A New Generation of Carbon-Containing Hypergolic Fuels Based on Water-Ignitable C-NaH Mixtures
Athanasios B Bourlinos*
Physics Department, University of Ioannina, Ioannina, Greece
*Corresponding Author: Dr. Athanasios B Bourlinos, Physics Department, University of Ioannina, 45110 Ioannina, Greece.
Received: 29 December 2021; Accepted: 05 January 2022; Published: 13 January 2022
Article Information
Citation:
Athanasios B. Bourlinos. A New Generation of Carbon-Containing Hypergolic Fuels Based on Water-Ignitable C-NaH Mixtures. Journal of Nanotechnology Research 4 (2022): 001-009.
DOI: 10.26502/jnr.2688-85210026
View / Download Pdf Share at FacebookAbstract
Carbon is an energetic, plentiful, cheap, non-toxic and non-corrosive material that is however scarcely used as solid fuel in hypergolic propellants for rocket engines, probably due to the luck of hypergolicity and ignition difficulties. Taking into consideration that carbon hypergolicity is a rare phenomenon in the literature, herein is presented a new type of carbon-containing hypergolic compositions based on water-ignitable C-NaH mixtures. In these formulations, carbon might be fullerenes C60, multiwall carbon nanotubes MWNTs, charcoal or active carbon, whereas sodium hydride NaH and water react exothermically upon contact to trigger carbon combustion and ignition of the mixtures at ambient conditions. Hence, carbon allotropes appear as potentially new solid fuels in hypergolic propellant compositions, meriting further attention in this direction.
Keywords
<p>carbon allotropes; sodium hydride; water; hypergolic propellants; hypergolic reactions</p>
Article Details
1. Introduction
Hypergolic reactions are essential in lifting off rockets to space as the means through which hypergolic fuels or propellants work. Conceptually, in this type of reactions a fuel and a strong oxidizer ignite spontaneously upon contact at ambient conditions without external stimuli (figure 1). Unlike an explosion event, where energy is released in a violent manner, in hypergolic ignition the energy is released in a smooth and continuous manner, provided that the involved reagents are slowly mixed together.
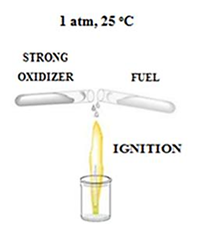
figure 1: Schematic representation of hypergolic reactions. This type of reactions is fast and spontaneous at ambient conditions.
An example refers to the aniline-fuming nitric acid HNO3 hypergolic pair shown in figure 2, which was used in 40’s to launch rocket WAC Corporal. Aniline and fuming nitric acid HNO3 react exothermically upon contact at ambient conditions as following:
5 C6H5NH2 + 31 HNO3 → 30 CO2 + 18 N2 + 33 H2O (1)
Where HNO3 serves as the source of O2 for the exothermic combustion of aniline (ΔΗ < 0). Likewise the exothermic decomposition of explosives, the formation of N2 with a strong triple bond (940 kJ mol-1) additionally favors the exothermic character of the reaction. At the same time, the release of gases secures a positive entropy change as moving from the liquid reactants to the gaseous products (ΔS > 0). Based on the thermodynamic definition of a reaction’s free energy change (ΔG = ΔΗ-ΤΔS) and the algebraic signs of ΔΗ and ΔS, we conclude ΔG < 0, i.e., hypergolic reactions are thermodynamically spontaneous at ambient conditions. On the other hand, the reaction proceeds instantaneously as a result of zero spin change upon moving from reactants to products [e.g., reactants and products in equation (1) are all diamagnetic species having zero spin]. Such reactions are spin allowed and therefore associated with a small activation energy Ea and fast kinetics. What’s more, the high concentration of the reactants may also contribute to a fast reaction rate.

figure 2: Dropwise addition of fuming nitric acid HNO3 into aniline instantly triggers ignition of the hypergolic mixture inside the “rocket” test tube.
