. Archives of Internal Medicine Research. 8 (2025): 167-177.
View / Download Pdf Share at FacebookAbstract
Background:
Vitamin D supplementation has been proposed as a capacity intervention to reduce respiratory infections. However, the evidence remains inconsistent across diverse populations and settings. This systematic review and meta-evaluation aimed to assess the efficacy of vitamin D supplementation in decreasing contamination occurrence, infection length, hospitalization charges, and upper respiratory infection (URI) severity.
Methods:
A complete search of digital databases identified 4,597 articles, of which 16 randomized controlled trials (RCTs) met the inclusion criteria. Meta-analyses were carried out the usage of RevMan, and heterogeneity was assessed the use of the I² statistic. The hazard of bias was evaluated using the Cochrane ROB 2 tool.
Results:
1. Infection Incidence: The pooled threat ratio (RR) for contamination prevalence changed into 0.93 (95% CI: 0.83–1.03; p = 0.15), suggesting a non-large 7% discount in risk. Significant heterogeneity changed into found (I² = 77%).
2. Infection Duration: The standardized suggest difference (SMD) for contamination duration was 0.23 (95% CI: -0.49 to 0.94; p = 0.53), with extensive heterogeneity (I² = 83%).
3. Hospitalization Rates: The RR for hospitalization because of respiratory infections became 0.83 (95% CI: 0.56–1.21; p = 0.32), without heterogeneity (I² = 0%).
4. URI Severity: The pooled SMD for URI severity was -0.32 (95% CI: -1.17 to 0.52; p = 0.45), with slight heterogeneity (I² = 70%).
The normal threat of booklet bias became low, even though variability throughout research became obtrusive.
Conclusions:
Vitamin D supplementation demonstrated a protective trend towards breathing infections, but didn't attain statistical significance in outcomes. Significant heterogeneity in contamination prevalence and duration highlights the need for similarly research to clarify its efficacy and become aware of populations most probably to benefit. Standardized methodologies and rigorous trial designs are critical to better understand the function of nutrition D in respiration contamination prevention.
Keywords
<p>Vitamin D; Respiratory infections; Rare genetic disorders; Systematic review; Meta-analysis; Immunomodulation; Cystic fibrosis; Primary ciliary dyskinesia; Infection incidence</p>
Article Details
Introduction
Respiratory tract infections (RTIs) are a common worldwide health difficulty, contributing considerably to morbidity and mortality. In 2010, on my own, RTIs were chargeable for 2.8 million deaths globally [1]. The maximum common pathogens encompass the bacterium Streptococcus pneumoniae and the influenza virus. While vaccines focused on these microbes are to be had in certain regions, their efficacy may be restricted due to vaccine non-responders and mechanisms that allow pathogens to prevent vaccine-precipitated immunity. Current treatment alternatives, including symptomatic therapies, antibiotics, and antivirals, face demanding situations like rising drug resistance, undoubtedly limiting their destiny effectiveness. Consequently, there's a need for added strategies to prevent or mitigate RTIs, and modulating the host immune response provides a promising opportunity. Emerging research highlights the role of nutrition D in modulating immune pathways, enhancing mucosal defenses, even as curtailing immoderate irritation [2]. For example, diet D upregulates the expression of the antimicrobial peptide LL-37 [3], which reveals strong bactericidal activity in opposition to key pathogens, together with Mycobacterium tuberculosis and the influenza virus [4,5]. Notably, human macrophages rely on the nutrition D/LL-37 axis for effective mycobacterial killing, an impact that diminishes while the LL-37 gene is silenced using RNA interference [6,7]. A large range of hospitalized patients with breathing infections require respiration aid and in-depth care (ICU) remedy [8,9]. Several factors contribute to sickness progression, with aged and frail individuals being at the highest risk for unfavorable outcomes and headaches. Additionally, conditions inclusive of cardiovascular sickness, diabetes, malignancy, and weight problems in addition increase the probability of headaches from COVID-19 and other respiratory infections [10–12]. One extremely good thing related to worse outcomes, increased severity, and a higher incidence of headaches is vitamin D deficiency [13,14]. Vitamin D plays a critical role in modulating each innate and adaptive immune responses. It promotes the production of antimicrobial proteins and well-known shows anti anti-inflammatory residences with the aid of lowering viral replication and pro-inflammatory cytokine synthesis [15,16]. Vitamin D also regulates numerous thrombotic pathways, which might also assist in mitigating coagulopathy associated with COVID-19 [17]. Low vitamin D levels are related to a higher occurrence of respiratory infections, along with a 64% extended risk of nosocomial-acquired pneumonia in patients with levels below 50 nmol/L [18,19]. Deficiency in vitamin D has also been related to higher rates of infections, sepsis, and mortality [20–22]. Observational studies have further demonstrated associations between low vitamin D levels and accelerated susceptibility, severity, and mortality in COVID-19 instances, with severe hypovitaminosis D correlating with poorer prognoses and higher mortality rates [23,24].