In most hypergolic propellant compositions, hydrazine derivatives, heterocyclics, long-chain aliphatics, N-alkyl diamines, aromatics, sugars, ionic liquids, metal-organic frameworks (MOFs) and metal powders (Al, Mg) play the role of fuel, whereas fuming nitric acid HNO3, N2O4, NH4ClO4 (AP), KClO3, dry ice CO2, concentrated H2O2 and Na2O2 the role of strong oxidizer [1-12]. It is surprising though that combustible carbon with a relatively high energy value of ca. 30 kJ g-1 is hardly incorporated in hypergolic mixtures as solid fuel [13]. Actually, carbon hypergolicity is quite rare in the literature limiting to very few cases. first, activated charcoal has been reported to ignite upon contact with Na2O2 in the presence of a small amount of water (see Bretherick’s Handbook of Reactive Chemical Hazards). Second, fullerenes and Na2O2 have been shown to ignite upon mechanochemical mixing of the two reagents [14]. Third, carbon fine dust has been known to be explosive in air under certain circumstances [15]. Fourth, coal ignites and burns upon contact with F2 gas at ambient conditions [16]. fifth, flash ignition of carbon nanotubes provides another interesting example of carbon hypergolicity [17]. And sixth, we should not forget to mention the myth of “pyrophoric carbon” [18], i.e., a self-ignitable form of carbon obtained from mild pyrolysis of cut herbage at 250-300 oC. In all the above-mentioned cases, carbon ignition takes place spontaneously at ambient conditions without external stimuli. Although carbon is a plentiful, low-cost, non-corrosive and non-toxic high energy material, its application as solid fuel in rocket engines has remained largely unexplored. This could be partly ascribed to the fact that elemental carbon is difficult to ignite at ambient conditions without external stimuli, requiring very strong yet highly toxic and corrosive oxidizers, such as fluorine gas.
Herein we present some preliminary results regarding carbon hypergolicity in the presence of sodium hydride NaH and water, the latter two reacting exothermically upon contact to trigger carbon combustion at ambient conditions. All the components in the mixture are more abundant, cheaper and less hazardous than those employed in conventional hypergolic fuels. For instance, the ionic liquids and MOFs mentioned earlier are relatively scarce and expensive materials, while fuming nitric acid HNO3 and concentrated H2O2 are highly corrosive and explosive reagents. For making the water-ignitable C-NaH mixtures, sodium hydride was merely mixed with sought-after graphene allotropes, such as fullerenes C60 and multiwall carbon nanotubes MWNTs, as well as, with common types of amorphous carbons, such as charcoal and active carbon, followed by ignition of the mixtures with water. The present water-ignitable C-NaH mixtures may pave the way towards the advancement of new generation hypergolic propellants based on energetic carbon allotrope compositions.
2. Materials and Methods
Fullerenes C60, multiwall carbon nanotubes MWNTs, active carbon and sodium hydride NaH (dry, 90 %) were purchased from Aldrich whereas charcoal from Merck. All samples were in powder form (figure 3). De-ionized water was used for the ignition of the C-NaH mixtures. For safety reasons, all experiments were conducted in a fume hood using Pyrex glass test tubes 1.6 cm x 16 cm and small amounts of reagents. Each C-NaH mixture was made by mechanically mixing 110 mg of carbon (250 mg in the case of fullerenes C60) with 530 mg NaH in a glass test tube. Following, the mixtures were ignited in a fume hood by the dropwise addition of 1 mL de-ionized water (1 drop per second). Direct addition of water into a C-NaH mixture results in violent ignition and hence should be avoided.
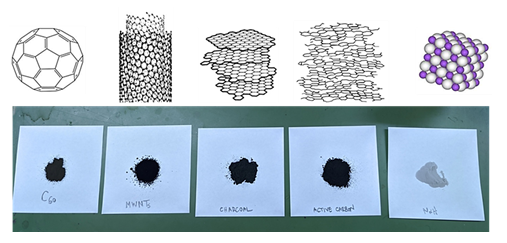
figure 3: Carbon materials used in the water-ignitable C-NaH mixtures: fullerenes C60, multiwall carbon nanotubes MWNTs, charcoal and active carbon. The grey powder on the right is sodium hydride.
3. Results and Discussion
The dropwise addition of water into a test tube containing the C-NaH mixture (C: fullerenes C60, multiwall carbon nanotubes MWNTs, charcoal or active carbon) triggered a fast and bright ignition with bursts of yellow flames as shown in figure 4 (the yellow color of the flames stems from sodium ions). Ignition was smooth and continuous for multiwall carbon nanotubes MWNTs and active carbon but more abrupt for fullerenes C60 and charcoal.

figure 4: Dropwise addition of water into a test tube containing a mixture of carbon (C60, MWNTs, charcoal or active carbon) and sodium hydride (left column) triggered a fast and bright ignition with bursts of yellow flames (right column).
In a blank experiment, the dropwise addition of 1 mL deionized water (1 drop per second) into a test tube (1.6 cm x 16 cm) containing 530 mg NaH (without carbon) gave a strongly exothermic reaction without ignition (figure 5). After reaction completion and dissolution of the hydride solid, a hot concentrated aqueous solution of NaOH was obtained. On the other hand, carbon materials such as detonation nanodiamonds, graphite and carbon fibers gave no ignition in the presence of NaH and water under identical conditions. Based on literature data on the Thermal Gravimetric Analysis (TGA) profiles under air of the studied carbons, such disparity could be ascribed to the fact that air-combustion of fullerenes C60, multiwall carbon nanotubes MWNTs, charcoal and active carbon generally commences below 500 oC [19-21], whereas that of nanodiamonds, graphite and carbon fibers above 500 oC [22-24], meaning that ignition of the former is easier than that of the latter.
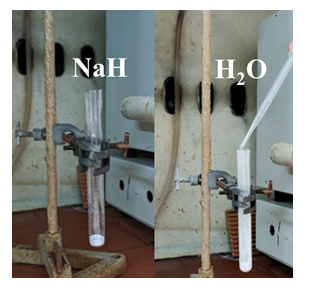
figure 5: In a blank experiment, the dropwise addition of water into a test tube containing NaH (without carbon) triggered a strongly exothermic reaction without ignition.
The following set of reactions concurrently occurs during ignition of the C-NaH mixture by water:
NaH + H2O → NaOH + H2 (2)
H2 + ½ O2 → H2O (3)
C + O2 → CO2 (4)
CO2 + 2 NaOH → Na2CO3 + H2O (5)
By multiplying (2) and (3) with two and algebraically adding equations (2)-(5), the following neat reaction is obtained:
C + 2 NaH + 2 O2 → Na2CO3 + H2O (6)
, for which ΔH = -1260 kJ, ΔS = -0.156 kJ/K and ΔG = -1214 kJ (1 atm, 298 K) (i.e., the reaction is exothermic and spontaneous at ambient conditions in spite of the negative sign of ΔS). Simultaneously, under the extreme ignition conditions, diamagnetic singlet O2 is expected to actively involved in equation (6) [25,26], thus reducing the activation energy Ea of the spin-allowed reaction as moving from diamagnetic reactants to diamagnetic products. Moreover, water is consumed and formed according to equations (2) and (3), while at the same time appears as product but not reactant in equation (6). Therefore it is likely that water acts as an autocatalyst, thus further decreasing the activation energy Ea of the reaction. The use of water as an ignition catalyst is not new in the literature, with typical examples including the water-ignitable Al-I2 mixture, the flash reaction (Mg-AgNO3) and the “Negative X” agent (Zn-NH4NO3-NaCl). Lastly, it is interesting to note that the released CO2 gas in the above set of reactions is finally converted into Na2CO3, thus presenting another advantage of the water-ignitable C-NaH system in reducing greenhouse emissions.
4. Conclusions
A new generation of carbon-containing hypergolic fuels has been presented based on water-ignitable C-NaH mixtures (C: fullerenes C60, multiwall carbon nanotubes MWNTs, charcoal, active carbon). Such a hypergolic pair uniquely combines high energy value, low-toxicity, low corrosiveness and low-cost carbon allotropes with sodium hydride, giving fast and spontaneous ignitions at ambient conditions by the dropwise addition of water catalyst, and producing fewer CO2 emissions due to conversion of the greenhouse gas into Na2CO3. Since carbon hypergolicity is a rare phenomenon in the literature, these findings serve as a proof-of-concept that could stimulate further research in developing a larger series of carbon-based hypergolic compositions for rocket engines. As a future outlook, coal, hydroxylated fullerenes, graphite oxide, fluorinated graphite, single-walled carbon nanotubes, carbon nanofibers and carbon dots are some additional examples of carbon materials that could be successfully tested in this context.
Conflict of interest
The author declared that he has no conflicts of interest to this work.
References
- Rastogi RP, Munjal NL. Mechanism and kinetics of pre-ignition reactions: part I-aniline-red fuming nitric acid propellants. Indian J Chem 4 (1966): 463-468.
- Munjal NL. Ignition catalysts for furfuryl alcohol-red fuming nitric acid bipropellant. AIAA J 8 (1970): 980-981.
- Gune SG, Kulkarni SG, Panda SP. Synergistic hypergolic ignition of solid substituted anilines mixed with magnesium powder and red fuming nitric acid. Combust. Flame 61 (1985): 189-193.
- Schneider S, Hawkins T, Rosander M, et al. Ionic liquids as hypergolic fuels. Energy Fuels 22 (2008): 2871-2872.