There is evidence supporting the protective impact of vitamin D supplementation towards respiratory tract infections [25]. However, findings concerning its blessings in COVID-19 sufferers have been inconsistent. While some research has found decreased ailment severity and quicker recovery with supplementation, others have observed no large effect on outcomes [26–32]. To address vitamin D deficiency, high-dose supplementation is frequently required [33]. A sort of dosing regimen is to be had, but bolus doses with longer intervals are generally discouraged because of an accelerated risk of unfavorable consequences [34]. Instead, daily supplementation has been proven to effectively lessen the incidence of respiratory infections in the general populace [35]. For ICU patients, fashionable doses may be inadequate, as it is able to take too long to accurately correct hypovitaminosis D. Higher day-by-day doses or an initial loading dose can be important for timely correction [36]. Different dosing regimens can produce various medical effects, with daily dosing presenting steady availability of vitamin D and its metabolites, probably influencing consequences.
While there may be no consensus at the higher limit for diet D supplementation, day-by-day doses generally range from four hundred to 2,000 IU, with the Endocrine Society recommending a higher restriction of 10,000 IU [37,38]. Studies suggest that long-term daily supplementation of up to 10,000 IU is safe and does not cause detrimental effects in human beings [39,40]. Furthermore, vitamin D supplementation is a cheaper and commonly secure intervention with rare side effects and a huge protection margin, making it a viable choice for hospitalized patients.
Rationale: Respiratory infections are a significant cause of morbidity and mortality globally, and individuals with rare genetic issues, including cystic fibrosis, primary ciliary dyskinesia, or other related situations, face a far greater danger because of their underlying respiratory and immune disorder. These infections regularly result in intense headaches, which include common hospitalizations and decreased nice of life. Emerging proof indicates that vitamin D, a key regulator of immune function, performs an essential function in enhancing mucosal defenses, modulating infection, and reducing the severity of breathing infections. Observational studies have related vitamin D deficiency to higher susceptibility and worse results in respiratory illnesses, highlighting the capability of vitamin D supplementation as a preventive or therapeutic approach. While the broader populace has been studied drastically, there is a lack of centered research examining the effect of vitamin D on breathing infections, especially within the context of rare genetic disorders. This systematic review and meta-analysis aim to synthesize available evidence, examine the efficacy of diet D supplementation in this high-risk population, and offer insights into its function in enhancing medical results, filling an essential gap in the literature.
Objectives: The number one objective of this systematic review and meta-evaluation is to assess the efficacy of nutrition D supplementation in lowering the occurrence, severity, and complications of breathing infections in individuals with uncommon genetic problems, which include cystic fibrosis and primary ciliary dyskinesia. Secondary targets encompass assessing the effect of diet D on immune modulation, biomarkers of respiratory fitness, and average great of lifestyles in this population. By synthesizing facts from present studies, the evaluation pursuits to become aware of styles, quantify effects, and provide evidence-based hints for scientific practice and destiny research. Additionally, it seeks to explore capacity versions in outcomes primarily based on dosing regimens, baseline diet D reputation, and look at design, imparting a complete information of the role of nutrition D in handling respiratory infections in this vulnerable group.
Methodology:
The method follows the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Protocols and Registration:
No registration or ethical approval was required for this systematic review and meta-analysis, as it is based on previously published studies.
Eligibility Criteria: Inclusion standards: Studies ought to include people of any age with uncommon genetic issues linked to respiratory headaches, which include cystic fibrosis or primary ciliary dyskinesia. The intervention of interest is Vitamin D supplementation or treatment, such as bureaucracy like cholecalciferol or vitamin D2. Comparators can consist of a placebo, no treatment, widespread care, or alternative treatments. Outcomes of hobby encompass the prevalence, severity, and duration of respiratory infections, in addition to biomarkers of breathing fitness and pleasant of life. Eligible observational designs consist of randomized controlled trials, observational studies with a control group, and systematic reviews presenting original or pooled records. Publications have to be peer-reviewed, written in English, and ideally posted within the closing 10–15 years.
Exclusion standards: Studies that concentrate on populations with rare genetic problems or on respiratory conditions not of non-genetic origin will be excluded. Research examining interventions other than Vitamin D, case reviews, editorials, animal research, in vitro experiments, and convention abstracts without full-text availability can also be excluded. Non-English publications without to be had translations will no longer be considered.
|
Population |
Intervention |
Comparison |
Outcomes |
Study Design |
|
Adults with heart failure (HFrEF or HFpEF) |
Vitamin D supplementation or treatment |
Placebo, no treatment, standard care, or alternative therapies. |
Incidence, severity, and duration of respiratory infections. |
Randomized controlled trials |
Table 1: PICOS framework
Information Sources: A complete search for studies on the efficacy of Vitamin D on respiratory infections was conducted across more than one digital database, consisting of PubMed, Google Scholar, ScienceDirect, and Cochrane Library. Independent journals and other scholarly guides have also been covered. The seek method adhered to PRISMA tips to ensure comprehensive coverage.
Search Strategy: The search method concerned the use of Boolean operators (AND/OR) to combine phrases related to the study title. Filters have been applied to attention in randomized controlled trials and human research. The search yielded sixteen studies (n=16) that met the inclusion criteria.
|
Sr No. |
Databases |
Search String |
Number of studies |
|
1 |
PubMed |
("Vitamin D"[Mesh] OR "Cholecalciferol"[Mesh] OR "vitamin D supplementation" OR "vitamin D deficiency" OR "cholecalciferol" OR "ergocalciferol") AND ("Respiratory Tract Infections"[Mesh] OR "respiratory infection" OR "respiratory illness" OR "lung infection" OR "pneumonia" OR "bronchitis") AND ("Rare Diseases"[Mesh] OR "Genetic Disorders"[Mesh] OR "hereditary disease" OR "rare genetic disorder" OR "monogenic disorder" OR "Mendelian disorder") AND ("Clinical Trial" OR "Randomized Controlled Trial" OR "RCT" OR "meta-analysis" OR "systematic review") |
1817 |
|
2 |
Cochrane Library |
("Vitamin D" OR "Cholecalciferol" OR "Ergocalciferol") AND ("Respiratory Tract Infections" OR "respiratory infection" OR "lung infection") AND ("Rare Diseases" OR "Genetic Disorders" OR "rare genetic disorder" OR "monogenic disorder") |
371 |
|
3 |
Google Scholar |
"Vitamin D" AND "respiratory infection" AND ("rare genetic disorder" OR "hereditary disease" OR "monogenic disorder") |
2409 |
Table 2: Search strategy on individual databases
Selection Process: The article selection was accomplished in stages. First, titles and abstracts had been screened for relevance. In the second level, the full texts of the selected articles were reviewed to verify eligibility. Data on the primary creator, year of guide, observation layout, use of a sample size, results, and methods were extracted using a standardized records extraction tool.
Data Items: For every study, information on the sample size, study layout, effects, and statistical measures (means, standard deviations) was extracted. Data were synthesized and analyzed the usage of RevMan software for meta-analysis.
Study Risk of Bias Assessment: The Cochrane Risk-of-Bias (version 2) tool was used to evaluate the threat of bias throughout seven domains: random series era, allocation concealment, blinding, incomplete outcome records, selective reporting, and other biases. The risk of bias for every look was assessed as low, unclear, or excessive.
Statistical Analysis: Meta-analysis changed into completed the usage of Review Manager (RevMan) software (version 5.4). A random-consequences model was used due to predicted heterogeneity throughout the research. Heterogeneity was assessed the usage of the I² statistic, and meta-regression turned into performed where applicable.
Reporting Bias Assessment: Potential reporting biases have been minimized by means of selecting high-quality studies and undertaking a thorough search for all relevant publications. Funnel plots have been used to visually check for eBook bias.
RESULTS
Study Selection and Screening
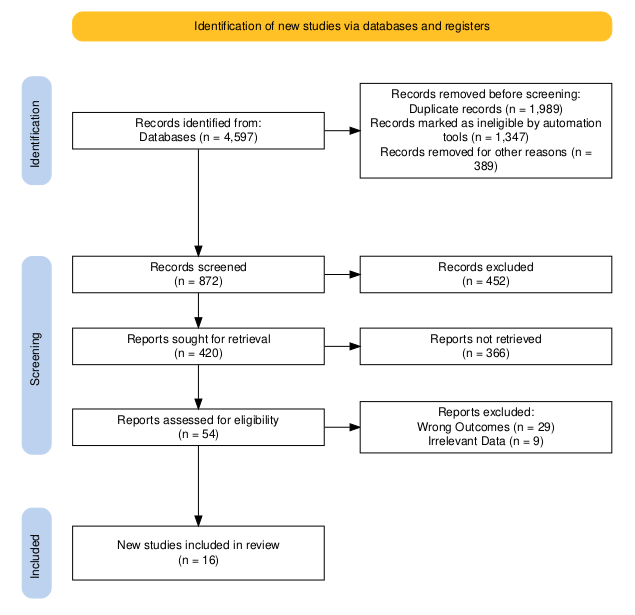
The initial search of the database yielded 4597 papers. After the removal of duplicates and applying the inclusion criteria total of 54 studies for selected for full-text analysis. Based on the methodological quality assessment and inclusion and exclusion criteria, a total of 16 articles finally met the criteria to be included in this systematic review and meta-analysis. Figure 1 presents the detailed PRISMA flowchart diagram of the selection process of the included studies.