- Kulkarni SG, Bagalkote VS, Patil SS, et al. Theoretical evaluation and experimental validation of performance parameters of new hypergolic liquid fuel blends with red fuming nitric acid as oxidizer. Propellants Explos Pyrotech 34 (2009): 520-525.
- Kulkarni S, Bagalkote V. Studies on pre-ignition reactions of hydrocarbon-based rocket fuels hypergolic with red fuming nitric acid as oxidizer. J Energet Mater 28 (2010): 173-188.
- Silva GD, Lha K. Hypergolic systems: a review in patents. J Aerosp Technol Manag 4 (2012): 407-412.
- Bhosale MVK, Kulkarni SG, Kulkarni PS. Ionic liquid and biofuel blend: a low-cost and high performance hypergolic fuel for propulsion application. ChemistrySelect 1 (2016): 1921-1925.
- Titi HM, Marrett JM, Dayaker G, et al. Hypergolic zeolitic imidazolate frameworks (ZIFs) as next-generation solid fuels: unlocking the latent energetic behavior of ZIFs. Sci Adv 5 (2019): 8.
- Yuan WL, Zhang L, Tao G-H, et al. Designing high-performance hypergolic propellants based on materials genome. Sci. Adv 6 (2020): 9.
- Maggi F, Zadra F. Combustion of nanoaluminum and magnesium in fuel-rich propellants. Propellants Explos. Pyrotech 45 (2020): 724-729.
- Xu Y, Wang Y, Zhong Y, et al. High-energy metal-organic frameworks with a dicyanamide linker for hypergolic fuels. Inorg. Chem 60 (2021): 5100-5106.
- Yan Q-L, Gozin M, Zhao FQ, et al. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 8 (2016): 4799-4851.
- Chalmpes N, Spyrou K, Vasilopoulos KC, et al. Hypergolics in carbon nanomaterials synthesis: new paradigms and perspectives. Molecules 25 (2020): 2207.
- Turkevich LA, Fernback J, Dastidar AG, et al. Potential explosion hazard of carbonaceous nanoparticles: screening of allotropes. Combust. Flame 167 (2016): 218-227.
- Huston JL, Scott RG, Studier MH. Reaction of fluorine gas with coal and the aromaticity of coal. Fuel 55 (1976): 281-286.
- Ajayan PM, Terrones M, De La Guardia A, et al. Nanotubes in a flash-ignition and reconstruction, Science 296 (2002): 705.
- Cuzzillo BR, Pagni PJ. The myth of pyrophoric carbon. fire Saf Sci 6 (2000): 301-312.
- Sabounchei SJ, Hashemi A, Hosseinzadeh M, et al. [60]Fullerene-based Pd(0) complexes of phosphorus ylides as efficient nanocatalyst for homo- and heterogeneous Mizoroki-Heck coupling reactions. Catal Lett 147 (2017): 2319-2331.
- Giraldo LF, Brostow W, Devaux E, et al. Scratch and wear resistance of polyamide 6 reinforced with multiwall carbon nanotubes. J Nanosci Nanotechnol 8 (2008): 1-8.
- Oliveira LCA, Pereira E, Guimaraes IR, et al. Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J. Hazard. Mater 165 (2009): 87-94.
- Laube Ch, Riyad YM, Lotnyk A, et al. Defined functionality and increased luminescence of nanodiamonds for sensing and diagnostic applications by targeted high temperature reactions and electron beam irradiation. Mater Chem Front 1 (2017): 2527-2540.
- Kim IT, Lee J, An JC, et al. Capacity improvement of tin-deposited on carbon-coated graphite anode for rechargeable lithium ion batteries. Int. J. Electrochem. Sci. 11 (2016): 5807-5818.
- Xu Z, Chen Y, Li W, et al. Preparation of boron nitride nanosheet-coated carbon fibres and their enhanced antioxidant and microwave-absorbing properties. RSC Adv 8 (2018): 17944-17949.
- Al-Nu’airat J, Altarawneh M, Oluwoye I, et al. Role of singlet oxygen in combustion initiation of aromatic fuels. Energy Fuels 32 (2018): 12851-12860.
- Al-Nu'airat J, Oluwoye I, Zeinali N, et al. Review of chemical reactivity of singlet oxygen with organic fuels and contaminants. Chem Rec 21 (2020): 315-342.

 Impact Factor: * 2.9
Impact Factor: * 2.9 Acceptance Rate: 78.36%
Acceptance Rate: 78.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 