Study Characteristics: Study Characteristics of all the included studies are given in Table 3.
Table 3: Characteristics of included studies.
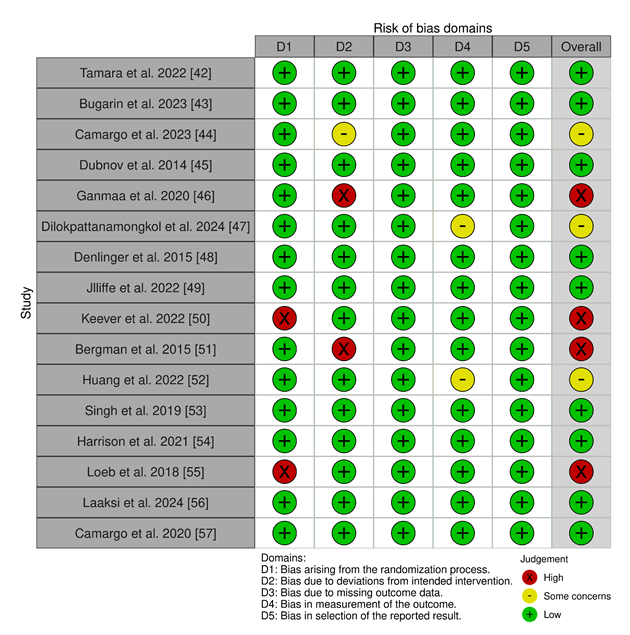
Risk of Bias: Risk of Bias [58] of the included studies was calculated using the Cochrane ROB 2 tool [59] since all of the included studies are Randomized Controlled Trials.
Meta-Analysis: RevMan was used to perform the meta-analysis for this study.
(i) Number of infections:
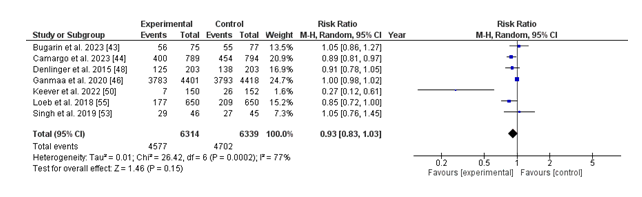
The forest plot illustrates the danger ratio (RR) for the number of infections in individuals receiving nutrition D supplementation in comparison to those receiving a placebo. The standard pooled risk ratio is 0.93 (95% CI: 0.83 to at least 1.03), indicating a 7% discount in the risk of infections with vitamin D supplementation, despite the fact that this result is not statistically significant (p = 0.15). Significant heterogeneity is present across the included studies (I² = 77%, p = 0.0002), suggesting variability in the effect sizes that may be due to variations in look at design, population characteristics, or intervention protocols. While man or woman research like Loeb et al. (2018) and Keever et al. (2022) display statistically huge discounts in infection hazard with vitamin D, other research reports no substantial difference. The ordinary findings suggest a protective capacity effect of diet D supplementation against infections, but the excessive heterogeneity highlights the need for similarly research to clarify its function and perceive populations' maximum in all likelihood to advantage.
(ii) Duration of infection:
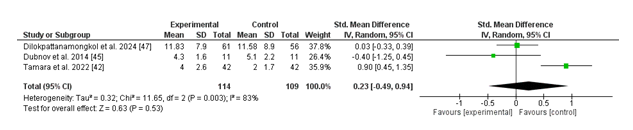
The Forest plot evaluates the effect of vitamin D supplementation as opposed to placebo on the length of infections, measured by the standardized suggest difference (SMD). The normal pooled SMD is zero.23 (95% CI: -0.49 to 0.94), indicating no statistically significant reduction in contamination duration with nutrition D supplementation (p = 0.53). High heterogeneity is located in most of the research (I² = 83%, p = zero.003), suggesting sizable variability in the consequences. Individual studies show combined results, and do not use a consistent fashion favoring both institutions. For instance, Tamara et al. (2022) mentioned an advantageous effect of vitamin D supplementation, whilst different studies like Dubnov et al. (2014) verified no sizable difference. These findings recommend that diet D supplementation does not continuously lessen contamination length, and the high heterogeneity underscores the need for additional research with standardized methodologies to affirm those results.
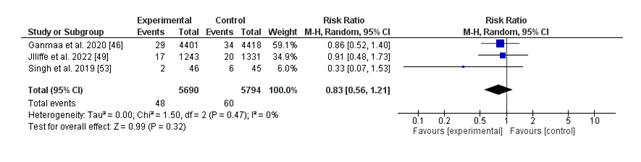
(iii) Hospitalization:
The forest plot summarizes the effect of vitamin D supplementation versus placebo on hospitalization costs due to breathing infections. The pooled chance ratio (RR) is 0.83 (95% CI: 0.56 to at least 1.21), suggesting a 17% discount in hospitalization chance with nutrition D supplementation; however, this end result isn't always statistically significant (p = 0.32). Notably, there is no proof of heterogeneity in a few of the protected studies (I² = 0%, p = 0.47), indicating consistency in the impact sizes suggested. While character research, which includes Ganmaa et al. (2020) and Jolliffe et al. (2022), displays non-great developments favoring nutrition D, the general findings suggest insufficient proof to conclude a defensive impact of diet D supplementation on reducing hospitalizations. Further research with large pattern sizes and extra rigorous take a look at designs is warranted to establish the capability position of nutrition D in this context.
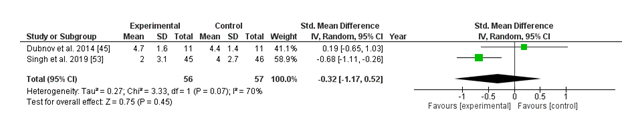
(iv) URI Severity:
The forest plot compares the standardized imply difference (SMD) in higher respiratory contamination (URI) severity between vitamin D supplementation and placebo groups. Two studies have been included in the evaluation, with Dubnov et al. (2014) showing a non-significant advantage of vitamin D (SMD: 0.19; 95% CI: -0.65 to at least one.03), at the same time as Singh et al. (2019) preferred the control group with a greater but still non-significant effect (SMD: -0.68; 95% CI: -1.11 to -0.26). The usual pooled impact estimate (SMD: -0.32; 95% CI: -1.17 to 0.52) suggests no statistically significant distinction in URI severity among the vitamin D and placebo groups (p = 0.45). Moderate heterogeneity was found (I² = 70%, p = 0.07), suggesting some inconsistency throughout research. Collectively, those findings mean that nutrition D supplementation won't appreciably reduce the severity of URIs as compared to placebo, even though variability among observed outcomes warrants further investigation.
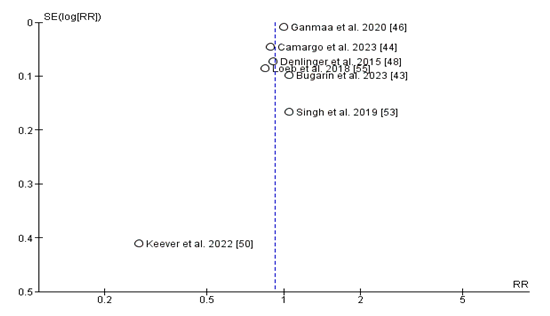
Publication Bias:
The funnel plot assessing publication bias for studies comparing the relative risk (RR) of diet D supplementation on top of upper respiratory infection (URI) incidence seems largely symmetrical around the line of no effect (RR = 1), suggesting a low chance of full-size publication bias. Most research clusters carefully around the valuable axis, with a slight spread in effect sizes that is predicted due to sampling variability. However, the study by means of Keever et al. (2022) seems as an outlier, located lower on the plot and in addition from the critical line, indicating a smaller scale look with bigger preferred errors and a more severe effect estimate. While this may improve some challenges for small-scale look at consequences, the overall distribution does not demonstrate the classic asymmetry function of big guide bias.
DISCUSSION:
This systematic review and meta-analysis assessed the impact of vitamin D supplementation on breathing infections, focusing on infection prevalence, contamination period, hospitalization, and top respiratory infection (URI) severity. While the findings propose ability advantages in some regions, the results have been commonly inconclusive, reflecting the heterogeneity of covered research and variability in the said outcomes.
The analysis of contamination incidence tested a 7% discount in danger with diet D supplementation (RR: 0.93; 95% CI: 0.83 to at least 1.03); however, this was not statistically significant (p = 0.15). Substantial heterogeneity (I² = 77%) changed into observed, in all likelihood stemming from variations in study designs, populations, and intervention protocols. While person research, together with Loeb et al. (2018) and Keever et al. (2022), showed sizeable discounts, others showed no impact, suggesting that the ability of the protective position of diet D can also rely upon unique populations or contexts.
For the contamination period, the pooled evaluation revealed no big reduction with diet D supplementation (SMD: 0.23; 95% CI: -0.49 to 0.94; p = 0.53). The high heterogeneity (I² = 83%) further complicates the translation of those findings. While some research, which includes Tamara et al. (2022), established a high-quality impact, others, like Dubnov et al. (2014), discovered no full-size distinction. This variability underscores the need for standardized methodologies and consistent outcome measures to better understand the position of vitamin D in reducing infection length.
Hospitalization rates because of respiratory infections have been additionally now not been extensively impacted by vitamin D supplementation (RR: 0.83; 95% CI: 0.56 to 1.21; p = 0.32), and not is no heterogeneity amongst research (I² = 0%). Although tendencies favoring diet D were observed in individual studies, together with Ganmaa et al. (2020) and Jolliffe et al. (2022), the general findings suggest inadequate evidence to assist its function in reducing hospitalizations. These effects highlight the need for large, first-rate studies to explore this capability advantage further.
The evaluation of URI severity similarly did not show great differences among the diet D and placebo groups (SMD: -0.32; 95% CI: -1.17 to 0.52; p = 0.45), with slight heterogeneity (I² = 70%). Notably, whilst Singh et al. (2019) said a trend favoring placebo, Dubnov et al. (2014) discovered no meaningful difference. This lack of consistency shows that vitamin D supplementation might not appreciably have an impact on URI severity, though similarly research is warranted to verify these findings.
A current systematic review and meta-analysis verified that vitamin D supplementation has a protective impact against respiratory tract infections (RTIs), with once-every-day dosing recommended because the simplest routine [61]. These findings align with the located developments in our analysis, which suggested a potential, though statistically non-enormous, protective role of vitamin D supplementation in opposition to contamination incidence and hospitalization. However, the heterogeneity discovered across blanket studies in both analyses underscores the demanding situations in drawing definitive conclusions. Differences in population characteristics, baseline diet reputation, dosing regimens, and study designs might also account for the range in results. Moreover, the ability for ebook bias, as stated in the preceding meta-analysis, in addition highlights the need for careful interpretation of these findings. Future studies have to focus on big, properly-designed trials with standardized methodologies to confirm these results and refine recommendations concerning the highest quality dosing techniques and target populations.
The low risk of guide bias located in the funnel plot analysis strengthens the validity of these findings. However, the presence of an outlier (Keever et al. 2022) highlights the capability have an effect on of small-scale research to have larger effect estimates.
Conclusion:
In brief, this meta-analysis highlights mixed and largely non-significant consequences of nutrition D supplementation on breathing contamination effects, with high variability amongst studies. While some subgroups or specific populations can also benefit, the general proof remains inconclusive. Future studies have to focus on figuring out populations most likely to benefit from diet D supplementation, standardizing intervention protocols, and addressing the sources of heterogeneity to offer clearer guidance for medical practice.
References
- Lozano R, Naghavi M, Foreman K, et al.: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380 (2012): 2095-2128.
- Pfeffer PE, Hawrylowicz CM: Vitamin D and lung disease. Thorax 67 (2012): 1018–1020.
- Gombart AF, Borregaard N, Koeffler HP: Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. The FASEB Journal 19 (2005): 1067–1077.
- Barlow PG, Svoboda P, Mackellar A, et al.: Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLoS ONE 6 (2011): e25333.
- Rivas-Santiago B, Santiago CER, Castañeda-Delgado JE, et al: Activity of LL-37, CRAMP and antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium tuberculosis. International Journal of Antimicrobial Agents 41 (2012): 143-148.
- Liu PT, Stenger S, Li H, et al.: Toll-Like receptor triggering of a vitamin D-Mediated human antimicrobial response. Science 311 (2006): 1770–1773.
- Liu PT, Stenger S, Tang DH, et al: Cutting Edge: Vitamin D-Mediated Human Antimicrobial Activity against Mycobacterium tuberculosis Is Dependent on the Induction of Cathelicidin. The Journal of Immunology. 179 (2007): 2060–2063.
- Coronavirus disease (COVID-19) – World Health Organization. (2025). Accessed: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Campi I, Gennari L, Merlotti D, et al.: Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infectious Diseases. (2021): 21.
- Annweiler C, Beaudenon M, Gautier J, et al.: COVID-19 and high-dose Vitamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. (2020): 21.
- Hossein-Nezhad A, Holick MF: Vitamin D for Health: A Global Perspective. Mayo Clinic Proceedings 88 (2013): 720-755.
- Bassatne A, Basbous M, Chakhtoura M, et al: The link between COVID-19 and Vitamin D (VIVID): A systematic review and meta-analysis. Metabolism 119 (2021): 154753.
- Bouillon R, Marcocci C, Carmeliet G, et al.: Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocrine Reviews 40 (2018): 1109–1151.
- Quesada-Gomez JM, Entrenas-Castillo M, Bouillon R: Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections. The Journal of Steroid Biochemistry and Molecular Biology 202 (2020): 105719.
- Hasanloei MAV, Rahimlou M, Eivazloo A, et al: Effect of oral versus intramuscular vitamin D replacement on oxidative stress and outcomes in traumatic mechanical ventilated patients admitted to intensive care unit. Nutrition in Clinical Practice 35 (2019): 548–558.
- Fabbri A, Infante M, Ricordi C: Editorial - Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. DOAJ (DOAJ: Directory of Open Access Journals) 24 (2020): 4048-4052.
- Sengupta T, Majumder R, Majumder S: Role of vitamin D in treating COVID-19-associated coagulopathy: problems and perspectives. Molecular and Cellular Biochemistry. 2021, 476:2421–7. 10.1007/s11010-021-04093-6
- Ali N: Role of vitamin D in preventing COVID-19 infection, progression, and severity. Journal of Infection and Public Health 13 (2020): 1373-1380.
- Zhou Y-F, Luo B-A, Qin L-L: The association between vitamin D deficiency and community-acquired pneumonia. Medicine 98 (2019): e17252.
- Langlois PL, D’Aragon F, Manzanares W: Vitamin D in the ICU: More sun for critically ill adult patients? Nutrition. 2018, 61: 173-178.
- De Haan K, Groeneveld AJ, De Geus HR, et al: Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Critical Care. (2014): 18.
- Zhang Y-P, Wan Y-D, Sun T-W, et al: Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Critical Care. (2014): 18.
- Oscanoa T, Amado J, Vidal X, et al: The Relationship between the Severity and Mortality of SARS-COV-2 Infection and 25-hydroxyvitamin D Concentration—A Metaanalysis. Advances in Respiratory Medicine 89 (2021): 145-157.
- Kazemi A, Mohammadi V, Aghababaee SK, et al: Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: A Systematic Review and Meta-analysis. Advances in Nutrition 12 (2021): 1636-1658.
- Amrein K, Hoffmann M, Lobmeyr E, et al: Vitamin D in critical care: where are we now and what is next? Current Opinion in Critical Care 27 (2021): 378-384.
- Maghbooli Z, Sahraian MA, Jamalimoghadamsiahkali S, et al.: Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocrine Practice 27 (2021): 1242-1251.
- Pal R, Banerjee M, Bhadada SK, et al: Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. Journal of Endocrinological Investigation 45 (2021): 53-68.
- Christopher KB: Vitamin D supplementation in the ICU patient. Current Opinion in Clinical Nutrition & Metabolic Care 18 (2015): 187-192.
- Lan S-H, Lai C-C, Chang S-P, et al: Vitamin D supplementation and the outcomes of critically ill adult patients: a systematic review and meta-analysis of randomized controlled trials. Scientific Reports. (2020): 10.
- Murai IH, Fernandes AL, Sales LP, et al.: Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19. JAMA 325 (2021): 1053.
- Putzu A, Belletti A, Cassina T, et al: Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. Journal of Critical Care 38 (2016): 109-114.
- Stroehlein JK, Wallqvist J, Iannizzi C, et al.: Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Library. (2021).
- Christopher KB: Vitamin D and critical illness outcomes. Current Opinion in Critical Care. 22 (2016): 332–338.
- Sanders KM, Stuart AL, Williamson EJ, et al: Annual High-Dose oral vitamin D and falls and fractures in older women. JAMA 303 (2010): 1815.
- Martineau AR, Jolliffe DA, Hooper RL, et al.: Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017): i6583.
- Hollis BW, Wagner CL: The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. The Journal of Clinical Endocrinology & Metabolism 98 (2013): 4619-4628.
- Ross AC, Manson JE, Abrams SA, et al.: The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. The Journal of Clinical Endocrinology & Metabolism 96 (2010): 53-58.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.: Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 96 (2011): 1911-1930.
- Amrein K, Scherkl M, Hoffmann M, et al.: Vitamin D deficiency 2.0: an update on the current status worldwide. European Journal of Clinical Nutrition 74 (2020): 1498-1513.
- Billington EO, Burt LA, Rose MS, et al.: Safety of High-Dose Vitamin D supplementation: Secondary analysis of a randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism 105 (2019): 1261-1273.
- Haddaway NR, Page MJ, Pritchard CC, et al: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Systematic Reviews. (2022): 18.
- Tamara L, Kartasasmita CB, Alam A, et al: Effects of Vitamin D supplementation on resolution of fever and cough in children with pulmonary tuberculosis: A randomized double-blind controlled trial in Indonesia. Journal of Global Health. (2022): 12.
- Bugarin JD, Dosenovic S, Ilic D, et al.: Vitamin D Supplementation and Clinical Outcomes in Severe COVID-19 Patients—Randomized Controlled Trial. Nutrients 15 (2023): 1234.
- Camargo CA, Schaumberg DA, Friedenberg G, et al.: Effect of daily vitamin D supplementation on risk of upper respiratory infection in older adults: a randomized controlled trial. Clinical Infectious Diseases 78 (2023): 1162-1169.
- Dubnov-Raz G, Hemilä H, Cohen AH, Rinat B, Choleva L, Constantini NW: Vitamin D supplementation and upper respiratory tract infections in adolescent swimmers: a randomized controlled trial. Pediatric Exercise Science. 2014, 27:113–9.
- Ganmaa D, Uyanga B, Zhou X, et al.: Vitamin D supplements for prevention of tuberculosis infection and disease. New England Journal of Medicine. 2020, 383: 359-368. 10.
- Dilokpattanamongkol P, Yan C, Jayanama K, et al: Impact of vitamin D supplementation on the clinical outcomes of COVID-19 pneumonia patients: a single-center randomized controlled trial. BMC Complementary Medicine and Therapies 24 (2024).
- Denlinger LC, King TS, Cardet JC, et al.: Vitamin D Supplementation and the Risk of Colds in Patients with Asthma. American Journal of Respiratory and Critical Care Medicine 193 (2015): 634-641.
- Jolliffe DA, Holt H, Greenig M, et al.: Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ. (2022): e071230.
- Villasis-Keever MA, López-Alarcón MG, Miranda-Novales G, et al.: Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. a randomized clinical trial. Archives of Medical Research 53 (2022): 423-430.
- Bergman P, Norlin A-C, Hansen S, et al: Vitamin D supplementation to patients with frequent respiratory tract infections: a post hoc analysis of a randomized and placebo-controlled trial. BMC Research Notes 8 (2015).
- Huang Y-N, Chi H, Chiu N-C, et al: A randomized trial of vitamin D supplementation to prevent seasonal influenza and enterovirus infection in children. Journal of Microbiology Immunology and Infection 55 (2022): 803-811.
- Singh N, Kamble D, Mahantshetti NS: Effect of vitamin D supplementation in the prevention of recurrent pneumonia in Under-Five Children. The Indian Journal of Pediatrics 86 (2019): 1105–1111.
- Harrison SE, Oliver SJ, Kashi DS, et al.: Influence of Vitamin D Supplementation by Simulated Sunlight or Oral D3 on Respiratory Infection during Military Training. Medicine & Science in Sports & Exercise 53 (2021): 1505-1516.
- Loeb M, Dang AD, Thiem VD, et al.: Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influenza and Other Respiratory Viruses 13 (2018): 176–83.
- Laaksi A, Kyröläinen H, Pihlajamäki H, et al: Effects of vitamin D supplementation and baseline vitamin D status on acute respiratory infections and cathelicidin: a randomized controlled trial. Open Forum Infectious Diseases 11 (2024).
- Camargo CA, Sluyter J, Stewart AW, et al.: Effect of monthly High-Dose Vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clinical Infectious Diseases 71 (2019): 311-317.
- Ma L-L, Wang Y-Y, Yang Z-H, et al: Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Medical Research 7 (2020).
- Sterne J a C, Savovic J, Page MJ, et al.: RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019): l4898.
- Page MJ, Sterne J a. C, Higgins JPT, et al: Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: A review. Research Synthesis Methods 12 (2020): 248-259.
- Bergman P, Lindh ÅU, Björkhem-Bergman L, et al: Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 8 (2013): e65835.
Article Views: 1064
Journal Statistics




Discover More: Recent Articles
Grant Support Articles
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved!